Abstract
Recombinant adenylate cyclase toxoids are shown to deliver inserted foreign CD4+-T-cell epitopes into the major histocompatibility complex class II presentation pathway, inducing a specific CD4+-T-cell response in vivo and yielding in vitro stimulation of specific CD4+ T cells at a 100-times-higher molar efficiency than the free peptide containing the epitope.
The Bordetella pertussis adenylate cyclase (AC) toxin (ACT or CyaA) can penetrate a variety of cells, and upon reaching their cytosol, it perturbs cellular physiology by unregulated conversion of ATP to cyclic AMP (10). Genetic ablation of the AC activity, however, does not affect cell invasiveness of ACT (12). AC toxoids can hence be used as a potent new tool for delivery of vaccinal CD8+-T-cell epitopes into the cytosolic major histocompatibility complex (MHC) class I antigen presentation pathway (5). Moreover, it was recently found that ACT specifically targets the β2 integrin molecule CD11b, which is present on cells of the myeloid lineage and in particular on professional antigen-presenting cells (APCs), such as dendritic cells (6). This opens appealing possibilities of using the ACT toxoid as a nonreplicative vector for antigen delivery aiming at stimulation of specific cellular immune responses. We have, indeed, recently used recombinant ACT for delivery of foreign epitopes and for induction of protective antiviral, as well as therapeutic antitumoral CD8+ cytotoxic T-cell responses in mice (1, 2, 4, 7, 12, 13, 15, 16).
In certain cases, such as particularly for antitumor immunity, activation of CD4+ T-helper cells in parallel to induction of CD8+ T cytotoxic cells appears, however, necessary for optimal and long-lasting immune responses (9, 14, 17). The CD4+ T-helper cells are generally activated by exogenous antigens that have been taken up by APCs via phagocytosis or endocytosis and processed through the MHC class II antigen presentation pathway. After processing, antigenic peptides bound to MHC class II molecules are presented at the cell surface and are recognized by T-cell receptors of CD4+ T cells. Therefore, we tested whether ACT can deliver CD4+-T-cell epitopes for efficient endosomal processing and MHC-class II-restricted presentation, yielding specific stimulation of CD4+-T-cell responses.
We used the previously defined permissive insertion sites along the ACT molecule (12) and generated a set of 11 ACT constructs, which carried an H-2b/H-2d-restricted CD4+-T-cell epitope NGKLIAYPIAVEALS from the Escherichia coli maltose binding protein MalE (11). The recombinant ACT molecules were expressed in E. coli and purified, and their cell-invasive capacity was characterized, using sheep erythrocytes as the surrogate target cell model (Table 1). As expected, 9 of the 11 ACT/MalE constructs exhibited over 60% of membrane insertion (binding) and hemolytic capacity, and seven of these constructs conserved over 90% of the cell-invasive capacity of wild-type ACT. The capacity to bind and penetrate erythrocytes was nil for the ACT926/MalE construct, whereas the ACT594/MalE and ACT607/MalE proteins exhibited reduced hemolytic and cell-invasive activities. The ACT336/MalE construct did not exhibit any measurable AC activity, and its cell-binding and invasive activity could not be quantified. The full hemolytic activity, however, indicated that its cell-targeting capacity was intact.
TABLE 1.
Characteristics of ACT constructs with MalE epitope
| ACT proteina | Insertion point/flanking sequencesb | Binding activityc | Invasive AC activity c,d | Hemolytic activityc |
|---|---|---|---|---|
| ACT wild type | None | 100 ± 11 | 100 ± 12 | 100 ± 15 |
| ACT108/MaIE | AHG107VLNGKLIAYPIAVEALSVH108HTA | 97 ± 12 | 99 ± 10 | 98 ± 13 |
| ACT133/MaIE | TGM132VYGNGKLIAYPIAVEALSYT134DGV | 96 ± 11 | 98 ± 12 | 95 ± 17 |
| ACT233/MaIE | TGG232VLNGKLIAYPIAVEALSVH233LDR | 100 ± 8 | 98 ± 13 | 99 ± 8 |
| ACT336/MaIE | YIG335VLNGKLIAYPIAVEALSVH336QQR | NDe | ND | 96 ± 9 |
| ACT424/MaIE | SDM423VYGNGKLIAYPIAVEALSYT425AAV | 90 ± 15 | 99 ± 6 | 111 ± 20 |
| ACT594/MaIE | LLA593CTNGKLIAYPIAVEALSGT594RGV | 75 ± 23 | <2 | 20 ± 5 |
| ACT607/MaIE | GAS606VYGNGKLIAYPIAVEALSYT608GAA | 61 ± 18 | 15 ± 8 | 80 ± 34 |
| ACT751/MaIE | EQL750VYGNGKLIAYPIAVEALSYT752NS | 83 ± 11 | 93 ± 8 | 99 ± 15 |
| ACT926/MaIE | NAS925CTNGKLIAYPIAVEALSGT926RIH | 0 | 0 | 0 |
| ACT1334/MaIE | WFG1333VLNGKLIAYPIAVEALSVH1334NTQ | 88 ± 15 | 90 ± 11 | 99 ± 8 |
| ACT1648/MaIE | WYR1647VLNGKLIAYPIAVEALSVH1648DAD | 100 ± 9 | 98 ± 12 | 88 ± 7 |
Three double-stranded synthetic oligonucleotides in the required reading frames were inserted into the unique BsrGI sites located at different positions within the cyaA gene on a set of pT7CACT1-BsrGI plasmids (12). The orientation and exact sequence of all inserted oligonucleotides were verified by DNA sequencing, and the proteins were produced and purified as previously described (8, 16).
The MaIE epitope sequence is underscored, and the flanking residues are indicated in boldface.
Samples were tested using sheep erythrocytes as model target cells and expressed as percent wild-type ACT activity. The activities of the proteins were determined prior to ablation of the AC activity (insertion of a dipeptide insert between residues 188 and 189) as described previously (12). The activities are expressed in percent wild-type ACT activity and represent average values with standard deviations from at least three independent determinations performed in duplicates with three different toxin preparations (n = 6).
Invasive activity was determined as the AC activity translocated into sheep erythrocytes and protected against digestion by extracellularly added trypsin (12).
ND, not determined because of no measurable AC activity.
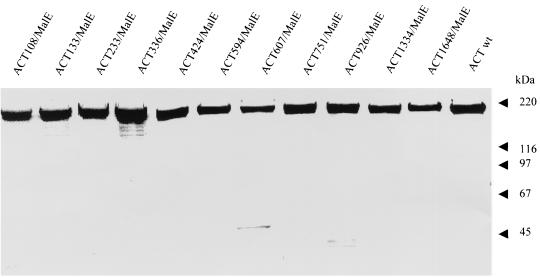
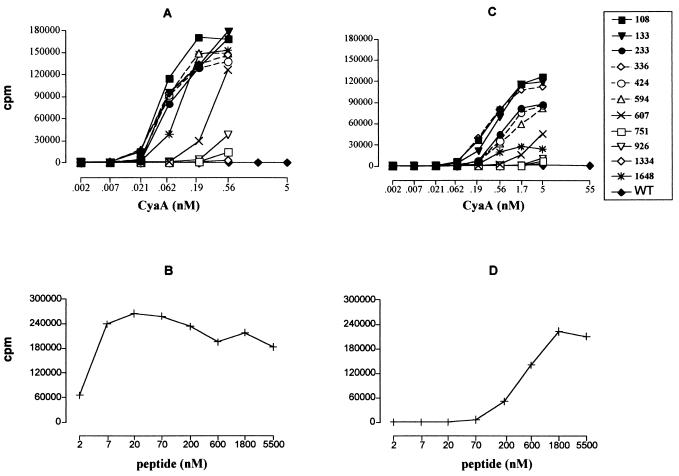
To avoid interference of the toxin activity of ACT (conversion of intracellular ATP to cyclic AMP) with the physiology of the cells used for in vitro presentation assays, the AC activity of the individual ACT/MalE constructs was genetically ablated by disruption of their respective ATP binding sites (4). The obtained toxoids were purified close to homogeneity, as documented in Fig. 1, and their capacity to deliver the inserted MalE CD4+-T-cell epitope into the MHC class II antigen processing and presentation pathway was first determined in vitro. This was measured as the potency of AC toxoids, incubated with mouse splenocytes as APCs, for stimulating interleukin 2 (IL-2) release from CRMC3 or FBCD1 T-cell hybridomas (11), which specifically recognize the NGKLIAYPIAVEALS peptide complexed at the surface of APCs with the MHC class II H-2b or H-2d molecules, respectively. As shown in Fig. 2, APCs incubated with up to 55 nM mock AC toxoid did not stimulate any IL-2 release from the two hybridoma T cells. In contrast, the six ACT/MalE proteins, carrying the epitope within the first 600 residues, mediated an efficient delivery and subsequent presentation of the MalE peptide to the H-2b-restricted CRMC3 T cells already at 0.06 nM toxoid concentrations (Fig. 2A). It should be noted that approximately hundred-times-higher concentrations of the free synthetic peptide were necessary to induce similar IL-2 production by the same hybridoma (Fig. 2B). The two toxoids bearing the CD4+-T-cell epitope at positions 607 and 1648 induced intermediate IL-2 production. Finally, the three toxoids with the MalE peptide at positions 751, 926, and 1334 induced very low responses of the CRMC3 T-cell hybridoma, even at the highest concentration tested (0.56 nM). A similar response pattern, albeit at higher AC toxoid concentrations, was observed also for the FBCD1 T-cell hybridoma, which recognizes the same epitope complexed with H-2d molecules (Fig. 2C and D). It can, therefore, be concluded that the constructs with the MalE epitope inserted within the first third of the toxoid molecule were particularly efficient in delivering the epitope for endosomal proteolytic processing and subsequent MHC-class II-dependent antigenic presentation resulting in productive recognition by T-cell hybridoma. These results suggest that the carrier ACT moiety was accounting for the delivery efficiency, by targeting the epitope into APCs.
FIG. 1.
SDS-PAGE analysis of the purified ACT/MalE preparations. The proteins were purified from urea extracts by DEAE- and phenyl-Sepharose chromatography as previously described (8), and 1 to 3 μg of each was analyzed together with the wild-type ACT on a 7.5% acrylamide gel stained with Coomassie blue.
FIG. 2.
Most of the ACT/MalE proteins efficiently deliver the inserted CD4+-T-cell epitope for presentation to specific T cells. Splenocytes from C57BL/6 (A) or BALB/c (C) mice were used as APCs. Various concentrations of the AC toxoids, harboring the MalE CD4+-T-cell epitope at different sites, were added to APCs. The APCs were then cocultured with 105 cells of CRMC3 (A) or FBCD1 (C) anti-MalE CD4+-T-cell hybridoma, which selectively recognize complexes of the H-2b or H-2d MHC class II molecules with bound MalE peptide (NGKLIAYPIAVEALS), respectively. As positive controls, C57BL/6 (B) or BALB/c (D) splenocytes were incubated with various concentrations of the MalE peptide and CRMC3 (B) or FBCD1 cells (D). After 18 h of culture, supernatants were frozen for at least 2 h at −80°C. The amount of IL-2 produced by the stimulated CRMC3 and FBCD1 cells was then determined by the CTLL proliferation method. Briefly, 104 cells of the IL-2-dependent CTLL line per well were cultured with 100 μl of supernatant in 200 μl of final volume. Twenty-four hours later, [3H]thymidine (50 μCi/well) was added and cells were harvested 6 h later with an automated cell harvester. Incorporated thymidine was detected by scintillation counting. Each point was done in duplicate, and the experiment was repeated four times. Results are expressed in change in counts per minute of incorporated [3H]thymidine (counts per minute in the presence − counts per minute in the absence of ACT). WT, wild type.
Interestingly, there was no influence of the residues flanking the epitope on MHC class II presentation. All three insertion contexts that were used (Table 1) allowed a comparable efficiency of T-cell hybridoma stimulation by inserts located within the first 600 residues of ACT (compare Table 1 and Fig. 2). Moreover, there was no clear-cut correlation between the capacities of ACT constructs to penetrate erythrocyte membrane and their capacities to deliver the MalE epitope for presentation on MHC class II molecules of APCs. Erythrocytes lack the ACT receptor (CD11b) and endocytosis mechanisms (6). The erythrocyte invasion assay allows, hence, only characterization of the membrane penetration activity of ACT constructs, which cannot fully reflect the more complex interactions of ACT with APCs. Nevertheless, the nil erythrocyte-binding and penetration activity may explain the very poor, if any, epitope delivery capacity of the ACT926/MalE toxoid. The ACT with the MalE insert at positions 751 and 1334 exhibited, however, full erythrocyte penetration capacity, while performing very poorly in the T-cell stimulation assay. It is possible that upon interaction with cells, only certain portions of the ACT polypeptide, such as its first 600 residues and the very C-terminal portion comprising an insert at position 1648, may effectively reach the endosomal entry of the MHC class II pathway of APCs. Presentation of the inserts at positions 751 and 1334 may also be inhibited by structural constraints preventing epitope excision within endosomes or by translocation of those ACT portions to the cytosolic side of the endosomal membrane.
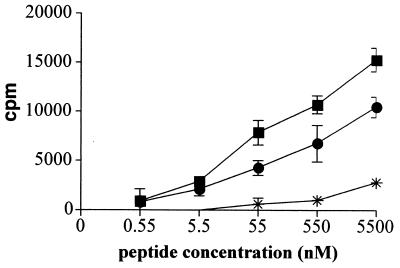
The present results, however, demonstrate that 6 out of 11 constructed AC toxoids delivered a model CD4+-T-cell epitope for presentation in vitro with high efficiency. Two of the best-performing constructs were, therefore, tested in vivo for the capacity to induce specific CD4+-T-cell responses. As shown in Fig. 3, 1 week after immunization the splenocytes of mice that intravenously received a single dose of 50 μg of the ACT108/MalE and ACT336/MalE toxoids exhibited a strong proliferative response to the cognate MalE peptide, as compared to splenocytes isolated from mice that received the same dose of mock AC toxoid. It can, hence be concluded that the ACT/MalE toxoids induced a CD4+-T-cell response specific for the MalE epitope.
FIG. 3.
In vivo induction of CD4+-T-cell responses by MalE/ACT proteins. C57BL/6 mice were intravenously injected with 50 μg of MalE/ACT proteins bearing the MalE epitope at site 108 (•) or 336 (▪). Mice were injected with mock AC toxoid (asterisks) as negative control. One week later, the mice were sacrificed and the splenocytes were in vitro stimulated with various concentrations of the MalE peptide. After 72 h of culture, [3H]thymidine (50 μCi/well) was added and cells were harvested 6 h later with an automated cell harvester. Incorporated thymidine was detected by scintillation counting. Results show the antigen-specific proliferation obtained for one representative mouse out of four tested in two independent experiments. Each point was done in triplicate and standard deviations are indicated by the bars (n = 3). Results are expressed in changes in counts per minute of incorporated [3H]thymidine (counts per minute in the presence of peptide − counts per minute in the absence of peptide).
The mechanism by which the MalE epitope inserted into ACT reaches the MHC-class II-dependent antigen presentation pathway remains to be elucidated. It is, however, plausible to assume that ACT endocytosis may be promoted by interaction with its β2 integrin (CD11b) receptor (6) and that this may direct the inserted MalE epitope to the endosomal pathway. Upon proteolytic processing, the excised epitope could then associate with MHC class II molecules, leading to presentation at the cell surface. Recently, ACT was also shown to efficiently deliver several CD8+-T-cell epitopes inserted within its AC moiety (3). These results suggest that ACT could be used for simultaneous delivery of CD4+- and CD8+-T-cell epitopes to professional APCs and for parallel induction of both T-helper and T cytotoxic responses. ACT appears, therefore, to be a promising candidate for immunotherapy protocols targeting cancer or chronic viral infections, where coactivation of specific CD4+- and CD8+-T-cell responses is required.
Acknowledgments
We thank Martina Hr̆ibová for help with AC assays and Gilles Dadaglio for critical reading of the manuscript.
This work was supported by ANRS and by grants no. 310/01/0934, A502907, and ME167 of the Grant Agency, the Academy of Science, and the Ministry of Education, Youth, and Sports of the Czech Republic, respectively, and QLK2-CT-1999-00556 from the 5th Framework Program of the European Union.
Editor: J. D. Clements
REFERENCES
- 1.Dadaglio, G., Z. Moukrim, R. Lo-Man, V. Sheshko, P. Sebo, and C. Leclerc. 2000. Induction of a polarized Th1 response by insertion of multiple copies of a viral T-cell epitope into adenylate cyclase of Bordetella pertussis. Infect. Immun. 68:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fayolle, C., D. Ladant, G. Karimova, A. Ullmann, and C. Leclerc. 1999. Therapy of murine tumors with recombinant Bordetella pertussis adenylate cyclase toxins carrying a cytotoxic T-cell epitope. J. Immunol. 162:4157-4162. [PubMed] [Google Scholar]
- 3.Fayolle, C., A. Osickova, R. Osicka, T. Henry, M.-J. Rojas, M.-F. Saron, P. Sebo, and C. Leclerc. 2001. Delivery of multiple epitopes by recombinant detoxified adenylate cyclase of Bordetella pertussis induces protective antiviral immunity. J. Virol. 75:7330-7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fayolle, C., P. Sebo, D. Ladant, A. Ullmann, and C. Leclerc. 1996. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J. Immunol. 156:4697-4706. [PubMed] [Google Scholar]
- 5.Guermonprez, P., C. Fayolle, G. Karimova, A. Ullmann, C. Leclerc, and D. Ladant. 2000. Bordetella pertussis adenylate cyclase toxin: a vehicle to deliver CD8-positive T-cell epitopes into antigen-presenting cells. Methods Enzymol. 326:527-542. [DOI] [PubMed] [Google Scholar]
- 6.Guermonprez, P., N. Khelef, E. Blouin, P. Rieu, P. Ricciardi-Castagnoli, N. Guiso, D. Ladant, and C. Leclerc. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (CD11b/CD18). J. Exp. Med. 193:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guermonprez, P., D. Ladant, G. Karimova, A. Ullmann, and C. Leclerc. 1999. Direct delivery of the Bordetella pertussis adenylate cyclase toxin to the MHC class I antigen presentation pathway. J. Immunol. 162:1910-1916. [PubMed] [Google Scholar]
- 8.Karimova, G., C. Fayolle, S. Gmira, A. Ullmann, C. Leclerc, and C. Ladant. 1998. Charge-dependent translocation of Bordetella pertussis adenylate cyclase toxin into eukaryotic cells: implication for the in vivo delivery of CD8+ T cell epitopes into antigen-presenting cells. Proc. Natl. Acad. Sci. USA 95:12532-12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern, D. E., J. P. Klarnet, M. C. Jensen, and P. D. Greenberg. 1986. Requirement for recognition of class II molecules and processed tumor antigen for optimal generation of syngeneic tumor-specific class I-restricted CTL. J. Immunol. 136:4303-4310. [PubMed] [Google Scholar]
- 10.Ladant, D., and A. Ullmann. 1999. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7:172-176. [DOI] [PubMed] [Google Scholar]
- 11.Lo-Man, R., J. P. M. Langeveld, E. Dériaud, M. Jehanno, M. Rojas, J. M. Clément, R. H. Meloen, M. Hofnung, and C. Leclerc. 2000. Extending the CD4+ T-cell epitope specificity of the Th1 immune response to an antigen using a Salmonella enterica serovar Typhimurium delivery vehicle. Infect. Immun. 68:3079-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osic̆ka, R., A. Osic̆kova, T. Basar, P. Guermonprez, M. Rojas, C. Leclerc, and P. S̆ebo. 2000. Delivery of CD8+ T-cell epitopes into major histocompatibility complex class I antigen presentation poathway by Bordetella pertussis adenylate cyclase: delineation of cell invasive structures and permissive insertion sites. Infect. Immun. 68:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saron, M. F., C. Fayolle, P. Sebo, D. Ladant, A. Ullmann, and C. Leclerc. 1997. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. USA 94:3314-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnell, S., J. W. Young, A. N. Houghton, and M. Sadelain. 2000. Retrovirally transduced mouse dendritic cells require CD4+ T cell help to elicit antitumor immunity: implications for the clinical use of dendritic cells. J. Immunol. 164:1243-1250. [DOI] [PubMed] [Google Scholar]
- 15.S̆ebo, P., C. Fayolle, O. d'Andria, D. Ladant, C. Leclerc, and A. Ullmann. 1995. Cell-invasive activity of epitope-tagged adenylate cyclase of Bordetella pertussis allows in vitro presentation of a foreign epitope to CD8+ cytotoxic T cells. Infect. Immun. 63:3851-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebo, P., Z. Moukrim, M. Kalhous, N. Schaft, G. Dadaglio, V. Sheshko, C. Fayolle, and C. Leclerc. 1999. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying multiple copies of a viral CD8+ T-cell epitope. FEMS Immunol. Med. Microbiol. 26:167-173. [DOI] [PubMed] [Google Scholar]
- 17.Toes, R. E., F. Ossendorp, R. Offringa, and C. J. Melief. 1999. CD4+ T cells and their role in antitumor immune responsesJ. Exp. Med. 189:753-756. [DOI] [PMC free article] [PubMed] [Google Scholar]