Abstract
Numerous investigations have shown that 70-kDa heat shock protein (Hsp70) homologs interact tightly with hydrophobic proteins and functionally assist proteins in membranous organelles and environments. One such protein is the Chlamydia trachomatis Hsp70 that is associated with isolated outer membrane complexes of infectious elementary bodies (EB). Previous observations have indicated that chlamydial Hsp70 plays a role in EB attachment to, or entry into, endometrial epithelial cells. In this study, immunofluorescence microscopy and transmission electron microscopy observations showed that chlamydial Hsp70 is not a surface-displayed ligand on purified EB. However, brief exposure of EB to the thiol reducing agent dithiothreitol (DTT) led to surface accessibility of the Hsp70 substrate-binding domain. Reduction of the highly disulfide-cross-linked EB outer membrane proteins with DTT resulted in a decrease in EB attachment and infectivity. Interestingly, exposure of EB to the membrane-impermeable thiol-alkylating reagent 5,5′-dithiobis(2-nitrobenzoic acid) enhanced attachment but compromised infectivity, suggesting that EB outer membrane proteins must be reduced for entry and productive infection. Together, our data suggest that (i) the structural integrity of the EB outer membrane, maintained by protein disulfide bonds, is important during the initial stages of attachment; (ii) reduction occurs within the localized microenvironment of host cell surfaces once intimate contact is established between EB and host cells; and (iii) subsequent conformational changes in EB ultrastructure allow productive infection in host cells. The accessibility of the Hsp70 substrate-binding domain may support the hypothesis that this protein plays a role in events following the initial stage of attachment instead of serving as a primary, surface-displayed adhesin.
A distinguishing feature of the chlamydiae is their transition between infectious elementary bodies (EB) that bind to and enter host cells and noninfectious reticulate bodies that replicate intracellularly within a membrane-bound inclusion. EB are small (diameter, 300 nm) particles with an unusually rigid ultrastructure due to cysteine-rich membrane proteins that exhibit intra- and intermolecular disulfide cross-linking in the envelope (22, 36). Several chlamydial molecules, including the cysteine-rich proteins, have been examined to determine their roles in EB attachment to eukaryotic cells (25, 26, 38, 43, 46, 48, 49, 50, 51). There is evidence that both the 60-kDa cysteine-rich membrane protein and the major outer membrane protein (MOMP) serve as receptors for sulfated glycosaminoglycans in a tethering event between EB and host cell surfaces (46, 48, 49). Adherence mediated by the 40-kDa MOMP appears to be initiated by charge-charge interactions involving surface-exposed domains (50) and a high-mannose oligomannose oligosaccharide (26). It is likely that additional, unidentified surface components also participate in establishing contact between EB and the host cell surface.
Although many devoted investigators have provided key insights into how chlamydiae initiate infection, there is much that remains to be determined concerning the multifactorial processes used by EB to gain access to their intracellular habitat in susceptible host cells. No singular chlamydial component has been identified as a high-affinity surface ligand. The lack of a prominent adhesin is consistent with observations that chlamydiae enter host cells by multiple routes (39). Closely related chlamydial biovariants exhibit clear differences in the utilization of known adherence mechanisms, which is thought to reflect properties inherent in tissue tropism and directional spread of infection (10).
Several years ago, study findings demonstrated that the chlamydial 70-kDa heat shock protein (Hsp70) (38) and the cochaperone protein GrpE (44) are associated with isolated outer membrane complexes of Chlamydia trachomatis. Moreover, when these proteins were expressed in recombinant Escherichia coli, the recombinant attached to human endometrial epithelial cells in a manner mimicking that observed for C. trachomatis EB (43). As determined by electron microscopy, the adherent recombinant appeared to be anchored in clathrin-coated pits. Also similar to chlamydial EB, the recombinant E. coli exhibited increased adherence to estrogen-dominant primary endometrial epithelial cells and decreased adherence to progesterone-dominant primary endometrial epithelial cells (31, 43). These surprising observations prompted further investigation of the contribution of chlamydial Hsp70 to attachment to human genital epithelial cells.
In this study, antibodies were generated against three peptides representing different domains of C. trachomatis serovar E Hsp70, and these antibodies were used to investigate exposure of Hsp70 at the surface of purified EB. Although none of the domains was prominently displayed at the surface, the Hsp70 substrate-binding domain became selectively accessible following brief reduction of the cysteine-rich outer membrane protein lattice with the reducing reagent dithiothreitol (DTT). A thiol-alkylating reagent, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), was subsequently used to determine whether reduction of EB membrane proteins occurs at the surface of the host cell just prior to or during EB entry. DTNB is a membrane-impermeable reagent that covalently modifies surface sulfhydryls to prevent disulfide bond cleavage (41) and has been used to demonstrate that the rigid, disulfide-cross-linked proteins in the envelope of Sindbis virus are modified by host surface reductase activities for efficient viral penetration (1). In the present study, chlamydial infectivity, but not attachment, was compromised by DTNB, which suggests that reduction of EB disulfide-cross-linked envelope proteins occurs in the microenvironment of the host cell surface. This reductive event, in turn, exposes the chlamydial Hsp70 substrate-binding domain. Overall, our data indicate that chlamydial Hsp70 is not a primary, surface-displayed ligand and that although substrates for envelope-associated Hsp70 are not known yet, the role of Hsp70 in adherence may be to provide a more intimate interaction with host ligands following the initial stages of attachment. Alternatively, Hsp70 may assist in the conformation and presentation of other chlamydial adhesins during attachment and entry.
MATERIALS AND METHODS
Bacterial strains, host cells, and growth.
Stock preparations of the genital C. trachomatis serovar E/UW-5/CX were generated by using McCoy cells (CRL 1696; American Type Culture Collection) propagated in microcarrier bead cultures (42). All preparations were frozen at −70°C in storage buffer (0.02 M phosphate buffer, 0.2 M sucrose, 5 mM glutamine; pH 7.2) and subsequently titrated for infectivity on McCoy cell monolayers. EB were purified and metabolically radiolabeled with [35S]Cys/Met as described previously (10). For all assays of chlamydial attachment and neutralization, human endometrial epithelial carcinoma cell line HEC-1B (HTB-113; American Type Culture Collection) was used as a representative natural target host cell line and was cultured in Dulbecco’s modified Eagle medium with 10% fetal calf serum and 2 mM glutamine in a humidified atmosphere containing 5% CO2 at 35°C; 0.5 μg of cycloheximide per ml was added to the culture medium after infection.
The methods used to grow recombinant E. coli JM109(pPBW58) and the characteristics of this strain have been described elsewhere (38, 43, 44). Purified recombinant chlamydial Hsp70 used for proteolytic mapping was obtained from E. coli LMG194(pPBW120). The open reading frame encoding Hsp70 was amplified from the C. trachomatis serovar E genome by using the Expand High Fidelity PCR system (Boehringer Mannheim) and 5′-GGCGGTACCTTACTCAGGTTTATCAACAATTTCAACATCAGC-3′ and 5′-CGCCTCGAGATGAGCGAAAAAAGAAAGTCTAACAAAATTATTGG-3′ as the forward and reverse primers, respectively. The 1,983-kb product was ligated into the multiple cloning site of pBAD/HisA (Invitrogen Corp.) and was verified by sequencing. Chlamydial Hsp70 was overexpressed by inducing E. coli LMG194(pPBW120) with 0.002% (vol/vol) l-arabinose, and histidine-tagged Hsp70 was purified by nickel affinity chromatography by using the manufacturers’ instructions (Invitrogen Corp.).
Preparation of antibodies, protein analyses, and immunoblotting.
Monospecific polyclonal antibodies against three custom-designed 18-amino-acid peptides in the C. trachomatis serovar E Hsp70 sequence were generated in New Zealand White female rabbits by GenoSys Biotechnologies Inc.; preimmune serum from each rabbit was also provided. Enzyme immunoassays to determine the peptide reactivities of all antisera, as well as a double-diffusion agarose precipitation assay against keyhole limpet hemocyanin, were conducted by GenoSys as part of their quality control. After arrival in our laboratory, the immunoglobulin G (IgG) fraction of each serum was purified with protein G affinity matrices (Pharmacia LKB) by using 20 mM sodium phosphate (pH 7.0) as the running buffer. IgG was eluted in 0.1 M glycine-hydrochloric acid (pH 2.7) buffer, immediately neutralized with 1 M Tris-HCl (pH 9.0), and dialyzed overnight at 4°C against 10 mM sodium phosphate buffer (pH 7.0). Aliquots were subsequently stored at −20°C.
The total protein contents of all samples in this study were determined by the bicinchoninic acid microassay (Pierce Chemical Co.), using bovine serum albumin as the standard. The stock concentrations of IgG preparations were adjusted to 1 μg of total protein per μl before the preparations were diluted for all immunoassays. Purified C. trachomatis serovar E EB, as well as mid-logarithmic-growth-phase E. coli JM109(pUC19), JM109(pPBW58), and LMG194 (pPBW120), were washed by repetitive centrifugation and resuspended in phosphate-buffered saline (PBS) (pH 7.0) prior to protein analyses. Proteins were solubilized, resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) by using 12.5% polyacrylamide separation gels, and visualized by staining with silver or Coomassie blue or by Western blotting as described previously (38), using a 1:1,000 (vol/vol) dilution for each IgG preparation. A 1:2,500 (vol/vol) dilution of goat anti-rabbit alkaline phosphatase-conjugated antibodies was used for detection.
Partial proteolytic digests of affinity-purified chlamydial Hsp70 were obtained by incubating 66.0 μg of Hsp70 with 0.5 μg of endoproteinase Arg-C (Promega) for 30 s at 37°C (protein/protease ratio, 132:1). Proteolytic activity was stopped by adding 1 μM EDTA, and samples were resolved by SDS-PAGE prior to Western blot analysis; approximately 13.0 μg of total protein was loaded into each lane.
Immunoprecipitation.
To confirm that peptide antisera reacted with nondenatured Hsp70, cultures of E. coli JM109(pUC19) and JM109(pPBW58) were washed twice in Tris-buffered saline (TBS) (50 mM Tris-HCl [pH 7.4], 150 mM NaCl). Approximately 1 × 107 CFU of each sample per ml was suspended in cold (4°C) TBS containing 1% (vol/vol) Triton X-100, placed in a water bath sonicator, and sonicated intermittently over a 20-min period; samples were placed on ice between pulses. Each resultant lysate was cleared by centrifugation at 13,000 × g for 45 min at 4°C and incubated for 1 h with 50 μl of protein A-Sepharose (Pharmacia LKB) to reduce nonspecific binding. Cleared supernatants were subsequently divided into multiple 100-μl aliquots, 10 μg of each IgG preparation was added to an aliquot, and the aliquots were incubated overnight on a rocking platform at 4°C. Fifty microliters of protein A-Sepharose was then added to each sample, the preparation was incubated for 2 h, and beads were gently pelleted and washed three times with Triton X-100-TBS and once with TBS alone prior to solubilization and resolution by SDS-PAGE. The immunoprecipitated protein was confirmed to be Hsp70 by Western blotting by using a monospecific chlamydial Hsp70 antibody (38).
Attachment and neutralization assays.
Metabolically 35S-radiolabeled EB preparations were used to assess attachment to HEC-1B host cells, whereas nonlabeled EB preparations were used to determine neutralization of chlamydial infectivity by staining intracellular inclusions with a pool of fluorescein-conjugated monoclonal antibodies generated against the C. trachomatis MOMP (Syva) (10). All experiments were conducted at least three times, and each sample was assayed in triplicate or quadruplicate. Statistical significance was determined by using a two-tailed Student’s t test.
For attachment, 35S-labeled EB were suspended in diluent (a 1:1 [vol/vol] mixture of storage buffer and Dulbecco’s modified Eagle medium without fetal calf serum) and inoculated onto nearly confluent monolayers of HEC-1B cells that had been washed once with PBS. After 1 h, host cells with adherent EB were washed three times with cold PBS and solubilized in 2% (vol/vol) SDS prior to immersion in scintillation cocktail. Radioactive counts were obtained for adherent EB as well as nonadherent EB in the supernatants and the washes; the data were expressed as percentages of the EB population that bound to HEC-1B cells.
For neutralization of chlamydial infectivity by various antibodies, 10 or 100 μg of total IgG per ml was added to 50-μl aliquots of EB; samples with no antibodies served as controls. After inoculation and incubation, the number of infected cells was determined at 48 h by staining for inclusions; at least 30 microscopic fields per sample were examined. To determine whether antibodies interacted with the host cell membrane, uninfected HEC-1B cells were incubated with 100 μg of each antibody per ml, incubated with fluorescein-conjugated anti-rabbit whole IgG, counterstained with Evans blue, and examined by fluorescence microscopy.
The effect of the reducing agent DTT on chlamydial attachment was examined by exposing purified 35S-labeled EB to 20 mM DTT in PBS for 2, 5, 10, or 30 min at 25°C or by incubating 35S-labeled EB in PBS alone as a control; samples were processed and counted as described above. Nonradiolabeled EB were used to assess inhibition of chlamydial infectivity by DTT. Four conditions were used to investigate the role of DTNB (final concentration, 2.5 mM) on both chlamydial attachment and neutralization. These conditions included (i) exposure of EB to DTNB in diluent for 30 min at 35°C, followed by removal of the DTNB by centrifugation, resuspension of the exposed EB in diluent, and inoculation of HEC-1B cells with the EB; (ii) exposure of HEC-1B cell monolayers to DTNB in diluent for 30 min at 35°C, followed by one wash with PBS prior to inoculation with EB; (iii) addition of DTNB to the EB inoculum during the 1-h adsorption period; and (iv) addition of DTNB 30 min after EB inoculation. Importantly, a 1-h exposure to 5.0 mM DTNB was not cytotoxic for HEC-1B cells (LIVE/DEAD viability/cytotoxicity kit; Molecular Probes, Inc.).
Immunofluorescence and electron microscopy.
An immunofluorescence assay was developed to examine the reactivities of antisera with the surfaces of EB. Purified EB were pelleted by centrifugation and resuspended in sterile water to a concentration of 1,000 EB per 10 μl; then 10-μl drops were placed in 6-mm wells on an eight-well microscope slide and allowed to air dry for 10 min. Fifty-micoliter drops of PBS, with or without 20 mM DTT, were placed on the EB for 15 min at 25°C. This was followed by one wash with PBS, and then primary antisera (1:50 [vol/vol] dilutions in PBS) were added to the EB and the preparations were incubated for 1 h at 37°C. After removal of the primary antisera and three washes with PBS, a 1:300 (vol/vol) dilution of fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma Chemical Co.) in PBS was added to the EB, and the preparations were incubated for 1 h at 37°C. After final washes with PBS, samples were covered with polyvinyl alcohol mounting medium (Sigma Chemical Co.) and examined with a Zeiss Axiovert 10 microscope at a magnification of ×40. Images of fluorescent EB were recorded by computer-aided morphometry (Metamorph; Universal Imaging Corp.). A pool of fluorescein-labeled MOMP antibodies (Syva) and polyclonal antisera against whole C. trachomatis serovar E EB (43) served as positive controls for EB surface labeling; the EB particles in at least six microscopic fields per sample were counted. A monospecific polyclonal antibody against the chlamydial histone 2 protein (17) was used as an internal control to verify that DTT exposure did not result in lysis of the EB.
For transmission electron microscopic analyses, purified EB were suspended in PBS with or without 20 mM DTT and incubated for 15 min at 25°C. The EB were subsequently placed on Formvar-coated gold grids (300 mesh) and immunolabeled by inverting the grids over drops of each peptide antibody reagent (1:50, vol/vol) and incubating them for 1 h at 37°C. After washing in PBS and incubation with a secondary 30-nm gold-conjugated goat anti-rabbit detection antibody, the samples were washed in distilled water and negatively stained for 15 s with 1% (wt/vol) phosphotungstic acid (pH 7.0). Samples were examined with a Zeiss EM900 transmission electron microscope operating at 50 kV.
Assembly of data.
Fluorescent images analyzed by the Metamorph imaging software were exported into Adobe Photoshop 5.0 as PICT or TIFF files for assembly of composite figures. All additional gels, Western blots, and negatives obtained from the electron microscope were recorded with a Microtek ScanMaker III and assembled by using Adobe Photoshop 5.0 and Adobe Pagemaker 6.0 software for the Power Macintosh.
RESULTS
Properties and reactivities of the chlamydial Hsp70 peptide antisera.
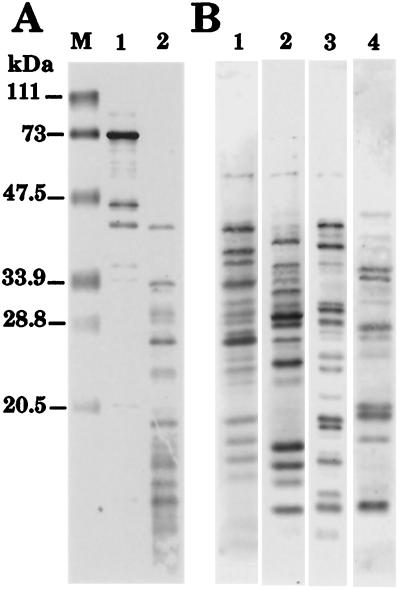
Antibodies were generated against three synthetic peptides based on the C. trachomatis serovar E Hsp70 sequence (Fig. 1). Each peptide was chosen based on predictive protein modeling (7, 24), the surface-exposed Hsp70 receptor on Strongylocentrosus purpuratus eggs for sperm binding (15), the three-dimensional structure of the 70-kDa bovine heat shock cognate (Hsc70) ATPase domain (14), and previously defined linear (3) and immunogenic (54) epitopes of the chlamydial Hsp70. The specificities of the IgG purified peptide antibody reagents and the corresponding preimmune antibodies were examined by Western blot analysis of total C. trachomatis EB proteins (Fig. 2). The peptide antibodies exhibited high degrees of specific reactivity with the C. trachomatis Hsp70 (Fig. 2B, lanes 2, 4, and 6). Although lower-molecular-mass species in lane 2 represented proteolytic fragments of Hsp70 that could be removed by adsorption with a polyclonal antiserum generated against whole Hsp70 (38), recognition of a high-molecular-weight protein by the carboxyl terminus antibody (lane 6) could not be eliminated by adsorption. BLAST analyses (2) of the C. trachomatis serovar D genomic database (47) for each Hsp70 peptide resulted in no significant hits with proteins other than Hsp70. However, a limited match with the chlamydial DNA gyrase subunit A was found with eight of the Hsp70 carboxyl terminus amino acids (i.e., SDVRDESD for DNA gyrase and SDVKNEAD for Hsp70); the predicted mass of the chlamydial gyrase subunit, 94 kDa, is consistent with the size of the cross-reactive protein visible in Fig. 2B, lane 6.
FIG. 1.
Description of C. trachomatis serovar E Hsp70 synthetic peptides. Three 18-amino-acid peptides (A) (solid boxes) at different locales in the chlamydial Hsp70 protein were used as immunogens to generate monospecific polyclonal antibodies. Each peptide sequence (B) (numbers indicate amino acid residues) was chosen based on specific, documented features of the region, as well as predictive protein modeling. The numbers in parentheses are reference numbers.
FIG. 2.
Western blot reactivity of chlamydial Hsp70 peptide antibodies with C. trachomatis serovar E EB proteins. Total EB protein was separated by SDS-PAGE, and the resultant profile was visualized by staining with Coomassie blue (A). A Western blot analysis (B) was conducted with total EB protein transferred to nitrocellulose by incubating blots with either preimmune antibodies (lanes 1, 3, and 5) as a control or antibodies from rabbits immunized with synthetic peptides targeted against the chlamydial Hsp70 amino terminus (lane 2), the midregion (lane 4), and the carboxyl terminus (lane 6).
The extents of cross-reactivity of the peptide antibodies with E. coli DnaK and human Hsp70 were also examined by Western blot analysis (data not shown). At low dilutions, both amino terminus and midregion peptide antibodies showed weak recognition of E. coli DnaK, but there was no reactivity with Hsp70s in the host endometrial epithelial cell line used in this study (HEC-1B). As expected, no cross-reactivity was observed for the carboxyl terminus peptide antibody with either E. coli DnaK or human Hsp70s; primary sequences in this domain are generally less conserved in Hsp70s.
Because heat shock proteins are potent antigens, two assays were conducted to confirm that the immune response generated against each peptide was an immune response directed against the peptide instead of a response directed against whole Hsp70. First, an enzyme immunoassay in which anti-IgG horseradish peroxidase was used as a secondary detection antibody revealed that the peptide antiserum dilutions required to produce 1.0 U of optical density at 450 nm ranged from 1:3,000 to 1:50,000 (vol/vol) when the peptide antiserum was assayed against the appropriate peptide; the only reactivity observed for antisera against unmatched peptides was the reactivity due to antibodies against keyhole limpet hemocyanin, which was conjugated to the synthetic peptides prior to immunization (GenoSys Biotechnologies, Inc.) (data not shown). The second approach used revealed specific Hsp70 peptide reactivities by Western blotting of partial proteolytic digests of purified recombinant chlamydial Hsp70 (Fig. 3). Purified Hsp70 was partly digested with endoproteinase Arg-C to produce a ladder of Hsp70 peptide fragments (Fig. 3A, lane 2). A polyclonal antiserum generated against whole recombinant chlamydial Hsp70 (38) showed antigenic recognition, in almost stoichiometric proportions, of the major peptides in the proteolytic digest (compare Fig. 3A, lane 2, with Fig. 3B, lane 1). As expected, the immunoreactive profiles obtained with each peptide antiserum were unique and reflected considerable differences in recognition within the pool of proteolytic peptide fragments (Fig. 3B, lanes 2 to 4).
FIG. 3.
Reactivities of peptide antibodies with Hsp70 fragments generated by partial proteolytic digestion. (A) Affinity-purified chlamydial Hsp70 from E. coli LMG194(pPBW120) (lane 1) was partially digested with endoproteinase Arg-C (lane 2), and SDS-PAGE-resolved peptides were visualized by staining with Coomassie blue. Lane M contained molecular mass markers. (B) Western blotting of duplicate samples of the Hsp70 proteolytic digest to examine the antigenic reactivities of Hsp70 antisera generated against the entire protein (lane 1), the amino terminus peptide (lane 2), the midregion peptide (lane 3), and the carboxyl terminus peptide (lane 4).
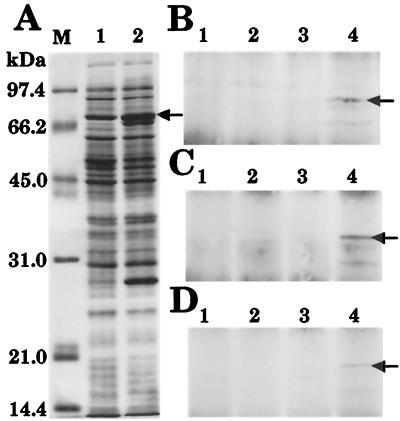
Before exposure of chlamydial Hsp70 at the surface of EB was studied, it was important to confirm that as predicted by protein modeling, each peptide antibody interacted with polypeptide domains that were accessible on nondenatured chlamydial Hsp70. E. coli JM109(pPBW58) was used as a source of chlamydial Hsp70 (Fig. 4A, lane 2) in an immunoprecipitation assay because (i) it is a source that provides abundant amounts, (ii) inclusion bodies are not formed by expression of Hsp70 in this recombinant, and (iii) chlamydial EB are resistant to solubilization with mild detergents. Total protein from E. coli JM109(pUC19) was included for comparison (Fig. 4A, lane 1). Soluble protein extracts were obtained by using the mild, nonionic, membrane detergent Triton X-100 and were incubated with each antibody prior to precipitation with protein A-Sepharose. All precipitates were subsequently resolved by SDS-PAGE and examined by silver staining, and the presence of chlamydial Hsp70 was confirmed by Western blotting. Each peptide antibody was able to selectively precipitate recombinant chlamydial Hsp70 (Fig. 4B to D, lanes 4), and no precipitation was observed when preimmune antibodies were used (Fig. 4B to D, lanes 3). No precipitation was evident in control extracts of E. coli JM109 (Fig. 4B to D, lanes 1 and 2). Thus, the Hsp70 peptide epitopes were recognized by their respective antibodies on nondenatured chlamydial Hsp70.
FIG. 4.
Peptide antibodies interact with nondenatured chlamydial Hsp70. Triton X-100-soluble protein from recombinant E. coli was precipitated from solution by using Hsp70 peptide antibodies. (A) Following resolution by SDS-PAGE, 20-μg portions of total protein from E. coli JM109 (lane 1) and E. coli JM109(pPBW58) (lane 2) were visualized by staining with Coomassie blue. The arrow indicates the position of recombinant C. trachomatis serovar E Hsp70. Lane M contained molecular mass markers. (B to D) For immunoprecipitation, both preimmune antibodies (lanes 1 and 3) and peptide antibodies (lanes 2 and 4) were incubated with protein from E. coli JM109 (lanes 1 and 2) and E. coli JM109(pPBW58) (lanes 3 and 4). Peptide antibodies generated against the amino terminus (B), the midregion (C), and the carboxyl terminus (D) were all able to precipitate recombinant chlamydial Hsp70 from solution (lanes 4, arrows). Western blot data are shown in panels B to D; visualization of the total protein by silver staining produced identical results (data not shown).
Peptide antibodies do not neutralize chlamydial infectivity.
Certain Hsp70 antibodies have been shown to inhibit chlamydial infectivity in vitro (9) and thus potentially reflect surface exposure of Hsp70 on EB. Using this approach with HEC-1B cells as target host cells, we observed no inhibition of chlamydial infectivity following preexposure of EB with the peptide antibodies (Fig. 5), whereas polyclonal antibodies generated against whole EB resulted in a limited, but significant (P < 0.01), reduction in chlamydial inclusion formation. The antibodies did not interact with a host cell Fc receptor, and none of the antibodies reacted with the surface of HEC-1B cells, as determined by immunofluorescence microscopy (data not shown). Overall, these data indicate that none of the Hsp70 peptide domains selected are likely to be directly displayed on the surfaces of EB.
FIG. 5.
C. trachomatis serovar E Hsp70 peptide antibodies do not neutralize chlamydial infectivity in vitro. Two concentrations (10 and 100 μg/ml) of preimmune IgG and anti-peptide IgG were added to C. trachomatis serovar E EB inocula prior to infection of HEC-1B epithelial cell monolayers. The numbers of intracellular chlamydial inclusions per ×40 microscopic field were determined after infected cell monolayers were stained with a pool of fluorescein-conjugated monoclonal antibodies generated against the C. trachomatis MOMP. The control EB inoculum contained no added antibodies, whereas addition of IgG generated against whole C. trachomatis serovar E EB (αEB) served as a positive control for neutralization. This experiment was conducted on three separate occasions, and each sample was assayed in triplicate or quadruplicate (n = 9 to 12); the error bars indicate one standard deviation of the mean. An asterisk indicates that the P value was <0.01.
Disulfide reduction of EB leads to surface accessibility of Hsp70.
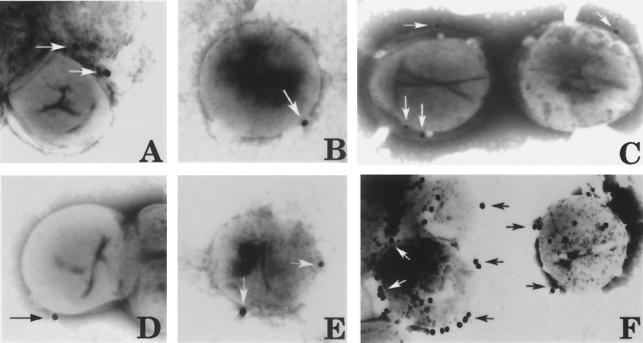
Because EB outer membrane proteins are highly disulfide cross-linked and Hsp70 is associated with the EB outer membrane complex, our next objective was to examine whether Hsp70 becomes accessible at the surface of EB following reduction with DTT. To begin this analysis, we devised an immunofluorescence assay to view a large population of DTT-exposed and unexposed EB. As positive controls, EB were incubated with antibodies against chlamydial MOMP (Syva) and against purified EB (Fig. 6A and B) (46 ± 9 EB particles per ×40 field). Incubation with an antiserum generated against chlamydial histone 2 protein (with permission of T. Hackstadt [17]) was used as an internal control (Fig. 6C and D) to demonstrate that lysis of EB did not occur following 15 min of reduction with DTT. EB were not labeled with the Hsp70 amino terminus and midregion peptide antibodies (Fig. 6E to H). However, labeling of a limited number of EB (11 ± 2 EB particles per ×40 field) was evident when the carboxyl terminus peptide antibody was used (Fig. 6I). Most importantly, the majority of EB within each microscopic field were detected with the carboxyl terminus antibody after 15 min of exposure of EB to DTT (Fig. 6J) (38 ± 5 EB particles per ×40 field).
FIG. 6.
Surface accessibility of each chlamydial Hsp70 peptide domain on purified C. trachomatis serovar E EB as visualized by immunofluorescence microscopy. Purified C. trachomatis serovar E EB were incubated with polyclonal antisera generated against MOMP (A) and whole EB (B) as positive controls for surface labeling. EB either were not exposed (C, E, G, and I) or were exposed (D, F, H, and J) to 20 mM DTT and incubated with antisera generated against chlamydial histone protein 2 (His 2) as an internal control (C and D). The Hsp70 amino terminus peptide (N-term) (E and F), the midregion peptide (G and H), and the carboxyl terminus peptide (C-term) (I and J) were examined. Fluorescein isothiocyanate-conjugated goat anti-rabbit IgG served as a secondary detection antibody, and images were captured by computer-assisted morphometry.
The best method to visualize immunolabeling on the surface of EB is to use whole, negatively stained EB and electron microscopy (Fig. 7). Only a few Hsp70 epitopes were available at the surfaces of EB exposed in PBS (Fig. 7A to C). In agreement with the immunofluorescence microscopy observations, exposure of the Hsp70 carboxyl terminus (Fig. 7F), but not exposure of the amino terminus (Fig. 7D) or the midregion (Fig. 7E), was clearly evident following reduction of EB with DTT. These results indicate that the envelope-associated chlamydial Hsp70 is beneath the outer surface of the EB. The selective accessibility of the Hsp70 carboxyl terminus peptide is particularly intriguing because this region is the domain responsible for binding to other proteins.
FIG. 7.
Surface accessibility of each chlamydial Hsp70 peptide domain on purified C. trachomatis serovar E EB, as visualized by transmission electron microscopy. Chlamydial Hsp70 peptide antibodies against the amino terminus (A and D), the midregion (B and E), and the carboxyl terminus (C and F) were used to examine labeling of the EB surface by negative staining with phosphotungstic acid and electron microscopy. A goat anti-rabbit 30-nm gold-conjugated antiserum was used for detection (arrows). EB either were not exposed (A to C) or were exposed (D to F) to 20 mM DTT. (A) Magnification, ×48,000; (B) ×50,000; (C) ×44,500; (D) ×43,500; (E) ×39,000; and (F) ×41,500.
Membrane protein disulfide bonds in EB attachment and infectivity.
Our next efforts were focused on the importance of cross-linking and reduction during the initial stages of host cell infection by EB in order to assess when conformational rearrangements may occur and thus expose the chlamydial Hsp70 carboxyl terminus. The membrane-impermeable thiol-alkylating reagent DTNB has been used by other investigators to show that reduction of viral capsomere protein disulfide cross-links occurs prior to penetration and release of viral nucleocapsids into the host cell (1). If a similar mechanism is used by chlamydiae, DTNB should not compromise EB binding, but penetration into host cells and inhibition of chlamydial inclusion formation should be observed.
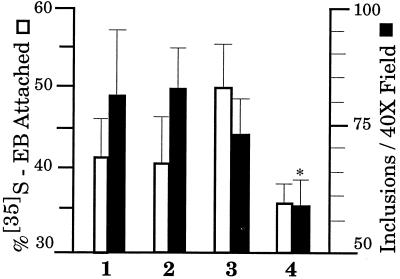
To examine the influence of DTNB on chlamydial infection, (i) purified EB were incubated for 30 min in the presence or absence of DTNB, (ii) HEC-1B cells were preexposed to DTNB for 30 min, or (iii) DTNB was added during inoculation of EB onto HEC-1B cells. The effect of DTNB on both EB attachment and infectivity (Fig. 8) was very similar to the effect reported previously in the virus study. DTNB did not interfere with EB attachment based on the experimental parameters examined. Indeed, preexposure of EB to DTNB resulted in a 20% increase in adherence of EB to HEC-1B cells (Fig. 8, lane 3), while chlamydial infectivity was reduced by 12%. Significant (P < 0.01) inhibition was observed when DTNB was present during the inoculation period. DTNB had no effect on either attachment or infectivity if it was added to infected HEC-1B cells 30 min postinoculation (data not shown), confirming that DTNB did not penetrate the host cell plasma membrane. In addition, no cytotoxicity toward HEC-1B cells was observed upon DTNB exposure. These data suggest that reduction of EB outer membrane protein disulfide bonds occurs prior to or during the entry process that leads to a productive chlamydial infection.
FIG. 8.
Effect of the thiol-alkylating reagent DTNB on C. trachomatis serovar E EB attachment to and infectivity in HEC-1B cells. C. trachomatis serovar E EB either were not exposed (bars 1) or were preexposed (bars 3) to 2.5 mM DTNB prior to inoculation onto HEC-1B cells to assess attachment and inhibition of inclusion formation. The samples used also included preparations in which HEC-1B cells were preexposed to 2.5 mM DTNB prior to inoculation with EB (bars 2) and preparations to which 2.5 mM DTNB was added during the inoculation period (bars 4). Attachment and infectivity assays were conducted as described in Materials and Methods. This experiment was conducted on three separate occasions, and each sample was assayed in triplicate or quadruplicate (n = 9 to 12); the error bars indicate one standard deviation of the mean. An asterisk indicates that the P value was <0.01.
It has long been recognized that preexposure of EB to DTT inhibits chlamydial infectivity (18). In Sindbis virus, DTT has been used to show that capsomere protein disulfide cross-linking is required for attachment to host cells and that bridges must be linked properly to preserve the functional conformation of viral envelopes so that the virus remains infectious (1). In our study, preexposure of EB to DTT for 30 min was detrimental for both EB attachment to and infectivity in host cells (Fig. 9).
FIG. 9.
Effect of preexposure of EB to 20 mM DTT on C. trachomatis serovar E EB attachment to and infectivity in HEC-1B cells. C. trachomatis serovar E EB were exposed to DTT for 2, 5, 10, or 30 min, washed, and examined for attachment to HEC-1B cells and inclusion formation as described in Materials and Methods. This experiment was conducted on three separate occasions, and each sample was assayed in triplicate or quadruplicate (n = 9 to 12); the error bars indicate one standard deviation of the mean.
DISCUSSION
Several years ago Raulston et al. (38) and Schmiel et al. (43) presented evidence that the chlamydial Hsp70 homolog is associated with isolated outer membrane complexes of EB and may participate in attachment to eukaryotic host cells. Our initial observation was that expression of the C. trachomatis serovar E Hsp70 and GrpE protein in recombinant E. coli JM109 conferred an adherent phenotype that paralleled the EB phenotype under numerous in vitro conditions known to differentially influence the attachment of EB to endometrial epithelial cells (43). Danilition et al. (9) also reported evidence of surface localization of chlamydial Hsp70; their monospecific chlamydial Hsp70 antiserum (i) partially neutralized chlamydial infectivity in vitro and (ii) labeled the surface of EB, as determined by immunofluorescence microscopy. These findings were perplexing at the time because heat shock proteins were thought to remain within the soluble, aqueous compartments of cells for protein folding. However, it is now clear that Hsp70s are diversified in terms of their subcellular compartmentalization and functional roles due to the promiscuous nature of their substrate binding capabilities. Numerous investigations have shown that Hsp70s participate in external ligand-binding events (15), in host cell attachment by other bacteria (4, 19, 21, 23, 28), and in ubiquitous membrane interactions that range from translocation of other proteins across membrane barriers to mobilization of processed antigen at the surface of antigen-presenting cells (6, 11, 16, 29, 52; for reviews see references 20, 34, and 55).
The purpose of this study was to determine whether the chlamydial Hsp70 is displayed on the surface of EB, as would be expected for a primary adhesin. We examined three peptide regions at different locales in the C. trachomatis serovar E Hsp70 that, based on predictive modeling, were candidates for surface exposure. Our data show that each of the epitopes selected was only minimally exposed on purified EB. Interestingly, a brief incubation with the reducing reagent DTT led to selective surface exposure of the Hsp70 carboxyl terminus. It is well known that this Hsp70 domain mediates binding to other protein substrates and exhibits a preference for extended hydrophobic polypeptides (5). Whether the chlamydial Hsp70 is bound to another protein substrate, such as a separate chlamydial outer membrane protein, is not known.
The related issue addressed in this study is the status of the structural integrity of EB envelopes, relative to protein disulfide cross-linking, during EB attachment to and infection of human endometrial epithelial cells. Our most notable finding is that exposure of EB to the thiol-alkylating reagent DTNB inhibits chlamydial infectivity with no detrimental effect on attachment to host cells. This observation parallels the previously reported effects of DTNB on attachment and entry of Sindbis virus in eukaryotic host cells (1). Both EB and Sindbis virus are unique in terms of the extent of disulfide-cross-linked proteins in their envelopes; the envelopes are better described as rigid external matrices with associated lipids than as fluid lipid bilayers with associated proteins. Similar to the observations made with Sindbis virus, DTNB reduced but did not eliminate entry and infectivity of chlamydiae in host cells. The partial inhibition is attributed to the rapid rate of the thiol disulfide exchange reactions (once every 10−6 s) that occur within and between cysteine-containing polypeptides that are located close to each other (8); DTNB competes inefficiently for transiently exposed disulfide cross-links.
The greatest level of inhibition of chlamydial infectivity (30%) occurred if DTNB was present during the inoculation period, suggesting that disulfide bonds within the EB outer membrane protein complex become exposed during localized surface reduction. DTNB does not penetrate host cell plasma membranes. In a hallmark study, DTNB was shown to inhibit the host cell cytotoxicity of diphtheria toxin, which must be cleaved into A and B subunits at the surfaces of host cells; conversely, DTNB has no effect on the cytotoxicity of ricin toxin, which is cleaved into subunits only after internalization by host cells (41).
The source of reductase activity that elicits conformational changes in the EB envelope is not known. However, reductases are present at the surfaces of eukaryotic plasma membranes (13, 30). One leading candidate is a eukaryotic protein disulfide isomerase (PDI) that not only is present at the surfaces of host cells but also is required for optimal functioning of estrogen receptors (27). No host cell receptors have been conclusively defined for C. trachomatis EB, but it is notable that EB attach with greater avidity to estrogen-dominant primary endometrial epithelial cells than to progesterone-dominant cells (31). An alternative possibility is that chlamydial EB have an inherent reductase activity that might be stimulated by contact with the host cell surface.
Overall, the data presented in this report suggest that (i) the conformation of the EB envelope, maintained by protein disulfide cross-linking, is important during the primary stage of adherence, (ii) reduction of disulfide bonds occurs at the host cell surface, and (iii) reduction is necessary for efficient and productive EB entry and infectivity. Exposure of the chlamydial Hsp70 substrate-binding domain should occur after the primary stage of attachment but before or during EB entry. Therefore, the role of chlamydial Hsp70 may be to establish a more intimate interaction with host cell ligands rather than to serve as a primary adhesin. Alternatively, Hsp70 and cochaperones may direct conformational rearrangements among other EB outer membrane adhesin candidates (25, 26, 46, 48, 49, 50, 51) so that primary and/or secondary ligands are correctly presented for entry into susceptible target host cells. E. coli DnaK exhibits strong binding affinities for hydrophobic domains of integral outer membrane proteins and is thought to play a crucial role in the biogenesis and functionality of the envelope of this gram-negative organism (11).
Conformation-dependent interactions of Hsp70s with other protein substrates in a complex environment have been reported by several investigators (11, 12, 35, 40). We are not the first researchers to suggest that rearrangements occur within the envelope of EB during attachment to host cells; Su et al. (50) have shown that the MOMP undergoes conformational changes during the initial stages of infection. There is also evidence that adherence mediated by the 60-kDa cysteine-rich protein depends on conformation (51). Although the driving force behind these changes in chlamydial outer membrane proteins is not yet known, the chlamydial chaperones Hsp70, GrpE, and DnaJ are logical candidates. Chaperone-mediated conformational changes would require energy driven by ATP hydrolysis, and there is evidence for (i) inducible ATPase activity in EB (33), (ii) penetration of ATP via reduced MOMP (53), and (iii) inhibition of chlamydial infectivity by a nonhydrolyzable ATP analog (37). Moreover, it is notable that during the maturation of Sindbis virus into infectious particles, the Hsp70 paralogue BiP is required for proper formation of disulfide bonds within Sindbis virus membrane glycoprotein E1 (32); likewise, BiP prevents the formation of improper disulfide cross-links in influenza virus hemagglutinin (45). Thus, there is a precedent for the hypothesis that Hsp70s mediate conformational requirements for crucial functional roles, such as infectivity, in other pathogens that are encased by envelopes containing highly disulfide-cross-linked proteins.
Because our study was based on only three representative Hsp70 peptide regions, it is possible that other epitopes of Hsp70 are accessible at the EB surface. However, given the data presented here, one might consider the possibility that EB surface labeling by chlamydial Hsp70 antibodies reflects localized areas on the outer membrane where disulfide-cross-linked proteins are reduced and Hsp70 is transiently exposed. Neutralizing Hsp70 antibodies may actually interfere with entry rather than with the initial attachment of EB to a host cell.
Acknowledgments
We gratefully acknowledge Johnny Carson of the Department of Pediatrics Electron Microscopy Core Facility, University of North Carolina School of Medicine, for use of transmission electron microscope and darkroom facilities. Stephen T. Knight is thanked for his assistance with the fluorescence imaging. We thank Ted Hackstadt (Rocky Mountain Laboratories, Hamilton, Mont.) for the kind gift of chlamydial histone protein antiserum.
This work was supported by Public Health Service grant AI13446.
Editor: D. L. Burns
REFERENCES
- 1.Abell, B. A., and D. T. Brown. 1993. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J. Virol. 67:5496–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. [DOI] [PubMed] [Google Scholar]
- 3.Birkelund. S., B. Larsen, A. Holm, A. G. Lundemose, and G. Christiansen. 1994. Characterization of a linear epitope on Chlamydia trachomatis serovar L2 DnaK-like protein. Infect. Immun. 62:2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulanger, J., D. Faulds, E. M. Eddy, and C. A. Lingwood. 1995. Members of the 70kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and Mycoplasma-related infertility. J. Cell Physiol. 165:7–17. [DOI] [PubMed] [Google Scholar]
- 5.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366. [DOI] [PubMed] [Google Scholar]
- 6.Chappell, T. G., W. J. Welch, D. M. Schlossmann, K. B. Palter, M. J. Schlesinger, and J. E. Rothman. 1986. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell 45:3–13. [DOI] [PubMed] [Google Scholar]
- 7.Chou, P. Y., and G. D. Fasman. 1978. Empirical predictions of protein conformation. Annu. Rev. Biochem. 47:251–276. [DOI] [PubMed] [Google Scholar]
- 8.Creighton, T. E. 1984. Disulfide bond formation in proteins. Methods Enzymol. 107:305–329. [DOI] [PubMed] [Google Scholar]
- 9.Danilition, S. L., I. W. Maclean, R. Peeling, S. Winston, and R. C. Brunham. 1990. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect. Immun. 58:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, C. H., and P. B. Wyrick. 1997. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect. Immun. 65:2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Crouy-Chanel, A., M. Kohiyama, and G. Richarme. 1999. Interaction of DnaK with native proteins and membrane proteins correlates with their accessible hydrophobicity. Gene 230:163–170. [DOI] [PubMed] [Google Scholar]
- 12.DeLuca-Flaherty, C., D. B. McKay, P. Parham, and B. L. Hill. 1990. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell 62:875–887. [DOI] [PubMed] [Google Scholar]
- 13.Feener, E. P., W. Shen, and H. J.-P. Ryser. 1990. Cleavage of disulfide bonds in endocytosed macromolecules. A processing not associated with lysosomes or endosomes. J. Biol. Chem. 265:18780–18785. [PubMed] [Google Scholar]
- 14.Flaherty, K. M., C. DeLuca-Flaherty, and D. B. McKay. 1990. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature 346:623–628. [DOI] [PubMed] [Google Scholar]
- 15.Foltz, K. R., J. S. Partin, and W. J. Lennarz. 1993. Sea urchin egg receptor for sperm: sequence similarity of binding domain and hsp70. Science 259:1421–1425. [DOI] [PubMed] [Google Scholar]
- 16.Gambill, B. D., W. Voos, P. J. Kang, B. Miao, T. Langer, E. A. Craig, and N. Pfanner. 1993. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J. Cell Biol. 123:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackstadt, T., T. J. Brickman, C. E. Barry III, and J. Sager. 1993. Diversity in the Chlamydia trachomatis histone homologue Hc2. Gene 132:137–141. [DOI] [PubMed] [Google Scholar]
- 18.Hackstadt, T., W. J. Todd, and H. D. Caldwell. 1985. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J. Bacteriol. 161:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanawana, T., M. Fukuda, H. Kawakami, H. Hirano, S. Kamiya, and T. Yamamoto. 1999. The Listeria monocytogenes DnaK chaperone is required for stress tolerance and efficient phagocytosis with macrophages. Cell Stress Chap. 4:118–128. [PMC free article] [PubMed] [Google Scholar]
- 20.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571–580. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann, E., and C. Lingwood. 1997. Brief heat shock treatment induces a long-lasting alteration in the glycolipid receptor binding specificity and growth rate of Haemophilus influenzae. Infect. Immun. 65:1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatch, T. P. 1996. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae? J. Bacteriol. 178:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huesca, M., S. Borgia, P. Hoffman, and C. A. Lingwood. 1996. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect. Immun. 64:2643–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson, B. A., and H. Wolf. 1988. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 4:181–186. [DOI] [PubMed] [Google Scholar]
- 25.Joseph, T. D., and S. K. Bose. 1991. A heat-labile protein of Chlamydia trachomatis binds to HeLa cells and inhibits the adherence of chlamydiae. Proc. Natl. Acad. Sci. USA 88:4054–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo, C.-C., N. Takahashi, A. F. Swanson, Y. Ozeki, and S.-I. Hakomori. 1996. An N-linked high mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Investig. 98:2813–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landel, C. C., P. J. Kushner, and G. L. Greene. 1995. Estrogen receptor accessory proteins: effects on receptor-DNA interactions. Environ. Health Perspect. 103:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamelak, D., M. Mylvaganam, H. Whetstone, E. Hartmann, W. Lennarz, P. B. Wyrick, J. Raulston, H. Han, P. Hoffman, and C. A. Lingwood. 2001. Hsp70s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic Hsp70 family members. Biochemistry 40:3572–3582. [DOI] [PubMed] [Google Scholar]
- 29.Manara, G. C., P. Sansoni, L. Badiali-De Giorgi, G. Gallinella, C. Ferrari, V. Brianti, F. F. Fagnoni, C. L. Ruegg, G. De Panfilis, and G. Pasquinelli. 1993. New insights suggesting a possible role of a heat shock protein 70-kD family-related protein in antigen processing/presentation phenomenon in humans. Blood 82:2865–2871. [PubMed] [Google Scholar]
- 30.Mandel, R., H. J.-P. Ryser, F. Ghani, M. Wu, and D. Peak. 1993. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Cell Biol. 90:4112–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maslow, A. S., C. H. Davis, J. Choong, and P. B. Wyrick. 1988. Estrogen enhances attachment of Chlamydia trachomatis to human endometrial epithelial cells in vitro. Am. J. Obstet. Gynecol. 159:1006–1014. [DOI] [PubMed] [Google Scholar]
- 32.Mulvey, M., and D. T. Brown. 1995. Involvement of the molecular chaperone BiP in maturation of Sindbis virus envelope glycoproteins. J. Virol. 69:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeling, R. W., J. Peeling, and R. C. Brunham. 1989. High-resolution 31P nuclear magnetic resonance study of Chlamydia trachomatis: induction of ATPase activity in elementary bodies. Infect. Immun. 57:3338–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce, S. K. 1994. Molecular chaperones in the processing and presentation of antigen to helper T cells. Experientia 50:1026–1030. [DOI] [PubMed] [Google Scholar]
- 35.Pratt, W. B., and M. J. Welsh. 1994. Chaperone functions of the heat shock proteins associated with steroid receptors. Semin. Cell Biol. 5:83–93. [DOI] [PubMed] [Google Scholar]
- 36.Raulston, J. E. 1995. Chlamydial envelope components and pathogen-host cell interactions. Mol. Microbiol. 15:607–616. [DOI] [PubMed] [Google Scholar]
- 37.Raulston, J. E., C. H. Davis, T. R. Paul, and P. B. Wyrick. 1998. Heat shock protein 70kDa and the chlamydial envelope: an entry-level position?, p.83–86. In R. S. Stephens, G. I. Byrne, G. Christiansen, I. Clarke, J. T. Grayston, R. G. Rank, G. L. Ridgway, P. Saikku, J. Schachter, and W. E. Stamm (ed.), Proceedings of the 9th International Symposium on Human Chlamydial Infection. Berkeley Press, San Francisco, Calif.
- 38.Raulston, J. E., C. H. Davis, D. H. Schmiel, M. W. Morgan, and P. B. Wyrick. 1993. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J. Biol. Chem. 268:23139–23147. [PubMed] [Google Scholar]
- 39.Reynolds, D. J., and J. H. Pearce. 1991. Endocytic mechanisms utilized by chlamydiae and their influence on induction of productive infection. Infect. Immun. 59:3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roman, E., C. Moreno, and D. Young. 1994. Mapping of Hsp70-binding sites on protein antigens. Eur. J. Biochem. 222:65–73. [DOI] [PubMed] [Google Scholar]
- 41.Ryser, H. J.-P., R. Mandel, and F. Ghani. 1991. Cell surface sulfhydryls are required for the cytotoxicity of diphtheria toxin but not of ricin in Chinese hamster ovary cells. J. Biol. Chem. 266:18439–18442. [PubMed] [Google Scholar]
- 42.Schachter, J., and P. B. Wyrick. 1994. Culture and isolation of Chlamydia trachomatis. Methods Enzymol. 236:377–390. [DOI] [PubMed] [Google Scholar]
- 43.Schmiel, D. H., S. T. Knight, J. E. Raulston, J. Choong, C. H. Davis, and P. B. Wyrick. 1991. Recombinant Escherichia coli clones expressing Chlamydia trachomatis gene products attach to human endometrial epithelial cells. Infect. Immun. 59:4001–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmiel, D. H., J. E. Raulston, E. Fox, and P. B. Wyrick. 1995. Characterization, expression and envelope association of a Chlamydia trachomatis 28 kDa protein. Microb. Pathog. 19:227–236. [DOI] [PubMed] [Google Scholar]
- 45.Segal, M. S., J. M. Bye, J. F. Sambrook, and M.-J. Gething. 1992. Disulfide bond formation during the folding of influenza virus hemagglutinin. J. Cell Biol. 118:227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens, R. S. 1994. Molecular mimicry and Chlamydia trachomatis infection in eukaryotic cells. Trends Microbiol. 2:99–101. [DOI] [PubMed] [Google Scholar]
- 47.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759. [DOI] [PubMed] [Google Scholar]
- 48.Stephens, R. S., K. Koshiyama, E. Lewis, and A. Kubo. 2001. Heparin-binding outer membrane protein of chlamydiae. Mol. Microbiol. 40:691–699. [DOI] [PubMed] [Google Scholar]
- 49.Su, H., L. Raymond, D. D. Rockey, E. Fischer, T. Hackstadt, and H. D. Caldwell. 1996. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 93:11143–11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su, H., N. G. Watkins, Y.-X. Zhang, and H. D. Caldwell. 1990. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect. Immun. 58:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ting, L.-M., R.-C. Hsia, C. G. Haidaris, and P. M. Bavoil. 1995. Interaction of outer envelope proteins of Chlamydia psittaci GPIC with the HeLa cell surface. Infect. Immun 63:3600–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voos, W., O. von Ahsen, H. Muller, B. Guiard, J. Rassow, and N. Pfanner. 1996. Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins. EMBO J. 15:2668–2677. [PMC free article] [PubMed] [Google Scholar]
- 53.Wyllie, S., R. H. Ashley, D. Longbottom, and A. J. Herring. 1998. The major outer membrane protein of Chlamydia psittaci functions as a porin-like ion channel. Infect. Immun. 66:5202–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong, G., and R. C. Brunham. 1992. Antigenic analysis of the chlamydial 75-kilodalton protein. Infect. Immun. 60:1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zugel, U., and S. H. E. Kaufmann. 1999. Role of heat shock proteins in protection from pathogenesis of infectious diseases. Clin. Microbiol. Rev. 12:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]