Abstract
Adherence of lymphocytes to the fungus is the first step in the direct lymphocyte-mediated antifungal effect against Candida albicans. In this study we identified macrophage-1 antigen (Mac-1) (CD11b/CD18, αM/β2) as the lymphocyte surface structure responsible for the adhesion of activated lymphocytes to the hyphal form of the fungus. Antibodies specific for epitopes of the α-subunit (CD11b) and the β2-subunit (CD18) of Mac-1 were shown to completely eliminate lymphocyte adhesion to C. albicans hyphae. Lymphocyte adhesion to C. albicans was also inhibited significantly by known ligands of Mac-1, including the extracellular matrix proteins laminin and fibrinogen, as well as engineered peptides containing arginine-glycine-aspartic acid sequences and the disintegrin echistatin. N-Acetyl-d-glucosamine and β-glucan, which inhibit Mac-1-mediated adhesion to the yeast, blocked lymphocyte adhesion to hyphae. NIH 3T3 fibroblast transfectants expressing human CD11b/CD18 bound to C. albicans, and their binding was inhibited by antibodies specific for CD11b/CD18. Finally, antibodies specific for CD11b/CD18 effectively inhibited the capacity of activated lymphocytes to have an antifungal effect against hyphae. Our results clearly identify Mac-1 (CD11b/CD18) as the lymphocyte surface structure that mediates activated lymphocyte adhesion to C. albicans and the resultant antifungal effect of the lymphocytes.
Fungal infections are a serious public health hazard, particularly for the growing population of immunocompromised individuals. Fungi are the cause of significant morbidity and/or mortality in patients with AIDS, patients undergoing chemotherapy for cancer treatment or for the prevention of transplanted organ rejection, burn patients, surgical patients, trauma patients, the very young, and the aged (18, 19, 39). One of the leading causes of such infections is Candida albicans. C. albicans is a dimorphic fungus which is found in mammals as part of the normal microbial flora. This fungus is an opportunistic pathogen which can produce life-threatening disease in mammalian hosts who are immunocompromised (12). Typically, individuals who carry the microorganism do not display symptoms of infection unless they are weakened by an underlying disease or disorder that reduces resistance to microbial invasion. Concern regarding fungal infection is justified and is a consequence of the lack of second-tier drugs and the increasing resistance of fungi to older antifungal drugs (38, 44). Treatments are palliative and unfortunately, must include strategies for long-term administration of toxic and irritating antifungal agents (14). Thus, there is strong motive to understand the immune response to this opportunistic pathogen.
Cell-mediated immunity (CMI) mediated by lymphocytes is an important form of host defense against fungi and is probably the principal defense at mucosal and epidermal surfaces (4, 11, 20, 28). During the CMI response to fungi, lymphocytes can release cytokines that not only enhance CMI but also modulate the antifungal activity of polymorphonuclear leukocytes and macrophages (47). In addition, natural killer (NK) cells and interleukin-2 (IL-2)-activated lymphocytes (IAL) have been shown to interact directly with and inhibit the growth of fungi (6, 8, 29, 36). The role of each of these forms of lymphocyte-mediated, antifungal host defense is dependent upon the immune status of the host and upon the individual fungal pathogen (11, 34, 35).
Previous observations in our laboratory have shown that IAL inhibit hyphal growth of C. albicans (6, 8). These activated lymphocytes have a large granular lymphocyte appearance, and it has been shown that they must directly contact C. albicans hyphae to inhibit growth (6, 22, 23). The interaction between lymphocytes and C. albicans has been demonstrated in a number of ways, including competitive inhibition of mammalian cell binding to the fungus (6, 55), direct measurement of adhesion of lymphocytes to fungal hyphae (22, 23), and yeast cell stimulation of cytokine synthesis (5, 9, 31). However, the interactive nature of the lymphocyte surface structures which mediate adhesion to C. albicans have not been well characterized. This investigation was undertaken to identify the structures on IAL that mediate adhesion to C. albicans.
MATERIALS AND METHODS
Mice.
C57BL/6 and BALB/c female mice that were 6 to 7 weeks old were obtained from Jackson Laboratory, Bar Harbor, Maine. The mice were 6 to 12 weeks old when they were used in experiments. BALB/c mice were used solely for preparation of monoclonal antibody (mAb)-containing ascites fluid.
Fungal culture.
C. albicans ATCC 58716 (American Type Culture Collection, Rockville, Md.) was obtained from T. Hashimoto, Loyola University of Chicago, Maywood, Ill., and was used throughout this investigation. Cultures were stored at 25°C on Sabouraud’s dextrose agar (SDA) (Becton Dickinson, Lincoln Park, N.J.). Cells used for experiments were cultured overnight at 37°C on SDA, collected as isolated colonies, and washed once in complete Hanks’ balanced salt solution (HBSS) containing sodium bicarbonate (pH 7.4) (GIBCO, Grand Island, N.Y.). Yeast cultures were enumerated microscopically.
IL-2 activation of murine and human lymphocytes.
To prepare murine IAL (mIAL), spleens were aseptically removed from untreated mice. Single-cell suspensions were prepared by dissociating the spleens by using a 60-gauge wire mesh and the hub of a syringe. The spleen cells were washed once in HBSS before they were placed in culture medium containing 50 μM 2-mercaptoethanol and 1,500 U of IL-2 (Hoffman-LaRoche, Nutley, N.J.) per ml in Falcon 24-well plates (Becton Dickinson) as described previously (8); the cell concentration was 2.5 × 106 cells per ml. Nonadherent cells were harvested following incubation for 6 days at 37°C, overlaid on lymphocyte separation medium (Litton Bionetics, Kensington, Md.), and centrifuged at 1,000 × g for 20 min. The cells at the interface were washed twice with HBSS prior to assessment of growth-inhibiting activity. These splenocytes were >99% lymphocytes, as judged by Wright-Giemsa staining.
To prepare human IAL (hIAL), peripheral blood mononuclear cells were obtained by venipuncture from healthy volunteers and isolated with lymphocyte separation medium as described above. The cells were placed in cultures with IL-2 and processed as described above for mouse splenocytes. As judged by flow cytometry at the end of the cell culture period, murine CD8+ lymphocytes accounted for 56.3% ± 5.3% and NK1.1+ lymphocytes accounted for 32.8% ± 6.0% of the total IAL population. The CD8+ lymphocytes were all CD11b+; approximately 85% of the NK1.1+ lymphocytes were CD11b+, and 15% of the NK1.1+ lymphocytes were CD11b−. Human CD8+ lymphocytes accounted for 64.3% ± 7.3% and CD56+ lymphocytes accounted for 26.7% ± 7.1% of the total IAL population. The CD8+ lymphocytes were all CD11b+; 80% of the CD56+ lymphocytes were CD11b+, and 20% of the CD56+ lymphocytes were CD11b−. The culture conditions employed favored production of activated CD8+ lymphocytes and NK cells, and CD4+ and CD19+ lymphocytes each accounted for approximately 5% of the final IAL population (8). The procedures and reagents used for immunofluorescent analysis by flow cytometry have been described previously (8, 37).
NIH 3T3 fibroblast transfected clones.
In the experiments with NIH 3T3 cells (National Institutes of Health, Bethesda, Md.) we utilized two transfected clones of this murine fibroblast cell line which were gifts from Robert F. Todd III (University of Michigan School of Medicine, Ann Arbor). These cells have been described in detail previously (27). Clone 3-1 (3T3-1 fibroblasts) expressed no surface CD11b/CD18 or CD16 but did contain the transfected PSV2neo plasmid. Clone 3-19 (3T3-19 fibroblasts) expressed abundant surface CD11b/CD18. These phenotypes were confirmed upon receipt and at regular intervals by immunofluorescent microscopy by using anti-human CD11b mAb OKM1 and anti-human CD18 mAb TS1/18 as described below.
The NIH 3T3 cells were maintained at 37°C in Falcon 75-mm2 tissue culture flasks (Becton Dickinson) in Dulbecco’s modified Eagle’s medium containing 4,500 mg of glucose per liter, sodium pyruvate, and l-glutamine (GIBCO) supplemented with 10% fetal bovine serum (Sigma Chemical Co., St. Louis, Mo.), 50 μM 2-mercaptoethanol, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 25 ng of amphotericin B per ml, 100 μM nonessential amino acids, and 2 mM l-glutamine (all obtained from GIBCO). Cells received fresh medium every 3 days and were passaged every 7 to 14 days when they were nearly confluent. Cells used for passage or for experiments were removed from the tissue culture flasks with HBSS without Ca2+ or Mg2+ (HBSS−/−) (GIBCO) (pH 7.4) containing Tris base to adjust the pH and 10 mM EDTA (Sigma Chemical Co.). Nonadherent NIH 3T3 fibroblasts were then washed once in HBSS−/− (pH 7.4) without EDTA and resuspended in Dulbecco’s modified Eagle’s medium for passage or in HBSS−/− to minimize clumping during labeling with 51Cr for use in an adhesion assay. Cells were labeled with 51Cr as described below for lymphocytes and then washed and resuspended in HBSS (for at least 1 h) as described below; the resultant 51Cr uptake and radioactive labeling were equal to approximately 5 × 104 cpm per 5 × 104 cells.
C. albicans growth inhibition.
The antifungal activity of lymphocytes for C. albicans was determined as described previously (7). Briefly, the fungal cells used for the experiments were collected from isolated, overnight SDA colonies and washed once in HBSS. The yeasts were resuspended at a concentration of 2 × 105/ml in RPMI 1640, and 1 × 104 cells were added to individual wells of 96-well, flat-bottom plates (catalog no. 25861; Corning, Corning, N.Y.). C. albicans hyphal forms were obtained by incubation at 37°C in the presence of 5% CO2 for 2 h. Effector cells were then added at ratios ranging from 100:1 to 2.5:1. After 3 h of incubation at 37°C in the presence of 5% CO2, effector cells were lysed and removed by washing the preparations with water by using a PHD cell harvester (Cambridge Technology, Cambridge, Mass.). RPMI 1640 (50 μl) containing 1 μCi of [3H]uridine (ICN Radiochemicals, Irvine, Calif.) was added to individual wells. Following 1 h of incubation at 37°C in the presence of 5% CO2, 25 U of lyticase (Sigma Chemical Co.) in 50 μl of HBSS was added to individual wells, and the preparations were incubated for 0.5 h at 25°C. Hyphae were harvested, and the associated radioactivity was determined by determining the percentage of inhibition of C. albicans growth, as follows: {[disintegrations per minute for C. albicans control − (disintegrations per minute for effector and C. albicans − disintegrations per minute for effector control)] ÷ disintegrations per minute for C. albicans control × 100. Mean percentages of inhibition based on triplicate values obtained in two or more experiments were calculated.
Adhesion of lymphocytes and NIH 3T3 fibroblasts to C. albicans hyphae.
An assay to examine adhesion of lymphocytes and NIH 3T3 fibroblasts to C. albicans hyphae was performed as described previously (23). Briefly, C. albicans hyphae were prepared by growth in RPMI 1640 for 3 h at 37°C in flat-bottom, 96-well plastic plates (Corning). After 3 h 90 to 100% hyphal confluence was obtained when 105 yeast cells were inoculated initially into each well of the assay plates. Mammalian cells labeled with 51Cr (NEN, Dupont Inc., Wilmington, Del.) were added to individual wells of the assay plates and incubated in a 5% CO2 incubator at 37°C for 1 h. The assay was terminated by washing and removal of unbound mammalian cells from each well with a Pasteur pipette or a PHD cell harvester. The assay wells were washed three times with HBSS or 0.9% saline, 200 μl of 0.5% NP-40 (Sigma Chemical Co.) was added to each well, and the preparations were incubated for 20 min. The 0.5% NP-40-containing supernatants were removed with a Pasteur pipette, and the associated radioactivity was measured by determining the percentage of lymphocyte adhesion to C. albicans, as follows: [(experimental counts per minute − background counts per minute) ÷ (maximum counts per minute − background counts per minute)] × 100. The maximum release of radioactivity was obtained by adding 0.5% NP-40 directly to radioactively labeled mammalian cells. The typical maximum radioactivity for 5 × 104 NIH 3T3 fibroblasts was approximately 5 × 104 cpm, while the typical maximum radioactivity for 105 mIAL and hIAL was approximately 4 × 104 cpm. The typical background values were usually less than 1% of the maximum values for all experiments and were approximately 500 cpm for NIH 3T3 fibroblast experiments and approximately 400 cpm for the IAL experiments. Mean percentages of adhesion based on triplicate values obtained in two or more experiments were calculated.
Competition for binding of lymphocytes and 3T3-19 fibroblasts to C. albicans hyphae.
Competition for binding of lymphocytes and 3T3-19 fibroblasts to C. albicans hyphae was examined as described above for adhesion of lymphocytes to C. albicans, except that 5 × 104 radiolabeled NIH 3T3 cells or 105 IAL were preincubated for 1 h with proteins, peptides, carbohydrates, or antibodies at 37°C in 200 μl of HBSS (22). This preincubation step was carried out in a 96-well polystyrene plate (Corning) that had been pretreated with sterile 1% bovine serum albumin (Sigma Chemical Co.) in HBSS at 25°C overnight and washed once with HBSS prior to addition of lymphocytes or NIH 3T3 cells. Each 200-μl preincubation mixture was transferred to a well containing C. albicans unless indicated otherwise. The mixtures were incubated with proteins, peptides, or antibodies in HBSS for 1 h at 37°C by using the concentrations indicated below. For all inhibition experiments, the associated radioactivity was determined and was expressed as a percentage of inhibition of mammalian cell adhesion, calculated as follows: {1 − [(counts per minute for treated experimental preparation − background counts per minute) ÷ (counts per minute for untreated experimental preparation − background counts per minute)]} × 100. Mean percentages of inhibition based on triplicate values obtained in two or more experiments were calculated.
Proteins, peptides, and carbohydrates.
The following proteins and peptides were used in this investigation: echistatin, human factor X, human fibrinogen, fibronectin-like engineered protein (FEP), fibrinogen binding inhibitory protein (FBIP) (HHLGGAKQAGDV; residues 400 to 411 from human fibrinogen-γ fragment), heparin sulfate, major histocompatibility complex (MHC) antigen H-2Kb fragment 163–174 (TCVEWLRRYLKN), and peptides GRGDSPK, GRGDTP, GRYDS, RGD, and RGDS (Sigma Chemical Co.); human vitronectin, GRGDSP peptide, and cyclic GPenGRGDSPCA (GRGDSPc) peptide (Telios Pharmaceuticals, San Diego, Calif.); mouse laminin from Engelbreth Holm Swarm (EHS) cells (GIBCO); and GRGDSP peptide (Peninsula Laboratories, Belmont, Calif.). The carbohydrates used were N-acetyl-d-glucosamine (NADG) and β-glucan (from baker’s yeast and barley; prepared as described by Ross et al. [41]), as well as d-glucose, methyl-α-d-mannopyranoside, and sucrose (Sigma Chemical Co.).
mAbs.
Anti-murine mAbs were prepared from hybridoma culture supernatants and were purified by using a column containing recombinant protein A/G as described by the manufacturer (Pierce, Rockford, Ill.). Hybridoma cells were grown under the conditions specified by the American Type Culture Collection, from which all of the hybridoma cells were obtained. The anti-murine mAb hybridomas used and animals of origin were as follows: M1/70.15 (rat anti-mouse CD11b, immunoglobulin G2b [IgG2b]), 5C6 clone 1 (hamster anti-mouse CD11b, IgG), M18/2.A (rat anti-mouse CD18, IgG2a), 2E6 (hamster anti-mouse CD18, IgG), M17/4.4 (rat anti-mouse CD11a, IgG2b), N418 (hamster anti-mouse CD11c, IgG), and MB23G2 (rat anti-mouse CD45RB, IgG2a). The purchased mAbs used were fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG antibody (Becton Dickinson) and FITC-conjugated goat anti-hamster IgG antibody (Accurate Chemical and Scientific Corp., Westbury, N.Y.).
The mouse anti-human mAbs used were OKM1 (anti-CD11b, IgG2b), TS1/18 (anti-CD18, IgG1), and BBM.1 (anti-β2 microglobulin, IgG2b) (American Type Culture Collection). These antibodies were purified from mouse ascites by using a column containing recombinant protein A/G as described by the manufacturer (Pierce). HEF1 (anti-CD30, IgG1) was used as a purified antibody and was a generous gift from Hans-Martin Jäck (Loyola University of Chicago). TS1/22 (anti-CD11a, IgG1) and TS2/9 (anti-CD58, IgG1) were used as purified antibodies and were generous gifts from Tom Ellis (Loyola University of Chicago). Purified anti-human β1 integrin CD29, IgG1, clone P4C10 was purchased from Telios Pharmaceuticals, and anti-human p150,95 (CD11c), IgG1, clone SHCL-3 was purchased from Becton Dickinson. All anti-human antibodies were of mouse origin, and the secondary antibody used for immunofluorescence analysis was FITC-conjugated goat anti-mouse antibody (Accurate Chemical and Scientific Corp.). Protein concentrations were determined by the bicinchoninic acid assay (Pierce).
RESULTS
ECM and RGD-mimetic peptides inhibit mIAL adhesion to C. albicans hyphae.
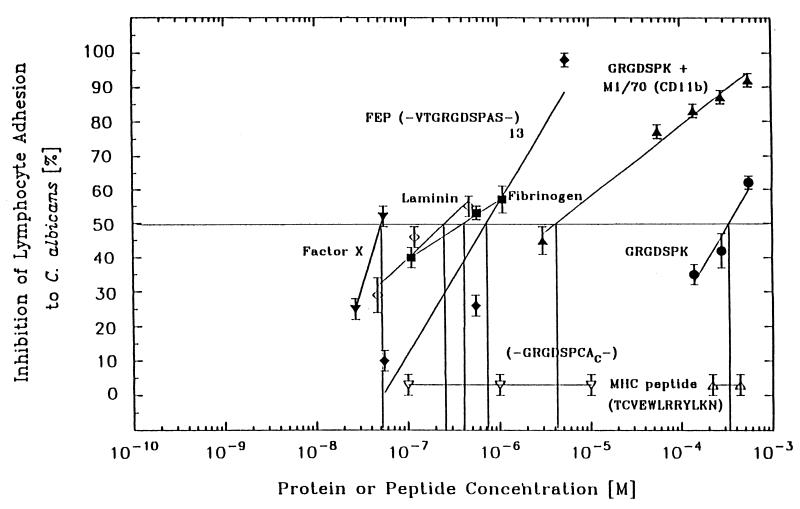
Extracellular matrix (ECM) proteins and RGD-mimetic peptides were tested to determine their abilities to inhibit binding of mIAL to C. albicans hyphae (Fig. 1). The two ECM proteins examined were murine EHS laminin and human fibrinogen. Clear dose-dependent inhibition of mIAL adhesion to hyphae was observed with both of these ECM proteins. The EHS laminin was a slightly more potent inhibitor of mIAL adhesion; the 50% inhibitory concentration (IC50) of this protein was 200 nM while a fibrinogen concentration of 500 nM was required for 50% inhibition of mIAL adhesion. The macrophage-1 antigen (Mac-1) ligand factor X (3) of the blood coagulation cascade was the most potent inhibitor on a molar basis and showed clear dose-dependent inhibition of mIAL adhesion to C. albicans hyphae, with 50% inhibition at a concentration of 55 nM and 25% inhibition at a concentration of 27 nM. In addition, the complex glycoprotein heparin sulfate, which is known to be a ligand for Mac-1 (15), inhibited adhesion of mIAL to C. albicans hyphae (IC50, 100 μM) (data not shown).
FIG. 1.
ECM proteins and RGD-mimetic peptides inhibit adhesion of mIAL to C. albicans. Adhesion of mIAL to C. albicans was assessed by measuring the retention of 51Cr-labeled lymphocytes in the presence of the following proteins and RGD-mimetic peptides: GRGDSPK (•), GRGDSPK plus 30 μg of anti-CD11b mAb M1/70.15 (▴), FEP (⧫), fibrinogen (▪), EHS laminin (◊), factor X (▾), GRGDSPCAc (▿), H-2Kb MHC peptide TCVEWLRRYLKN (▵). The conditions and adhesion assessment protocol used are described in Materials and Methods. The data are means ± standard deviations based on two or more experiments. The vertical lines intersecting the abscissa indicate the IC50 for the molecules evaluated.
Several RGD-mimetic peptides were also examined to determine their abilities to inhibit mIAL adhesion to C. albicans hyphae (Fig. 1). The GRGDSPK peptide had a dose-dependent inhibitory effect on mIAL adhesion to C. albicans hyphae, with an IC50 of 300 μM. The FEP multimer peptide also had a clear dose-dependent inhibitory effect on mIAL adhesion to hyphae; a concentration of 5.5 μM completely eliminated adhesion of mIAL to C. albicans hyphae, and the IC50 was 800 nM. Experiments were carried out to determine whether an additive inhibitory effect could be obtained with the GRGDSPK peptide and mAb M1/70.15 to an epitope on murine CD11b outside the RGD-binding I domain which may be associated with Mac-1 adhesion to hydrophobic ligands (30). Combinations of mAb M1/70.15 (30 μg) and the concentrations of GRGDSPK peptide that were used alone did result in dramatic dose-dependent increases in inhibition by the GRGDSPK peptide; the GRGDSPK concentration that resulted in 50% inhibition of adhesion was 4 μM.
An irrelevant bioactive peptide fragment containing 12 amino acids (molecular weight, 1,580; sequence, TCVEWLRRYLKN) which inhibited allorecognition and adhesion of C57BL/6 T-cell receptors to the murine MHC H-2Kb receptor was found to have no effect on mIAL adhesion to C. albicans at concentrations of 400 and 200 μM (45). Several other RGD-mimetic peptides were poor inhibitors of mIAL adhesion to C. albicans; 900 μM RGDS and 700 μM GRGDTP each inhibited adhesion by only 30%, and 700 μM GRYDS and 700 μM GRGDSP had no effect on mIAL adhesion to C. albicans hyphae (data not shown).
mAbs to murine CD11b/CD18 block adhesion of mIAL to C. albicans hyphae.
The mAb that inhibited mIAL adhesion to C. albicans hyphae most completely was OKM1 (mouse anti-human antibody which also binds to murine CD11b, IgG2b) (Fig. 2). OKM1 virtually eliminated mIAL adhesion to hyphae, and inhibition was more than 90% when 45 μg (1.2 μM) was used. OKM1 had a clear dose-dependent inhibitory effect on mIAL adhesion to hyphae. The next-most-potent anti-murine CD11b mAb was mAb 5C6 (hamster anti-mouse CD11b, IgG), which inhibited adhesion of mIAL by 70% when 45 μg was used and by 60% when 25 μg (670 nM) was used (data not shown). Finally, mAb M1/70.15 (rat anti-mouse CD11b, IgG2b), which also binds to human CD11b, inhibited adhesion of mIAL to C. albicans hyphae by 60% when 45 μg was used, by 50% when 30 μg was used, and by 25% when 15 μg was used (data not shown). Two anti-murine CD18 mAbs were tested, and each had a dose-dependent inhibitory effect on mIAL adhesion to C. albicans hyphae. The anti-CD18 mAb 2E6 (hamster anti-mouse CD18, IgG) inhibited adhesion by 60% when 45 μg was used, by 40% when 25 μg was used, and by 20% when 10 μg was used (data not shown). The anti-CD18 mAb M18/2.A (rat anti-mouse CD18, IgG2a) inhibited mIAL adhesion to hyphae by 50% when 45 μg was used, by 40% when 25 μg was used, and by 20% when 10 μg was used (data not shown). Combinations of anti-CD11b and anti-CD18 mAbs very effectively inhibited mIAL adhesion to hyphae; 25 μg of OKM1 plus 15 μg of 2E6 inhibited adhesion by 80%, and 25 μg of OKM1 plus 15 μg of M18/2.A inhibited adhesion by 70%. A combination of 25 μg of OKM1 plus 15 μg of M1/70.15 resulted in 53% inhibition, and 25 μg of OKM1 plus 15 μg of 5C6 inhibited mIAL adhesion to hyphae by 57% (data not shown). The irrelevant mAbs used for the mIAL adhesion inhibition experiments were M17/4.4 (rat anti-mouse CD11a, IgG2b), N418 (hamster anti-mouse CD11c, IgG), and MB23G2 (rat anti-mouse CD45RB, IgG2a) (data not shown). When 45 and 25 μg were used, these antibodies had no inhibitory effect on mIAL adhesion to hyphae other than the 10% inhibition observed with 45 μg of M17/4.4 Lack of inhibition by these antibodies demonstrated that there was no nonspecific and Fc-mediated inhibition. The data demonstrated that mIAL uses CD11b/CD18 for adhesion to C. albicans hyphae.
FIG. 2.
mAbs to CD11b/CD18 inhibit adhesion of mIAL to C. albicans. Adhesion of mIAL to C. albicans was assessed by measuring the retention of 51Cr-labeled lymphocytes in the presence of the following mAbs to CD antigens: for CD11b, OKM1 (mouse anti-human, IgG2b), M1/70.15 (rat anti-mouse, IgG2b), and 5C6 (hamster anti-mouse, IgG); for CD18, M18/2.A (rat anti-mouse, IgG2a [κ]), and 2E6 (hamster anti-mouse, IgG); for CD11a, M17/4.4 (rat anti-mouse, IgG2b[κ]); and for CD11c, N418 (hamster anti-mouse, IgG). The conditions and adhesion assessment protocol used are described in Materials and Methods.
Carbohydrate ligands of Mac-1 selectively inhibit mIAL and hIAL adhesion to C. albicans hyphae.
It has been shown that selected carbohydrates block adhesion of neutrophil CD11b/CD18 to the yeast Saccharomyces cerevisiae (41, 49). These carbohydrates were examined to determine their abilities to inhibit mIAL adhesion to C. albicans hyphae (Table 1). A clear dose-dependent inhibitory effect was observed when NADG was used. Although 150 mM d-mannose inhibited mIAL adhesion by only 40%, the combination of 75 mM NADG plus 75 mM d-mannose inhibited mIAL adhesion to hyphae by almost 75%. The carbohydrate α-methyl mannoside also had a dose-dependent inhibitory effect, with 150 mM inhibiting mIAL adhesion to hyphae by 45% and 75 mM inhibiting mIAL adhesion to hyphae by 19%. Yeast β-glucan at a concentration of 4 mg/ml inhibited mIAL adhesion by 75%. The irrelevant carbohydrates (41) sucrose and d-glucose did not inhibit mIAL adhesion to hyphae at a concentration of 150 or 75 mM.
TABLE 1.
Carbohydrates as competitive blockers of adherence of mIAL and hIAL to C. albicansa
| IAL | Carbohydrate | Concn | % Inhibition of binding (mean ± SD) |
|---|---|---|---|
| mIAL | NADG | 7.5 mM | 25 ± 3 |
| NADG | 75 mM | 50 ± 2 | |
| NADG | 150 mM | 85 ± 3 | |
| d-Mannose | 150 mM | 40 ± 5 | |
| NADG +d-mannose | 75 mM + 75 mM | 74 ± 2 | |
| α-Methyl mannoside | 75 mM | 19 ± 4 | |
| α-Methyl mannoside | 150 mM | 45 ± 2 | |
| d-Glucose | 150 mM | 5 ± 3 | |
| Sucrose | 150 mM | 0 ± 5 | |
| hIAL | d-Glucose | 150 mM | 3 ± 5 |
| β-Glucan (yeast) | 4 mg/ml (40 μM) | 70 ± 5 | |
| NADG | 150 mM | 66 ± 2 |
The effects of several carbohydrates on adherence were assessed by the competitive binding assay as described in the legend to Fig. 2. The data are the data for the maximum concentrations used; multiple carbohydrate concentrations were tested.
Carbohydrates that characteristically inhibit neutrophil Mac-1-mediated adhesion to yeasts (41) also inhibited hIAL adhesion to hyphae at concentrations that inhibited mIAL adhesion to C. albicans (Table 1). NADG at a concentration of 150 mM inhibited hIAL adhesion to C. albicans hyphae by 66%, and β-glucan from S. cerevisiae at a concentration of 4 mg/ml inhibited adhesion of hIAL to hyphae by 70%. d-Glucose again had no effect on adhesion. The profile of carbohydrate inhibition at these concentrations is characteristic of adhesion mediated by the CD11b/CD18 lectin-like domain (41, 46, 49).
hIAL adhesion to C. albicans hyphae is inhibited by ECM proteins and RGD-mimetic peptides and mAbs to CD11b/CD18.
Experiments were conducted with a subset of the reagents and anti-human CD11b/CD18 mAbs described above to examine whether hIAL from the peripheral blood of 12 healthy donors also utilized Mac-1 for adhesion to C. albicans hyphae (Fig. 3). The ECM protein and RGD-mimetic peptide inhibition analysis was not as extensive as the mIAL analysis; however, the fibrinogen fragment FBIP inhibited hIAL adhesion to hyphae, and the IC50 was the same (340 μM). The disintegrin echistatin inhibited adhesion of hIAL to hyphae by 50% at a concentration of 2 μM, an effect identical to its effect on mIAL adhesion to hyphae. The Mac-1 ligand and complex glycoprotein heparin sulfate was found to inhibit hIAL by 45% at a concentration of 100 μM, which also inhibited mIAL adhesion to hyphae by 50% (Table 1). The GRGDSPc cyclic peptide had no effect on hIAL adhesion to hyphae.
FIG. 3.
mAbs to CD11b/CD18 and RGD-mimetic proteins inhibit adhesion of hIAL to C. albicans. Adhesion of hIAL to C. albicans was assessed by measuring the retention of 51Cr-labeled lymphocytes in the presence of the following mAbs to CD antigens and RGD-mimetic proteins: for CD11b, OKM1 (mouse anti-human, IgG2b) and M1/70.15 (rat anti-mouse, IgG2b); for CD18, TS1/18 (mouse anti-human, IgG1); for CD58, TS2/9 (mouse anti-human LFA-3, IgG1); for CD11a, TS1/22 (mouse anti-human LFA-1, IgG1); FBIP; echistatin, a disintegrin RGD-specific integrin inhibitor; heparin, an RGD-containing ECM protein; and GRGDSPc, a cyclical molecule with the sequence GPenGRGDSPCA (where Pen is penicillamine). The conditions and adhesion assessments protocol used are described in Materials and Methods. The data are the data from 12 separate preparations obtained with human peripheral blood mononuclear cells and are means ± standard deviations.
A restricted number of anti-human CD11b/CD18 mAbs, as previously described for a human lymphocyte cell line (YT) (22), were examined to determine their abilities to inhibit hIAL adhesion to C. albicans hyphae (Fig. 3). mAb OKM1 (mouse anti-human CD11b, IgG2b) had a dose-dependent inhibitory effect on adhesion; 45 μg virtually eliminated hIAL adhesion to hyphae (83% inhibition), when 25 and 15 μg were used, adhesion was inhibited by 72 and 28%, respectively. mAb M1/70.15 (rat anti-mouse CD11b, IgG2b) was tested only at a dose of 45 μg, which inhibited hIAL adhesion to hyphae by 42%. The combination of 25 μg of OKM1 plus 25 μg of M1/70 inhibited adhesion of hIAL by 75%. Anti-human CD18 mAb TS1/18 had a dose-dependent inhibitory effect on hIAL adhesion; 45 μg inhibited hIAL adhesion to hyphae by 68%, while 25 μg inhibited adhesion of these lymphocytes by 22%. The combination of 25 μg of OKM1 plus 25 μg of TS1/18 inhibited hIAL adhesion to C. albicans hyphae by 82%. The irrelevant mAbs utilized in the hIAL experiments were TS2/9 (mouse anti-human CD58, IgG1) (43), BBM.1 (mouse anti-human β2 microglobulin, IgG2b), P4C10 (mouse anti-human CD29, IgG1), TS1/22 (mouse anti-human CD11a, IgG1) (16), and MB23G2 (rat anti-mouse CD45RB, IgG2a) (data not shown). TS1/22 mAb inhibited hIAL adhesion by 10% when 45 μg was used and had no effect on hIAL adhesion when 25 μg was used (data not shown). The other mAbs had no effect on hIAL adhesion to C. albicans hyphae when either 45 or 25 μg was used (data not shown). Overall, the hIAL data are in agreement with the mIAL inhibition data and identify CD11b/CD18 as the means by which lymphocytes adhere to C. albicans.
Murine 3T3-19 fibroblasts expressing transfected human Mac-1 exhibit Mac-1-specific adhesion to C. albicans hyphae.
The experiments with mIAL and hIAL described above indicated that Mac-1 (CD11b/CD18) is the principal adhesion molecule which mediates binding of these activated lymphocytes to the hyphae of C. albicans. To confirm the ability of Mac-1 to mediate this adhesion, mouse NIH 3T3 fibroblasts expressing transfected human CD11b/CD18 (3T3-19 fibroblasts) were examined to determine their adhesion to C. albicans hyphae compared to the adhesion of NIH 3T3 fibroblasts expressing no CD11b/CD18 (3T3-1 fibroblasts). 51Cr-radiolabeled fibroblasts (5 × 104 cells) expressing CD11b/CD18 (3T3-19) bound C. albicans hyphae with 49,210 ± 2,160 cpm (mean ± standard deviation), while only 512 ± 120 cpm was associated with plastic wells containing the same fibroblasts and no C. albicans. In contrast, 1,465 ± 42 cpm was associated with 5 × 104 51Cr-radiolabeled 3T3-1 fibroblasts when they were incubated with C. albicans hyphae, and 595 ± 80 cpm was associated when they were incubated in plastic wells without C. albicans. Competitive inhibition of adhesion of 3T3-19 fibroblasts to C. albicans is shown in Fig. 4. Anti-CD11b mAb OKM1 was tested over a broad range of concentrations and was found to inhibit adhesion of 3T3-19 fibroblasts to C. albicans in a clearly concentration-dependent manner. mAb OKM1 inhibited adhesion by 67% when 45 μg was used, by 57% when 40 μg was used, by 38% when 25 μg was used, by 32% when 20 μg was used, by 22% when 15 μg was used, and by 10% when 10 μg was used and had no effect on 3T3-19 fibroblast adhesion to hyphae when 5 μg was used. Anti-CD18 mAb TS1/18 also had a concentration-dependent inhibitory effect on 3T3-19 adhesion to C. albicans; 45 μg of TS1/18 inhibited 3T3-19 adhesion to hyphae by 28%, while 25 μg of TS1/18 inhibited 3T3-19 adhesion to hyphae by only 9%. That 3T3-19 adhesion to C. albicans hyphae is CD11b/CD18 specific was also demonstrated by the dramatic 85% inhibition of adhesion by 25 μg of OKM1 plus 25 μg of TS1/18. Further verification of the specificity was obtained with the anti-murine CD29 mAb, which reacted with the murine β1 integrin chain on the surface of the 3T3-19 fibroblasts and had no effect on 3T3-19 adhesion to C. albicans (Fig. 4). The data confirm that CD11b/CD18 can mediate specific adhesion to C. albicans hyphae. To demonstrate that adhesion of 3T3-19 transfectants to hyphae is Mac-1 specific, NIH 3T3 fibroblasts that were subjected to the transfection protocol but did not express Mac-1 (3T3-1 fibroblasts) were also examined. The 3T3-1 fibroblasts exhibited no significant adhesion to C. albicans hyphae above the background levels, and the results were not different when the anti-CD11b mAb OKM1 or the anti-CD18 mAb TS1/18 was added (Fig. 4). These data demonstrate that only transfectants expressing CD11b/CD18 bind to C. albicans hyphae.
FIG. 4.
mAbs to CD11b/CD18 inhibit adhesion of 3T3-19 (Mac-1+) transfectants to C. albicans. Adhesion to C. albicans of NIH 3T3 fibroblasts expressing transfected human CD11b/CD18 (3T3-19 fibroblasts) and of NIH 3T3 fibroblasts not expressing CD11b/CD18 (3T3-1 fibroblasts) was assessed by measuring the retention of 51Cr-labeled transfectants in the presence of the following mAbs to CD antigens: for CD11b, OKM1 (mouse anti-human, IgG2b); for CD18, TS1/18 (mouse anti-human, IgG1); and for CD29, clone 551125 (rat anti-mouse β1 integrin, IgG1). The conditions and adhesion assessment protocol used are described in Materials and Methods. The typical maximum amount of radioactivity for 5 × 104 NIH 3T3 fibroblasts, either 3T3-19 (Mac-1+) or (3T3-1), was approximately 5 × 104 cpm.
RGD-mimetic peptides alone and in combination with mAbs to CD11b/CD18 inhibit adhesion of 3T3-19 (Mac-1+) transfectants to C. albicans.
To confirm the observations described above, experiments were performed with 3T3-19 transfectants to determine whether inhibition of lymphocyte adhesion to C. albicans hyphae by RGD-mimetic peptides was specific for Mac-1-mediated adhesion (Fig. 5). The FBIP peptide at a concentration of 340 μM and the GRGDSPK peptide at a concentration of 140 μM had no effect on 3T3-19 adhesion to C. albicans hyphae. Because these two peptides clearly inhibited adhesion of IAL to hyphae by 50% at these concentrations, the peptides were tested with low concentrations of mAbs to CD11b, as shown in Fig. 1. The anti-CD11b mAb M1/70.15 inhibited adhesion of 3T3-19 fibroblasts to hyphae by 25% when 30 μg was used, but when 30 μg of anti-CD11b mAb M1/70.15 was combined with 340 and 170 μM FBIP peptide, 3T3-19 adhesion was inhibited by 70 and 53%, respectively. The anti-CD11b mAb OKM1 inhibited 3T3-19 adhesion to hyphae by 22% when 15 μg was used alone, but when 15 μg of this mAb was combined with 140 μM GRGDSPK peptide, 3T3-19 fibroblast adhesion to hyphae was inhibited by 40%. A similar effect was observed for the OKM1 mAb and the anti-human CD18 mAb TS1/18 (Fig. 5). When 45 μg of TS1/18 was used, 3T3-19 adhesion was inhibited by 25%, but 25 μg of TS1/18 had no effect on 3T3-19 adhesion to hyphae (data not shown). However, 15 μg of OKM1 plus 15 μg of OKM1 plus 15 μg of TS1/18 resulted in enhanced inhibition (84% inhibition of 3T3-19 fibroblast adhesion to C. albicans hyphae). Similarly, 15 μg of OKM1 plus 15 μg of M1/70.15 inhibited 3T3-19 transfectant adhesion by 58%.
FIG. 5.
RGD-mimetic peptides in combination with mAbs to CD11b/CD18 inhibit adhesion of 3T3-19 (Mac-1+) transfectants to C. albicans. Adhesion to C. albicans of NIH 3T3 fibroblast transfectants expressing human CD11b/CD18 (3T3-19 fibroblasts) was assessed by measuring the retention of 51Cr-labeled transfectants in the presence of the following mAbs to CD antigens and RGD-mimetic peptides: for CD11b, OKM1 (mouse anti-human, IgG2b) and M1/70.15 (rat anti-mouse, IgG2b); for CD18, TS1/18 (mouse anti-human, IgG1); GRGDSPK; and FBIP. The conditions and adhesion assessment protocol used are described in Materials and Methods. The typical maximum amount of radioactivity for 5 × 104 NIH 3T3 fibroblasts, either 3T3-19 (Mac-1+) or (3T3-1), was approximately 5 × 104 cpm.
mAbs to CD11b/CD18 block growth inhibition of C. albicans hyphae by mIAL.
Finally, experiments were carried out to investigate whether inhibition of CD11b/CD18-mediated adhesion of mIAL to C. albicans hyphae was functionally relevant to inhibition of the growth of the fungus by these IAL (Fig. 6). The growth inhibition assay utilizes uptake of [3H]uridine to compare growth of treated and untreated hyphae (7). The mIAL utilized for these experiments were preincubated either with no mAb or with an mAb for 1 h; then they were added to hyphae for 3 h or removed, and [3H]uridine was added for 2 h. Baseline values were obtained by using wells containing no mIAL and wells containing mIAL but no mAb (Fig. 6, bars 1 and 2). Some C. albicans hyphae also were treated with anti-CD11b mAbs M1/70.15 and OKM1 without mIAL (Fig. 6, bars 3 and 6, respectively). This treatment had no significant effect on fungal growth (P > 0.05, as determined by Student’s t test). Similarly, mIAL treated with the irrelevant mAbs to murine CD11a (M17/4.4) (Fig. 6, bars 9 and 10) and CD11c (N418) (Fig. 6, bars 11 and 12) inhibited hyphal growth, and their effects were not significantly different (P > 0.05) from the effects of untreated mIAL (Fig. 6, bar 2). With both anti-CD11b mAbs, dose-dependent blocking of mIAL inhibition of C. albicans growth was observed. OKM1 and M1/70.15, tested separately, at a dose of 45 μg, completely eliminated mIAL inhibition of growth of C. albicans hyphae (Fig. 6, bars 4 and 7). There was not a significant difference (P > 0.05) between the values obtained in these experiments and the baseline values obtained without added mIAL either with or without mAbs (Fig. 6, bars 1, 3, and 6). These data showed that the anti-CD11b mAbs had a significant (P < 0.05) (Fig. 6, bar 4 versus bar 2) blocking effect on mIAL inhibition of C. albicans growth. Significant (P < 0.05) growth inhibition by mIAL was evident when 25 μg of OKM1 was used (43%) (bar 8 versus bar 6) and when 25 μg of M1/70.15 was used (19%) (bar 5 versus bar 3), although the level of growth inhibition obtained with each of these mAbs was significantly less (P < 0.05) than the 58% inhibition observed with mIAL alone (Fig. 6, bar 2).
FIG. 6.
mAbs to CD11b/CD18 block inhibition of growth of C. albicans hyphae by mIAL. Inhibition of C. albicans growth was assessed by measuring the incorporation of [3H]uridine after treatment with mIAL. mAbs either were added to hyphae alone or were preincubated with mIAL as shown in Fig. 1. The following mAbs to CD antigens were used: for CD11b, OKM1 (mouse anti-human, IgG2b) and M1/70.15 (rat anti-mouse, IgG2b); for CD11a, M17/4.4 (rat anti-mouse, IgG2b [κ]); and for CD11c, N418 (hamster anti-mouse, IgG). An asterisk indicates that data are statistically significantly different (P < 0.05), as determined by Student’s independent t test. An asterisk with a superscript a indicates that there was statistically significant blocking of mIAL-mediated growth inhibition by 45 μg of mAb M1/70 (bar 4) or OKM1 (bar 7). An asterisk with a superscript b indicates that there was statistically significant growth inhibition by mIAL treated with 25 μg of M1/70 (bar 5 versus bar 2 or 3) or 25 μg of OKM1 (bar 8 versus bar 2 or 6). Note that the baseline mean growth inhibition value, 58% (bar 2), was compared to four values (bars 9, 10, 11, and 12) to test significance, and the differences were found to be not significant (P > 0.05). The data are means ± standard deviations based on two or more experiments.
DISCUSSION
Direct, lymphocyte-mediated effects on C. albicans are absolutely dependent on intimate contact between lymphocytes and the fungus (8, 31). In this study we found that this contact can be inhibited by mAbs specific for epitopes of the αM subunit (CD11b) and the β2 subunit (CD18) of the integrin Mac-1. Furthermore, significant inhibition of lymphocyte adhesion to C. albicans was achieved with ECM proteins, as well as with peptides containing arginine-glycine-aspartic acid (RGD) sequences and with NADG and β-glucan. It has been shown previously that IL-2-activated CD8+ lymphocytes are capable of directly interacting with and inhibiting the growth of the fungus (8) and that IL-2-activated NK cells are also capable of directly interacting with the fungus, resulting in the release of gamma interferon (31). The IL-2-activated CD8+ lymphocyte populations are broadly cytolytic for mammalian tumor cells and have lymphokine-activated killer cell-like activites (8). These IL-2-activated NK cells have tumor-cell-lytic activities as well, which are consistent with those of activated NK cells (8). The results presented here clearly demonstrate that the major means by which these highly activated lymphocytes interact with C. albicans is through CD11b/CD18. Moreover, it is not surprising that the Mac-1 receptor mediates adhesion of lymphocytes to C. albicans. Several integrins have been shown to mediate adhesion to microorganisms (13). The β2 leukocyte integrins are expressed only on cells of the immune system (48). Mac-1 is expressed on macrophages, dendritic cells, neutrophils, eosinophils, basophils, mast cells, large granular lymphocytes (including NK cells), B cells (especially CD5+ cells), and T cells (especially CD8+ cells) (26). CD11b/CD18 is dramatically upregulated on both mIAL and hIAL (17, 32, 50), and the structures of murine and human CD11b/CD18 molecules are very similar (21).
With regard to the domains of the CD11b/CD18 molecule that bind to C. albicans, the I domain has been shown to be sufficient to support binding of lymphocytes to C. albicans (24). However, the anti-murine CD11b mAb M1/70.15 has been shown to map outside domain I of human CD11b to an area possibly responsible for Mac-1 hydrophobic adhesion (54). Also, the anti-human CD11b mAb OKM1 is known to bind the lectin domain of human Mac-1 (16, 49). Each of these mAbs blocked mIAL and hIAL at similar levels; the maximum inhibition by M1/70 was in the 35 to 45% range, and the maximum inhibition by OKM1 was 84% for hIAL and 92% for mIAL. In addition, β-glucan and NADG (as the chitin polymer) are known ligands for the lectin-like domain of Mac-1 and are predominant components of the surface of C. albicans hyphae (25). Therefore, it is likely that the interaction between lymphocytes and C. albicans hyphae is complex with multiple interactive interfaces. Nonetheless, it is clear that lymphocytes from both mice and humans utilize CD11b/CD18 molecules as the principal structures for adhesion to C. albicans hyphae.
Several ECM and blood proteins, which are documented ligands for Mac-1, inhibited mIAL and hIAL adhesion to C. albicans hyphae. The complex glycoprotein heparin has been determined to be a Mac-1 ligand and inhibited adhesion well; the IC50 for mIAL was 100 μM, compared to an IC50 for blocking Mac-1 adhesion to heparin-coated plastic of 9.0 μM (15). Fibrinogen has been shown by many workers to be a ligand for Mac-1 and is bound only by activated Mac-1 (2, 52). For mIAL, the documented Mac-1 ligand factor X had a clear dose-dependent inhibitory effect on adhesion to hyphae, and the IC50 was similar to previously published values (1, 42). RGD-mimetic peptides also inhibited lymphocyte adhesion to C. albicans. GRGDSPK peptide sequences from fibronectin (53) had a dose-dependent inhibitory effect on mIAL adhesion to hyphae. The IC50 for this peptide, 300 μM, is similar to 400 μM, the IC50 for inhibition of C3bi-coated erythrocytes (EC3bi) with an RGD-mimetic peptide containing the C3bi sequence TRYRGDQDATMS (51). The engineered GRGDSP-containing peptide FEP inhibited lymphocyte adhesion to C. albicans hyphae. Tertiary structure is clearly important, as emphasized by the results obtained with the αvβ3-specific circular GRGDSP peptide, which had an identical RGD sequence but had no effect on adhesion (40). The potent inhibitory activity of FEP may also be due to the multiple GRGDSP repeats contained within each molecule which can interact with clustered integrins (33). Finally, the integrin inhibitor echistatin inhibited adhesion of mIAL and hIAL potently, with an IC50 of 2.0 μM. To our knowledge, this is the first demonstration of inhibition of Mac-1 by any disintegrin. Echistatin contains two RGD-mimetic sequences in a circular peptide, CKRARGDDMDDYC (10). In summary, the RGD-mimetic peptide, ECM, and blood protein inhibition data support the hypothesis that Mac-1 has a principal role in mediating activated lymphocyte adhesion to C. albicans hyphae.
The 3T3-1 fibroblasts exhibited only background adhesion to C. albicans hyphae. In contrast, 3T3-19 fibroblasts bound to C. albicans hyphae and were inhibited in a dose-dependent manner by OKM1 and TS1/18. Adhesion of 3T3-19 cells was not affected by an mAb to murine CD29 (β1 integrin) that reacted with the cell line. GRGDSPK and FBIP, two RGD-mimetic peptides that inhibited mIAL adhesion to hyphae, had no inhibitory effect on 3T3-19 adhesion to the fungus when they were used alone. However, in combination with OKM1 and M1/70.15 (both of which are anti-CD11b mAbs) both GRGDSPK and FBIP inhibited adhesion of 3T3-19 transfectants in a concentration-dependent manner. The data from the 3T3-19 transfectant mAb inhibition studies confirmed that CD11b/CD18 expressed on mammalian cells is capable of mediating specific, mAb-inhibitable adhesion to C. albicans hyphae. These data suggest that there are differences between activated lymphocytes and the fibroblasts, but confirm that the integrin inhibitable with RGD-mimetic peptides on mIAL and hIAL is indeed CD11b/CD18, and support the hypothesis that cross-linking has a role in activation of Mac-1 adhesion to such peptides.
To confirm that Mac-1 has a primary physiological role during mIAL-mediated inhibition of growth of C. albicans hyphae, experiments were conducted to examine the effect of mAbs to CD11b/CD18 on mIAL antifungal activity. A 45-μg dose of OKM1 (anti-CD11b), which inhibited mIAL adhesion by 92%, completely eliminated mIAL inhibition of C. albicans growth. A concentration-dependent effect for OKM1 inhibition was demonstrated by using 25 μg of OKM1, which resulted in 43% inhibition of growth by mIAL. This effect was significantly different (P < 0.05, as determined by a t test) from the 58% inhibition obtained with mIAL alone and thus represented an intermediate level of inhibition compared to the data obtained with 45 μg of OKM1. Similar results were obtained with mAb M1/70.15 (anti-CD11b), which eliminated mIAL inhibition of growth when 45 μg was used and had a significant (P < 0.05) concentration-dependent effect, further demonstrating specificity, similar to the effect of OKM1. The anti-CD11b mAbs OKM1 and M1/70.15 did not inhibit C. albicans growth when they were used alone without mIAL. mAbs to murine CD11a (M17/4.4) and murine CD11c (N418) had no effect on mIAL anticandidal activity. These data confirmed that CD11b/CD18 has a principal role in mediating adhesion of mIAL to C. albicans hyphae during mIAL-mediated growth inhibition of the fungus.
Acknowledgments
This work was supported in part by USPHS grant AI-31127 and by the Cancer Federation.
Editor: T. R. Kozel
REFERENCES
- 1.Altieri, D. C., and T. S. Edgington. 1988. A monoclonal antibody reacting with distinct adhesion molecules defines a transition in the functional state of the receptor CD11b/CD18 (Mac-1). J. Immunol. 141:2656–2660. [PubMed] [Google Scholar]
- 2.Altieri, D. C., J. Plescia, and E. F. Plow. 1993. The structural motif glycine 190-valine 202 of the fibrinogen gamma chain interacts with CD11b/CD18 integrin (alpha M beta 2, Mac-1) and promotes leukocyte adhesion. J. Biol. Chem. 268:1847–1853. [PubMed] [Google Scholar]
- 3.Anderson, D. C. 1994. The role of B2 integrins and intracellular adhesion molecule type 1 in inflammation, p.29–70. In C. D. Wegner (ed.), Adhesion molecules. Academic Press, San Diego, Calif.
- 4.Ashman, R. B. 1990. Murine candidiasis: cell-mediated immune responses correlate directly with susceptibility and resistance to infection. Immunol. Cell Biol. 68:15–20. [DOI] [PubMed] [Google Scholar]
- 5.Ausiello, C. M., F. Urbani, S. Gessani, G. C. Spagnoli, M. J. Gomez, and A. Cassone. 1993. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents from Candida albicans. Infect. Immun. 61:4105–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beno, D. W., and H. L. Mathews. 1992. Growth inhibition of Candida albicans by interleukin-2-activated splenocytes. Infect. Immun. 60:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beno, D. W., and H. L. Mathews. 1993. Quantitative measurement of lymphocyte mediated growth inhibition of Candida albicans. J. Immunol. Methods 164:155–164. [DOI] [PubMed] [Google Scholar]
- 8.Beno, D. W., A. G. Stover, and H. L. Mathews. 1995. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J. Immunol. 154:5273–5281. [PubMed] [Google Scholar]
- 9.Blanchard, D. K., M. B. Michelini-Norris, and J. Y. Djeu. 1991. Production of granulocyte-macrophage colony-stimulating factor by large granular lymphocytes stimulated with Candida albicans: role in activation of human neutrophil function. Blood 77:2259–2265. [PubMed] [Google Scholar]
- 10.Blobel, C. P., and J. M. White. 1992. Structure, function and evolutionary relationship of proteins containing a disintegrin domain. Curr. Opin. Cell Biol. 4:760–765. [DOI] [PubMed] [Google Scholar]
- 11.Calderone, R., R. Diamond, J. M. Senet, J. Warmington, S. Filler, and J. E. Edwards. 1994. Host cell-fungal cell interactions. J. Med. Vet. Mycol. 32:151–168. [DOI] [PubMed] [Google Scholar]
- 12.Cho, Y., and H. Y. Choi. 1991. Opportunistic fungal infection among cancer patients. A ten year autopsy study. Am. J. Clin. Pathol. 72:617–621. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, N. R. 1994. New aspects of complement structure and function. Anna Erdei, Austin, Tex.
- 14.Davies, S. F. 1990. Fungal diseases. Introduction. Semin. Respir. Infect. 5:91–92. [PubMed] [Google Scholar]
- 15.Diamond, M. S., R. Alon, C. A. Parkos, M. T. Quinn, and T. A. Springer. 1995. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD1). J. Cell Biol. 130:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond, M. S., J. Garcia-Aguilar, J. K. Bickford, A. L. Corbi, and T. A. Springer. 1993. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J. Cell Biol. 120:1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dianzani, U., D. Zarcone, V. Pistoia, C. E. Grossi, A. Pileri, M. Massaia, and M. Ferrarini. 1989. CD8+ CD11b+ peripheral blood T lymphocytes contain lymphokine-activated killer cell precursors. Eur. J. Immunol. 19:1037–1044. [DOI] [PubMed] [Google Scholar]
- 18.Dictar, M. O., E. Maiolo, B. Alexander, N. Jacob, and M. T. Veron. 2000. Mycoses in the transplanted patient. Med. Mycol. 38:251–258. [PubMed] [Google Scholar]
- 19.Dupont, B., H. H. Crewe Brown, K. Westermann, M. D. Martins, J. H. Rex, O. Lortholary, and C. A. Kauffmann. 2000. Mycoses in AIDS. Med. Mycol. 38:259–267. [PubMed] [Google Scholar]
- 20.Fidel, P. L., Jr., and J. D. Sobel. 1994. The role of cell-mediated immunity in candidiasis. Trends Microbiol. 2:202–206. [DOI] [PubMed] [Google Scholar]
- 21.Fleming, J. C., H. L. Pahl, D. A. Gonzalez, T. F. Smith, and D. G. Tenen. 1993. Structural analysis of the CD11b gene and phylogenetic analysis of the alpha-integrin gene family demonstrate remarkable conservation of genomic organization and suggest early diversification during evolution. J. Immunol. 150:480–490. [PubMed] [Google Scholar]
- 22.Forsyth, C. B., and H. L. Mathews. 1996. Lymphocytes utilize CD11b/CD18 for adhesion to Candida albicans. Cell. Immunol. 170:91–100. [DOI] [PubMed] [Google Scholar]
- 23.Forsyth, C. B., and H. L. Mathews. 1993. A quantitative radiometric assay to measure mammalian cell binding to hyphae of Candida albicans. J. Immunol. Methods 165:113–119. [DOI] [PubMed] [Google Scholar]
- 24.Forsyth, C. B., E. F. Plow, and L. Zhang. 1998. Interaction of the fungal pathogen Candida albicans with integrin CD11b/CD18: recognition by the I domain is modulated by the lectin-like domain and the CD18 subunit. J. Immunol. 161:6198–6205. [PubMed] [Google Scholar]
- 25.Georgopapadakou, N. H., and J. S. Tkaez. 1995. The fungal cell wall as a drug target. Trends Microbiol. 3:98–104. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino, T., A. Yamada, J. Honda, Y. Imai, M. Nakao, M. Inoue, K. Sagawa, M. M. Yokoyama, K. Oizumi, and K. Itoh. 1993. Tissue-specific distribution and age-dependent increase of human CD11b+ T cells. J. Immunol. 151:2237–2246. [PubMed] [Google Scholar]
- 27.Krauss, J. C., H. Pool, W. Xue, L. Mayo-Bond, R. F. Todd, 3rd, and H. R. Petty. 1994. Reconstitution of antibody-dependent phagocytosis in fibroblasts expressing Fc gamma receptor IIIB and the complement receptor type 3. J. Immunol. 153:1769–1777. [PubMed] [Google Scholar]
- 28.Levitz, S. M., and M. P. Dupont. 1993. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J. Clin. Investig. 91:1490–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitz, S. M., M. P. Dupont, and E. H. Smail. 1994. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect. Immun. 62:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, R., J. Xie, C. Kantor, V. Koistinen, D. C. Altieri, P. Nortamo, and C. G. Gahmberg. 1995. A peptide derived from the intercellular adhesion molecule-2 regulates the avidity of the leukocyte integrins CD11b/CD18 and CD11c/CD18. J. Cell Biol. 129:1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathews, H., and L. Witek-Janusek. 1998. Effect of NK1.1+ lymphocytes on Candida albicans. J. Med. Microbiol. 47:1–8. [DOI] [PubMed] [Google Scholar]
- 32.McFarland, H. I., S. R. Nahill, J. W. Maciaszek, and R. M. Welsh. 1992. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J. Immunol. 149:1326–1333. [PubMed] [Google Scholar]
- 33.Miyamoto, S., H. Teramoto, O. A. Coso, J. S. Gutkind, P. D. Burbelo, S. K. Akiyama, and K. M. Yamada. 1995. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131:791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy, J. W. 1989. Immunity to fungi. Curr. Opin. Immunol. 2:360–367. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, J. W. 1991. Mechanisms of natural resistance to human pathogenic fungi. Annu. Rev. Microbiol. 45:509–538. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, J. W., M. R. Hidore, and S. C. Wong. 1993. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J. Clin. Investig. 91:1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagabhushan, M., H. L. Mathews, and L. Witek-Janusek. 2001. Aberrant nuclear expression of AP-1 and NFκB in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav. Immun. 15:78–84. [DOI] [PubMed] [Google Scholar]
- 38.Pfaller, M. A. 1994. Epidemiology and control of fungal infections. Clin. Infect. Dis. 19(Suppl.1):S8–S13. [DOI] [PubMed] [Google Scholar]
- 39.Pfaller, M. A., S. A. Messer, A. Houston, M. S. Rangel-Frausto, T. Wiblin, H. M. Blumberg, J. E. Edwards, W. Jarvis, M. A. Martin, H. C. Neu, L. Saiman, J. E. Patterson, J. C. Dibb, C. M. Roldan, M. G. Rinaldi, and R. P. Wenzel. 1998. National epidemiology of mycoses survey: a multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn. Microbiol. Infect. Dis. 31:289–296. [DOI] [PubMed] [Google Scholar]
- 40.Piersbacher, M., and E. Ruoslahti. 1987. Influence of stereochemistry of the sequence arg-gly-asp-xaa on binding specificity in cell adhesion. J. Biol. Chem. 262:17294–17298. [PubMed] [Google Scholar]
- 41.Ross, G. D., J. A. Cain, and P. J. Lachmann. 1985. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin and functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J. Immunol. 134:3307–3315. [PubMed] [Google Scholar]
- 42.Rozdzinski, E., J. Sandros, M. van der Flier, A. Young, B. Spellerberg, C. Bhattacharyya, J. Straub, G. Musso, S. Putney, R. Starzyk, et al. 1995. Inhibition of leukocyte-endothelial cell interactions and inflammation by peptides from a bacterial adhesin which mimic coagulation factor X. J. Clin. Investig. 95:1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Madrid, F., A. M. Krensky, C. F. Ware, E. Robbins, J. L. Strominger, S. J. Burakoff, and T. A. Springer. 1982. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc. Natl. Acad. Sci. USA 79:7489–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangeorzan, J. A., S. F. Bradley, X. He, L. T. Zarins, G. L. Ridenour, R. N. Tiballi, and C. A. Kauffman. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am. J. Med. 97:339–346. [DOI] [PubMed] [Google Scholar]
- 45.Schneck, J., T. Munitz, J. E. Coligan, W. L. Maloy, D. H. Margulies, and A. Singer. 1989. Inhibition of allorecognition by an H-2Kb-derived peptide is evidence for a T-cell binding region on a major histocompatibility complex molecule. Proc. Natl. Acad. Sci. USA 86:8516–8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sehgal, G., K. Zhang, R. F. Todd, 3rd, L. A. Boxer, and H. R. Petty. 1993. Lectin-like inhibition of immune complex receptor-mediated stimulation of neutrophils. Effects on cytosolic calcium release and superoxide production. J. Immunol. 150:4571–4580. [PubMed] [Google Scholar]
- 47.Spaccapelo, R., L. Romani, L. Tonnetti, E. Cenci, A. Mencacci, G. Del Sero, R. Tognellini, S. G. Reed, P. Puccetti, and F. Bistoni. 1995. TGF-beta is important in determining the in vivo patterns of susceptibility or resistance in mice infected with Candida albicans. J. Immunol. 155:1349–1360. [PubMed] [Google Scholar]
- 48.Springer, T. A. 1990. Adhesion receptors of the immune system. Nature 346:425–434. [DOI] [PubMed] [Google Scholar]
- 49.Thornton, B. P., V. Vetvicka, M. Pitman, R. C. Goldman, and G. D. Ross. 1996. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156:1235–1246. [PubMed] [Google Scholar]
- 50.Timonen, T., C. G. Gahmberg, and M. Patarroyo. 1990. Participation of CD11a-c/CD18, CD2 and RGD-binding receptors in endogenous and interleukin-2-stimulated NK activity of CD3-negative large granular lymphocytes. Int. J. Cancer 46:1035–1040. [DOI] [PubMed] [Google Scholar]
- 51.Wright, S. D., S. M. Levin, M. T. Jong, Z. Chad, and L. G. Kabbash. 1989. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J. Exp. Med. 169:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright, S. D., J. I. Weitz, A. J. Huang, S. M. Levin, S. C. Silverstein, and J. D. Loike. 1988. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc. Natl. Acad. Sci. USA 85:7734–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada, Y., and H. K. Kleinman. 1992. Functional domains of cell adhesion molecules. Curr. Opin. Cell Biol. 4:819–823. [DOI] [PubMed] [Google Scholar]
- 54.Zhou, L., D. H. Lee, J. Plescia, C. Y. Lau, and D. C. Altieri. 1994. Differential ligand binding specificities of recombinant CD11b/CD18 integrin I-domain. J. Biol. Chem. 269:17075–17079. [PubMed] [Google Scholar]
- 55.Zunino, S. J., and D. Hudig. 1988. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect. Immun. 56:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]