Abstract
The cytolethal distending toxin (CDT) from Actinobacillus actinomycetemcomitans has been shown to induce cell cycle arrest in the G2/M phase in HeLa cells. In the present study, the mechanism of CDT-induced cell cycle arrest was investigated by using HS-72 cells, a murine B-cell hybridoma cell line. Using flow cytometric analysis, we found that the recombinant CDT (rCDT) from A. actinomycetemcomitans induced G2 cell cycle arrest in HS-72 cells and that rCDT upregulated expression of the cyclin-dependent kinase inhibitor p21CIP1/WAF1 and the tumor suppressor protein p53. HS-72 cells transfected with the E6/E7 gene of human papillomavirus type 16, which lacked rCDT-induced accumulation of p53, exhibited expression of p21CIP1/WAF1 or G2 cell cycle arrest upon exposure to rCDT. Furthermore, ectopic expression of a dominant negative p53 mutant did not inhibit rCDT-mediated p21CIP1/WAF1 expression or G2 cell cycle arrest in HS-72 cells. These results suggest that the CDT from A. actinomycetemcomitans induces p21CIP1/WAF1 expression and G2 cell cycle arrest in B-lineage cells by p53-independent pathways. Together with additional observations made with HeLa cells and COS-1 cells cultured with the rCDT from A. actinomycetemcomitans, the results of this study indicate that CDT-induced p53 accumulation may not be required for G2 cell cycle arrest and that an increased level of p21CIP1/WAF1 may be important for sustaining G2 cell cycle arrest in several mammalian cells.
Actinobacillus actinomycetemcomitans, a gram-negative, nonmotile, capnophilic, fermentative coccobacillus, has been recovered from periodontally diseased gingival tissues (4). It is well known that this microorganism is implicated in the pathogenesis of severe juvenile and progressive periodontitis (30, 31) and various infectious diseases, such as endocarditis, pericarditis, meningitis, osteomyelitis, empyema, and subcutaneous abscesses (15). In addition, A. actinomycetemcomitans has been reported to produce multiple virulence factors and tissue-damaging toxins, such as a leukotoxin (34, 36), an epitheliotoxin (12), a bone resorption-inducing toxin (29), a cytolethal distending toxin (CDT) (33), and an apoptosis-inducing toxin (21).
CDT was first described as a distinct and novel toxin produced by Escherichia coli (14). The CDTs constitute a family of bacterial heat-labile toxins produced by several bacterial species, including Haemophilus ducreyi, Campylobacter species, Shigella dysenteriae, and Helicobacter hepaticus (23). CDT is encoded by three genes, designated cdtA, cdtB, and cdtC. These three genes encode the polypeptides CdtA (27 kDa), CdtB (29 kDa), and CdtC (20 kDa), which are responsible for the toxic activity (22, 25, 33). Recently, we cloned the cdtA, cdtB, and cdtC genes of A. actinomycetemcomitans, constructed an E. coli expression system, and purified the products of these genes, CdtA, CdtB, and CdtC (24).
Exposure of mammalian cells to several DNA-damaging agents evokes a complicated cellular response, including a reversible block in the cell cycle at the G1 and G2/M phases, and induces programmed cell death (11). The cell cycle arrest at the G1 and G2/M phases reflects the fact that mammalian cells need time to repair damaged DNA. After DNA damage, the cell cycle stops at the transition from the G1 phase to the S phase and at the transition from the G2 phase to the M phase, with DNA complements of 2n and 4n, respectively. It has been reported that transitions between different cell cycle phases are regulated at checkpoints controlled by cyclin-dependent kinases (CDKs), which are activated by cyclins (18). Recently, inhibitors of CDKs have been identified (27). There have been many studies of one of these inhibitors, p21CIP1/WAF1, which negates the kinase activities of cyclin-CDK by directly binding to the catalytically active kinase complexes.
Many investigators have reported that CDTs inhibit proliferation of several mammalian cell lines by inducing a block in the G2 phase of the cell cycle. E. coli CDT was found to block the cell cycle of HeLa cells at the G2/M transition by preventing CDK1 protein kinase dephosphorylation and activation (23). Recently, Shenker et al. (26) reported that lymphocytes treated with the CDT from A. actinomycetemcomitans expressed normal cyclin A and B1 levels but reduced levels of CDK1 and that most CDK1 was in an inactive form. Although recent studies of several investigators have indicated that CDT interferes with the cell cycle-dependent dephosphorylation of Cdc1, the catalytic subunit of cyclin B (5, 37), we are not aware of any reports concerning the contribution of CDK inhibitors to induction of G2 cell cycle arrest in mammalian cells treated with CDT.
The present study was undertaken to determine the mechanism by which the CDT from A. actinomycetemcomitans induces G2 cell cycle arrest in B-cell hybridoma cells. Our results show that the CDT from A. actinomycetemcomitans induces cell distension and G2 cell cycle arrest in HeLa cells and B-cell hybridoma cells. Furthermore, G2 cell cycle arrest may be induced by expression of p21CIP1/WAF1 in B-cell hybridoma cells treated with A. actinomycetemcomitans CDT.
MATERIALS AND METHODS
Cell lines and culture conditions.
Mouse hybridoma cell line HS-72 was maintained in Iscove’s modified Dulbecco’s medium (GIBCO BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml). HeLa, a human epithelioid carcinoma cell line, and COS-1, a monkey fibroblast-like cell line, were purchased from the American Type Culture Collection and were maintained in Dulbecco’s modified Eagle medium (GIBCO BRL) supplemented with 10% FBS and antibiotics. HS-72 cells were stably transfected with human papillomavirus type 16 (HPV-16) gene E6/E7 as described previously (39). HS-72 transfectants were selected on the basis of growth in the presence of G418 (1 mg/ml; GIBCO BRL), and individual clones were isolated by limiting dilution. HPV-16 E6/E7-transfected clones (E6/E7-HS-1 and E6/E7-HS-3) and control plasmid-transfected clones (neoHS-1 and neoHS-2) were grown in media containing 10% FBS and G418 (1 mg/ml). Stably proliferating cells were screened for E6/E7 mRNA expression by reverse transcription-PCR (39).
Preparation of rCDT from A. actinomycetemcomitans.
The pUC119ΔH vector and CDT-expressing plasmid pUC119ΔH-YI-PCR3, which contains three open reading frames (cdtA, cdtB, and cdtC), were inserted into E. coli JM109 as described previously (24). An E. coli strain (JM109/pUC119ΔH-YI-PCR3) which produced recombinant CDT (rCDT) and a control strain (JM109/pUC119ΔH) were grown in 2× YT medium supplemented with ampicillin (50 μg/ml). To obtain rCDT and a control preparation from extracts of JM109/pUC119ΔH-YI-PCR3 and JM109/pUC119ΔH, E. coli strains were grown at 30°C for 3 h, and the proteins were induced by adding 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside). After 4 h of incubation at 30°C, the bacteria were precipitated by centrifugation, resuspended in 10 ml of sterile phosphate-buffered saline, and disrupted with an Ultrasonic processor (Ultrasonic, Misonix, N.Y.). The supernatants were collected after centrifugation and filtered (Minisart; pore size, 0.20 μm; Sartorius, Göttingen, Germany). The growth-inhibitory activity was determined by the cell viability assay as described previously (21). The protein contents of rCDT and a control preparation were determined with a protein assay reagent (Bio-Rad Laboratories, Richmond, Calif.).
Plasmid and transfection.
A human p53 mutant (G273H) was a kind gift from B. Vogelstein (The Johns Hopkins Oncology Center, Baltimore, Md.) (16). HS-72 cells were transfected with the human p53 mutant by using Effectene transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. HS-72 transfectants were selected on the basis of growth in the presence of G418 (1 mg/ml), and individual clones were isolated by limiting dilution.
Cell cycle analysis.
The cells were suspended in a hypotonic solution (0.1% Triton X-100, 1 mM Tris-HCl [pH 8.0], 3.4 mM sodium citrate, 0.1 mM EDTA), stained with 5 μg of propidium iodide per ml, and analyzed with a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) (21). The population of cells in each cell cycle phase was determined by using ModFit LT software (Becton Dickinson Immunocytometry Systems).
Immunoblot analysis.
The cells were suspended in a lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.0], 1% NP-40). A sample (30 μg of protein) was separated on 12.5% polyacrylamide gels containing 0.1% sodium dodecyl sulfate and then electroblotted on polyvinylidine fluoride membranes. For analysis of p53, the cells were suspended in a lysis buffer (5 mM EDTA, 10 mM Tris-HCl [pH 7.4], 1% Triton X-100), and the lysate was separated on 10% polyacrylamide gels containing 0.1% sodium dodecyl sulfate. Immunodetection was performed with an ECL Western blot detection system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) according to the manufacturer’s instructions. Monoclonal anti-p21CIP1/WAF1 antibody (F-5), monoclonal anti-human p53 antibody (Bp53-12), and polyclonal anti-p53 antibody (PAb240) were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The protein contents of samples were determined with a protein assay reagent (Bio-Rad Laboratories). Blots were stained with Coomassie brilliant blue, and we confirmed that all lanes contained similar amounts of protein extract.
RESULTS
A. actinomycetemcomitans rCDT induces G2 cell cycle arrest and distension in HS-72 cells.
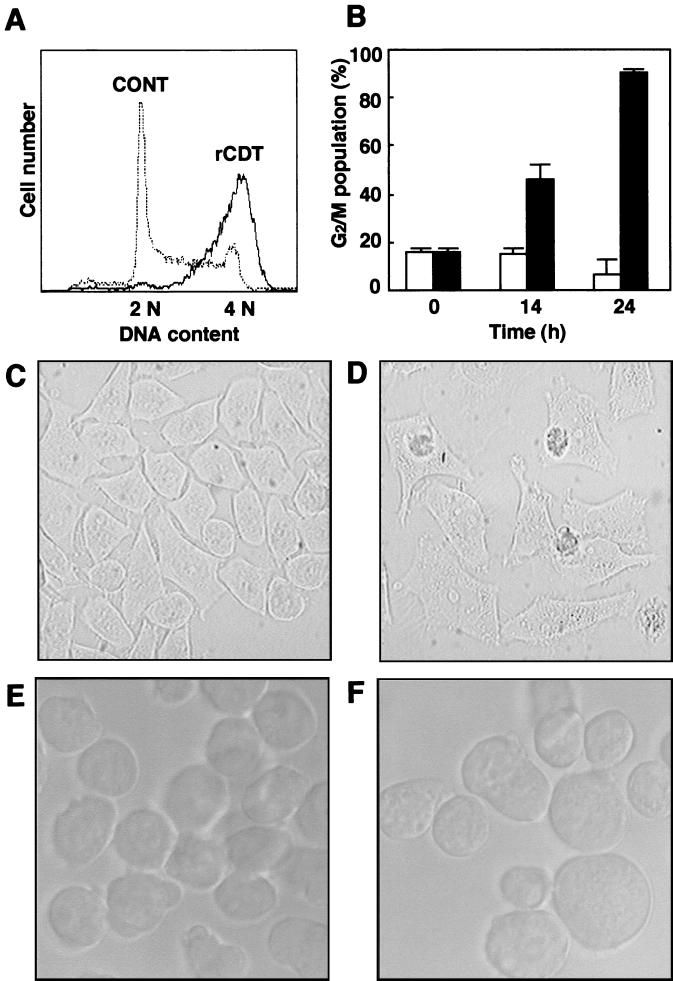
We first examined the effect of an rCDT preparation from an E. coli strain (JM109/pUC119ΔH-YI-PCR3) on G2 cell cycle arrest and distension in HS-72 cells. Cultivation with the rCDT (100 μg/ml) increased the population of HS-72 cells in the G2/M phase with time (Fig. 1A and B). As shown in Fig. 1, both HS-72 cells and HeLa cells were distended when they were treated with rCDT (100 μg/ml) for 24 h.
FIG. 1.
Morphology and cell cycle analysis of cells cultured with rCDT from A. actinomycetemcomitans. (A) HS-72 cells cultured with rCDT (100 μg/ml) for 24 h, stained with propidium iodide, and then analyzed with a flow cytometer. CONT, control. (B) DNA contents of the G2/M population in HS-72 cells determined with a flow cytometer at different times. The cells were stimulated with the control preparation (100 μg/ml) (open bars) or rCDT (100 μg/ml) (solid bars). The experiment was performed three times, and similar results were obtained in all experiments. (C to F) HeLa cells (C and D) and HS-72 cells (E and F) cultured with the control preparation (100 μg/ml) (C and E) or rCDT (100 μg/ml) (D and F) for 24 h. Cell morphology was analyzed by phase-contrast microscopy.
Expression of p21CIP1/WAF1 and p53 in HS-72 cells treated with rCDT.
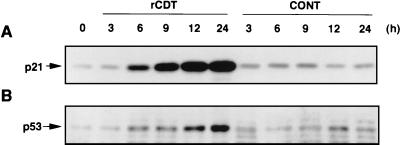
Immunoblot analysis showed that untreated HS-72 cells contained negligible levels of p21CIP1/WAF1 and p53 (Fig. 2). When cells were exposed to rCDT (100 μg/ml), p21CIP1/WAF1 and p53 were detected as early as 6 h, and the levels increased with time. On the other hand, the control extract from the E. coli strain (JM109/pUC119ΔH) had almost no effect on expression of p21CIP1/WAF1 or p53 at a protein concentration of 100 μg/ml (Fig. 2).
FIG. 2.
p21CIP1/WAF1 and p53 expression in HS-72 cells cultured with rCDT. HS-72 cells were cultured with the control preparation (100 μg/ml) (CONT) or rCDT (100 μg/ml) for different times and analyzed for expression of p21CIP1/WAF1 (A) and p53 (B) by immunoblotting. Monoclonal anti-p21CIP1/WAF1 antibody and polyclonal anti-p53 antibody were used as the primary antibodies to detect p21CIP1/WAF1 and p53, respectively.
Role of p53 in induction of p21CIP1/WAF1 and G2 cell cycle arrest.
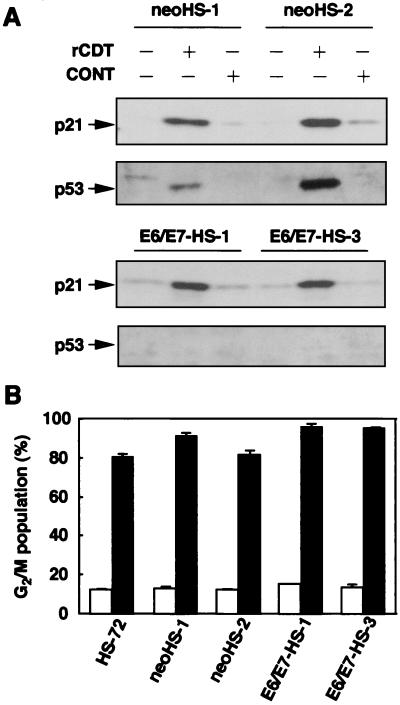
To analyze the role of p53 in induction of p21CIP1/WAF1 and G2 cell cycle arrest, we used HS-72 cells stably transfected with the E6/E7 gene of HPV-16, in which endogenous p53 is suppressed by the E6 oncogene. rCDT (100 μg/ml) induced both p53 expression and p21CIP1/WAF1 expression in control HS-72 cells (neoHS-1 and neoHS-2), as observed with parental cells. However, after exposure to rCDT for 18 h, E6/E7-transfected HS-72 cells (E6/E7-HS-1 and E6/E7-HS-3) contained increased levels of p21CIP1/WAF1, and there was no accumulation of p53 (Fig. 3A). To examine whether p53 was required for rCDT-induced G2 cell cycle arrest, cell cycle distribution was examined with E6/E7-HS-1 and E6/E7-HS-3 cells stimulated with rCDT. As shown in Fig. 3B, exposure to rCDT (100 μg/ml) for 18 h increased the population in the G2/M phase in control HS-72 cells and E6/E7-transfected HS-72 cells.
FIG. 3.
Effect of ectopic expression of E6/E7 on induction of p21CIP1/WAF1 and p53 and G2 cell cycle arrest in HS-72 cells. The cells were cultured with the control preparation (100 μg/ml) (CONT) or rCDT (100 μg/ml) for 18 h. (A) Immunoblot analysis of cell extracts with monoclonal anti-p21CIP1/WAF1 antibody and polyclonal anti-p53 antibody. (B) Flow cytometric analysis of the DNA content of the G2/M population in HS-72 cells stimulated with the control preparation (100 μg/ml) (open bars) or rCDT (100 μg/ml) (solid bars). The experiment was performed three times, and similar results were obtained in all experiments. neoHS-1 and neoHS-2 were control plasmid-transfected clones. E6/E7-HS-1 and E6/E7-HS-3 were human HPV-16 E6/E7-transfected clones.
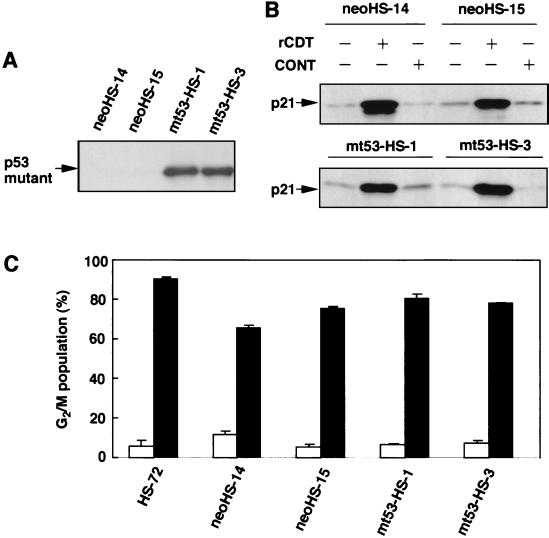
Next, we introduced a human p53 mutant (G273H) into HS-72 cells to block the normal p53 function. Figure 4A shows the expression of human p53 protein in mutant p53-transfected HS-72 cells (mt53-HS-1 and mt53-HS-3) when the cells were cultured without rCDT. Immunoblot analysis revealed that rCDT (100 μg/ml) induced p21CIP1/WAF1 expression in control HS-72 cells (neoHS-14 and neoHS-15) and mutant p53-transfected HS-72 cells (mt53-HS-1 and mt53-HS-3) (Fig. 4B). We also examined the effect of human mutant p53 on induction of G2 cell cycle arrest in HS-72 cells stimulated with rCDT. As shown in Fig. 4C, the control HS-72 cells (neoHS-14 and neoHS-15) and the human mutant p53-transfected HS-72 cells (mt53-HS-1and mt53-HS-3) showed G2 cell cycle arrest when they were cultured with rCDT (100 μg/ml) for 18 h.
FIG. 4.
Effect of ectopic expression of mutant p53 on induction of p21CIP1/WAF1 and p53 and G2 cell cycle arrest in HS-72 cells. (A) Immunoblot analysis of the cell extract with monoclonal anti-human p-53 antibody to detect expression of mutant p53. (B) The cells were cultured with the control preparation (100 μg/ml) (CONT) or rCDT (100 μg/ml) for 18 h, and an immunoblot analysis of the cell extract was performed with monoclonal anti-p21CIP1/WAF1 antibody. (C) Flow cytometric analysis of the DNA content of the G2/M population in HS-72 cells stimulated with the control preparation (100 μg/ml) (open bars) or rCDT (100 μg/ml) (solid bars). The experiment was performed three times, and similar results were obtained in all experiments. neoHS-14 and neoHS-15 were control plasmid-transfected clones. mt53-HS-1 and mt53-HS-3 were human p53 mutant-transfected clones.
rCDT induces p21CIP1/WAF1 expression and G2 cell cycle arrest in COS-1 cells and HeLa cells, as well as HS-72 cells.
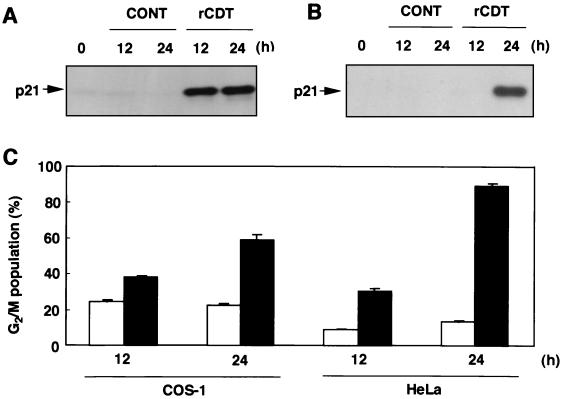
To examine whether induction of p21CIP1/WAF1 occurs in other types of cells undergoing rCDT-mediated G2 cell cycle arrest, expression of p21CIP1/WAF1 and cell cycle distribution in COS-1 cells and HeLa cells were analyzed. p21CIP1/WAF1 was detected in COS-1 cells 12 and 24 h after treatment with rCDT (100 μg/ml) (Fig. 5A). On the other hand, HeLa cells expressed p21CIP1/WAF1 proteins when the cells were cultured with rCDT (100 μg/ml) for 24 h (Fig. 5B). Flow cytometric analysis revealed that rCDT (100 μg/ml) increased the population in the G2/M phase in COS-1 and HeLa cultures when the cells were cultured for 12 and 24 h (Fig. 5C).
FIG. 5.
Effect of rCDT on p21CIP1/WAF1 expression and G2 cell cycle arrest in HeLa cells and COS-1 cells. The cells were cultured with the control preparation (100 μg/ml) (CONT) or rCDT (100 μg/ml) for different times. (A and B) Immunoblot analysis of extracts of COS-1 cells (A) and HeLa cells (B) with monoclonal anti-p21CIP1/WAF1 antibody. (C) Flow cytometric analysis of the DNA content of the G2/M population in COS-1 and HeLa cells stimulated with the control preparation (100 μg/ml) (open bars) or rCDT (100 μg/ml) (solid bars). The experiment was performed three times, and similar results were obtained in all experiments.
DISCUSSION
It is well known that DNA damage in proliferating cells induces a complex intracellular response that includes perturbation of the cell cycle and apoptotic cell death. The loss of cell cycle control and the inability of cells to repair DNA at cell cycle checkpoints result in propagation of genetic lesions. Irradiation of mammalian cells has been shown to delay completion of the S phase, and this is followed by extended G2/M arrest and apoptosis (1). Recently, Cortes-Bratti et al. (6) reported that the CDT from H. ducreyi, which causes chancroid, induces cell cycle arrest and apoptosis, suggesting that the response to this toxin resembles the checkpoint response induced by irradiation. It has been reported that A. actinomycetemcomitans produces a new member of the CDT family which induces growth arrest in the G2/M phase of cultured mammalian cells (33) and that CDT-treated T lymphocytes contain reduced levels of hypophosphorylated active CDK1 (26). Recently, some investigators have demonstrated that CdtB-associated DNase activity is responsible for CDT-induced cell cycle arrest, suggesting that DNase activity is essential for CDT toxicity (10, 17). In a preliminary study, we examined the levels of expression of cell cycle-related proteins, such as phosphorylated CDK1 and CDK2, cyclins, and Cdc25B, and found that CDT from A. actinomycetemcomitans strongly upregulated expression of p21CIP1/WAF1 and p53. Although a substantial amount of biological and biochemical information concerning CDT-mediated G2 cell cycle arrest is available, little attention has been paid to the contribution of the CDK inhibitor p21CIP1/WAF1 and the tumor suppressor protein p53 to induction of G2 cell cycle arrest in mammalian cells treated with bacterial CDT.
p21CIP1/WAF1 is a well-known negative regulator of CDKs (8, 38, 40). Expression of p21CIP1/WAF1 is regulated by various antimitogenic signals, such as members of the transforming growth factor β superfamily and p53 (7, 41). Recently, several observations have raised the possibility that p21CIP1/WAF1 plays important roles at several stages in the cell cycle (38). In the present study, we demonstrated that A. actinomycetemcomitans CDT induced G2 cell cycle arrest and enhanced expression of p21CIP1/WAF1 in HS-72 cells, suggesting that p21CIP1/WAF1 is necessary to sustain G2 cell cycle arrest in B-lineage cells. This is consistent with the finding that the p21CIP1/WAF1 protein was expressed in 12-O-tetradecanoylphorbol-13-acetate-treated A549 lung carcinoma cells, which also exhibited G2 cell cycle arrest (35).
We previously reported that in HS-72 cells activins and bone morphogenetic protein 2 (BMP-2) induced G1 arrest and apoptosis, which was associated with accumulation of p21CIP1/WAF1 (13, 40). A recent study indicated that expression of E6/E7 blocked inhibition of retinoblastoma protein (Rb) phosphorylation and G1 cell cycle arrest but did not attenuate apoptotic cell death in BMP-2-treated cells (39). It is well known that E7 protein binds to Rb (8, 20) and that this binding disrupts the interaction between Rb and the E2F transcription factor, allowing E2F to activate its downstream cellular target genes that are required for G1 cell cycle progression (3). In the present study, E6/E7 expression had no effect on expression of p21CIP1/WAF1 or G2 cell cycle arrest in CDT-stimulated cells. These findings are consistent with results showing that BMP-2-induced p21CIP1/WAF1 expression was not blocked in E6/E7-transfected HS-72 cells and had no effect on apoptotic cell death mediated by BMP-2 (39). Although Tchou et al. reported that 12-O-tetradecanoylphorbol-13-acetate-induced growth arrest in A549 cells was associated with induction of p21CIP1/WAF1 (35), further work is needed to elucidate the precise role of p21CIP1/WAF1 in induction of G2 cell cycle arrest in HS-72 cells stimulated with the CDT from A. actinomycetemcomitans.
Recently, it has been reported that activation of endogenous p53 by E7 can be reversed by simultaneous expression of E6 and that HS-72 cells express wild-type p53 mRNA and a very low level of p53 protein (39). In the present study, we found that ectopic expression of human E6/E7 eliminated CDT-induced expression of p53 (Fig. 3A). Song et al. (32) demonstrated that both E6, which inhibits p53 functions, and E7, which inhibits Rb phosphorylation, can also eliminate growth arrest in rodent cells. They also reported that no p53-positive cells were observed in E6 transgenic mice, suggesting that E6 may lead to efficient degradation of p53 (32). Taken together, these findings suggest that activation of endogenous p53 may be regulated by E6 and that overexpressed E6 in E6/E7-transfected HS-72 cells may degrade p53 protein.
p21CIP1/WAF1 is a universal CDK inhibitor, and p21CIP1/WAF1 gene expression is directly regulated by the p53 tumor suppressor protein (9). Bunz et al. (2) demonstrated that p53 and p21CIP1/WAF1 appear to be essential for maintaining the G2 checkpoint in human cells. It has been reported that p21CIP1/WAF1 can be regulated by p53-dependent or -independent pathways in several situations, including during normal tissue development, following serum stimulation, and in the cellular response to several stimulants (19, 41). It has been reported that a human p53 mutant (G273H) blocks normal p53 function (16, 28). In the present study, we examined the abilities of a human p53 mutant (G273H) in HS-72 cells stimulated with CDT from A. actinomycetemcomitans in order to determine the effect of p53 on induction of p21CIP1/WAF1 and G2 cell cycle arrest. As shown in Fig. 4, human dominant negative mutant p53-transfected HS-72 cells expressed p21CIP1/WAF1 protein, even when the cells were cultured with CDT. Furthermore, the dominant negative human mutant p53 had no effect on induction of G2 cell cycle arrest in CDT-stimulated HS-72 cells. These results suggest that p21CIP1/WAF1 expression and G2 cell cycle arrest in HS-72 cells stimulated with CDT may be regulated by p53-independent pathways.
In summary, our results indicate that CDT-induced p21CIP1/WAF1 promotes G2 cell cycle arrest in several types of mammalian cells, such as B-lineage cells and fibroblast-like cells. A. actinomycetemcomitans has been associated with not only periodontitis but also systemic infections in humans, such as endocarditis, pericarditis, meningitis, osteomyelitis, empyema, and subcutaneous abscesses. Our results might provide insight into the important pathological roles of the toxin from A. actinomycetemcomitans in the initiation and progression of severe systemic infectious diseases, as well as progressive periodontitis.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Science, and Culture of Japan and the Ministry of Health and Welfare of Japan.
Editor: E. I. Tuomanen
REFERENCES
- 1.Aloni-Grinstein, R., D. Schwartz, and V. Rotter. 1995. Accumulation of wild-type p53 protein upon γ-irradiation induces a G2 arrest-dependent immunoglobulin κ light chain gene expression. EMBO J. 14:1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497–1501. [DOI] [PubMed] [Google Scholar]
- 3.Chellappan, S., V. Kraus, B. Kroger, K. Munger, P. Howley, and J. Nevins. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 89:4549–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christersson, L. A., U. M. Wikesjo, B. Albini, J. J. Zambon, and R. J. Genco. 1987. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluorescence and culture techniques. J. Periodontol. 58:540–545. [DOI] [PubMed] [Google Scholar]
- 5.Comayras, C., C. Tasca, S. Y. Pérès, B. Ducommun, E. Oswald, and J. De Rycke. 1997. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect. Immun. 65:5088–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell-cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 276:5296–5302. [DOI] [PubMed] [Google Scholar]
- 7.Dulic, V., W. K. Kaufmann, S. J. Wilson, T. D. Tlsty, E. Lees, J. W. Harper, S. J. Elledge, and S. I. Reed. 1994. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76:1013–1023. [DOI] [PubMed] [Google Scholar]
- 8.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934–937. [DOI] [PubMed] [Google Scholar]
- 9.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825. [DOI] [PubMed] [Google Scholar]
- 10.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37:952–963. [DOI] [PubMed] [Google Scholar]
- 11.Hartwell, L. H., and T. A. Weinert. 1989. Checkpoints: controls that ensure the order of cell events. Science 246:629–634. [DOI] [PubMed] [Google Scholar]
- 12.Helgeland, K., and O. Nordby. 1993. Cell cycle-specific growth inhibitory effect on human gingival fibroblasts of a toxin isolated from the culture medium of Actinobacillus actinomycetemcomitans. J. Periodontal Res. 28:161–165. [DOI] [PubMed] [Google Scholar]
- 13.Ishisaki, A., K. Yamato, S. Hashimoto, A. Nakao, K. Tamaki, K. Nonaka, P. T. Dijke, H. Sugino, and T. Nishihara. 1999. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J. Biol. Chem. 274:13637–13642. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, W. M., and H. Lior. 1987. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol. Lett. 43:19–23. [Google Scholar]
- 15.Kaplan, A. H., D. J. Weber, E. Z. Oddone, and P. R. Perfect. 1989. Infection due to Actinobacillus actinomycetemcomitans:15 cases and review. Rev. Infect. Dis. 11:46–63. [DOI] [PubMed] [Google Scholar]
- 16.Kern, S. E., K. W. Kinzler, S. J. Baker, J. M. Nigro, V. Rotter, A. J. Levine, P. Friedman, C. Prives, and B. Vogelstein. 1991. Mutant p53 proteins bind DNA abnormally in vitro. Oncogene 6:131–136. [PubMed] [Google Scholar]
- 17.Lara-Tejero, M., and E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354–357. [DOI] [PubMed] [Google Scholar]
- 18.Lew, D. J., V. Dulic, and S. I. Reed. 1991. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66:1197–1206. [DOI] [PubMed] [Google Scholar]
- 19.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and -independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9:935–944. [DOI] [PubMed] [Google Scholar]
- 20.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohguchi, M., A. Ishisaki, N. Okahashi, M. Koide, T. Koseki, K. Yamato, T. Noguchi, and T. Nishihara. 1998. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in the G2/M phase and apoptosis. Infect. Immun. 66:5980–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickett, C. L., E. C. Pesci, D. L. Cottle, G. Russell, A. N. Erdem, and H. Zeytin. 1996. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect. Immun. 64:2070–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292–297. [DOI] [PubMed] [Google Scholar]
- 24.Saiki, K., K. Kinishi, T. Gomi, T. Nishihara, and M. Yoshikawa. 2001. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol. Immunol. 45:497–506. [DOI] [PubMed] [Google Scholar]
- 25.Scott, D. A., and J. B. Kaper. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenker, B. J., T. McKay, S. Datar, M. Miller, R. Chowhan, and D. Demuth. 1999. Actinobacillus actinomycetemcomitans immuno-suppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 162:4773–4780. [PubMed] [Google Scholar]
- 27.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinase. Genes Dev. 9:1149–1163. [DOI] [PubMed] [Google Scholar]
- 28.Shikh, M. S., X. S. Li, J. C. Chen, Z. M. Shao, J. V. Ordonez, and J. A. Fontana. 1994. Mechanisms of regulation of WAF1/CIP1 gene expression in human breast carcinoma: role of p53-dependent and independent signal transduction pathways. Oncogene 9:3407–3415. [PubMed] [Google Scholar]
- 29.Slots, J., and R. J. Genco. 1984. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J. Dent. Res. 63:412–421. [DOI] [PubMed] [Google Scholar]
- 30.Slots, J., and M. A. Listgarten. 1988. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J. Clin. Periodontol. 15:85–93. [DOI] [PubMed] [Google Scholar]
- 31.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song, S., G. A. Gulliver, and P. F. Lambert. 1998. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent pathways. Proc. Natl. Acad. Sci. USA 95:2290–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugai, M., T. Kawamoto, S. Y. Peres, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 66:5008–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taichman, N. S., R. T. Dean, and C. J. Sanderson. 1980. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect. Immun. 28:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tchou, W.-W., W. N. Rom, and K.-M. Tchou-Wong. 1996. Novel form of p21WAF1/CIP1/SDI1 protein in phorbol ester-induced G2/M arrest. J. Biol. Chem. 271:29556–29560. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, C. C., B. J. Shenker, J. M. DiRienzo, D. Malamud, and N. S. Taichman. 1984. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 43:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehouse, C. A., P. B. Balbo, E. C. Pesci, D. L. Cottle, P. M. Mirabito, and C. L. Pickett. 1998. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect. Immun. 66:1934–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong, Y., G. J. Hannon, H. Zhang, D. Casso, R. Kobayashi, and D. Beach. 1993. p21 is a universal inhibitor of cyclin kinases. Nature 366:701–704. [DOI] [PubMed] [Google Scholar]
- 39.Yamato, K., S. Hashimoto, N. Okahashi, A. Ishisaki, K. Nonaka, T. Koseki, M. Kizaki, Y. Ikeda, and T. Nishihara. 2000. Dissociation of bone morphogenetic protein-mediated growth arrest and apoptosis of mouse B cells by HPV-16 E6/E7. Exp. Cell Res. 257:198–205. [DOI] [PubMed] [Google Scholar]
- 40.Yamato, K., T. Koseki, M. Ohguchi, M. Kizaki, Y. Ikeda, and T. Nishihara. 1997. Activin A induction of cell cycle arrest involves modulation of cyclin D2 and p21CIP1/WAF1 in plasmacytic cell. Mol. Endocrinol. 11:1044–1052. [DOI] [PubMed] [Google Scholar]
- 41.Zeng, Y.-X., and W. S. El-Deiry. 1996. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene 12:1557–1564. [PubMed] [Google Scholar]