Abstract
Ehrlichia chaffeensis, a bacterium that cannot survive outside the eukaryotic cell, proliferates exclusively in human monocytes and macrophages. In this study, signaling events required for ehrlichial infection of human monocytic cell line THP-1 were characterized. Entry and proliferation of E. chaffeensis in THP-1 cells were significantly blocked by various inhibitors that can regulate calcium signaling, including 8-(diethylamino)octyl-3,4,5-trimethoxybenzoate and 2-aminoethoxydiphenyl borate (intracellular calcium mobilization inhibitors), verapamil and 1-{β-[3-(4-methoxyphenyl)propyl]-4-methoxyphenethyl}-1H-imidazole (SKF-96365) (calcium channel inhibitors), neomycin and 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione (U-73122) (phospholipase C [PLC] inhibitors), monodansylcadaverine (a transglutaminase [TGase] inhibitor), and genistein (a protein tyrosine kinase [PTK] inhibitor). Addition of E. chaffeensis resulted in rapid increases in the level of inositol 1,4,5-trisphosphate (IP3) and the level of cytosolic free calcium ([Ca2+]i) in THP-1 cells, which were prevented by pretreatment of THP-1 cells with inhibitors of TGase, PTK, and PLC. E. chaffeensis induced rapid tyrosine phosphorylation of PLC-γ2, and the presence of a PLC-γ2 antisense oligonucleotide in THP-1 cells significantly blocked ehrlichial infection. Furthermore, tyrosine-phosphorylated proteins and PLC-γ2 were colocalized with ehrlichial inclusions, as determined by double-immunofluorescence labeling. The heat-sensitive component of viable E. chaffeensis cells was essential for these signaling events. E. chaffeensis, therefore, can recruit interacting signal-transducing molecules and induce the following signaling events required for the establishment of infection in host cells: protein cross-linking by TGase, tyrosine phosphorylation, PLC-γ2 activation, IP3 production, and an increase in [Ca2+]i.
Intracellular bacteria are known to usurp many preexisting host cell signaling and membrane sorting mechanisms for entry, survival, and proliferation. Recent findings concerning a variety of molecular mechanisms of intracellular parasitism, therefore, have been enlightening not only in terms of microbial pathogenesis but also in terms of host cell biology (14, 18, 21). Ehrlichia chaffeensis is a recently discovered bacterium that is found in the United States, and it targets and multiplies in only primary host defensive cells, the monocytes and macrophages. E. chaffeensis causes a Rocky Mountain spotted fever-like illness called human monocytic ehrlichiosis, which can be fatal in immunocompromised patients or patients who are treated inappropriately (42). Unlike facultatively intracellular bacteria, such as Salmonella, Yersinia, Mycobacterium, etc., E. chaffeensis cannot survive extracellularly. Therefore, upon binding to host cells, E. chaffeensis must trigger rapid and precise signals and spatially assemble host molecules for internalization in a specific intracellular compartment, an early endosome (7, 32) conducive to proliferation. The majority of bacteria and various bacterial components, such as lipopolysaccharides, peptidoglycans, heat shock proteins, and CpG-encoding DNA, activate monocytes and macrophages through their pathogen-associated molecular patterns. In order to survive inside monocytes and macrophages, ehrlichiae must not induce activation signals, but they must selectively induce ehrlichial entry signals which are independent from macrophage activation signals. Most obligately and facultatively intracellular bacteria, such as Orientia tsutsugamushi (formerly Rickettsia tsutsugamushi [38]), Salmonella (17), Bartonella (20), and Listeria (24), mobilize microfilaments and enter host cells by phagocytosis. However, we found previously that entry of Neorickettsia risticii (formerly Ehrlichia risticii [15]), a monocytic obligately intracellular bacterium that causes Potomac horse fever (the level of 16S rRNA gene sequence identity between N. risticii and E. chaffeensis is 85% [42]), into host cells is unusual, since it consistently exhibits greater sensitivity to monodansylcadaverine (MDC), which is an inhibitor of transglutaminase (TGase) related to receptor-mediated endocytosis (1, 11, 27), and to taxol or colchicines, which are inhibitors of microtubules, than to cytochalasins, which are inhibitors of microfilament assembly (31, 40). We recently found that the entry of E. chaffeensis and entry of the granulocytic obligately intracellular bacterium Anaplasma phagocytophila (human granulocytic ehrlichiosis agent; the level of 16S rRNA gene sequence identity between A. phagocytophila and E. chaffeensis is 92.5% [15, 42]) are also sensitive to MDC (8, 33, 55).
Furthermore, N. risticii entry into host cells is unique since it is very sensitive to Ca2+ channel blockers, calmodulin antagonists (41), and protein tyrosine kinase (PTK) inhibitors (56). None of these inhibitors has direct inhibitory effects on generation of CO2 from l-glutamine by host-cell-free ehrlichiae (40). Ca2+ is required for internalization of Salmonella enterica serovar Typhimurium (35). PTK activities are required for internalization of enteropathogenic Escherichia coli (EPEC) (43), Listeria (52), Yersinia (44), and Campylobacter jejuni (54). However, except for the EPEC proteins, the identities of the tyrosine-phosphorylated proteins are largely unknown. N. risticii is distinct from these bacteria since it consistently requires both Ca2+ and PTK signals for internalization.
Since previous studies of N. risticii were limited to the use of inhibitors and the role of phospholipase C (PLC) was not examined and to determine whether the calcium signals are also required for infection by E. chaffeensis, in the present study we examined the effects of inhibitors of PTK, PLC, intracellular calcium mobilization, and calcium channel blockers on internalization and proliferation of E. chaffeensis in THP-1 cells, a human monocytic cell line. Also, the production of inositol 1,4,5-trisphosphate (IP3), the level of cytosolic free calcium ([Ca2+]i) in response to E. chaffeensis, and the effects of the inhibitors on IP3 generation and [Ca2+]i were determined. Finally, the isozyme of PLC involved in IP3 generation, its role in ehrlichial internalization, and localization of PLC and tyrosine-phosphorylated proteins were examined.
MATERIALS AND METHODS
E. chaffeensis and THP-1 cells.
E. chaffeensis Arkansas (12) was propagated in THP-1 cells (American Type Culture Collection, Rockville, Md.), a human monocytic leukemia cell line (50), in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 2% l-glutamine at 37°C in a 5% CO2−95% air atmosphere. No antibiotics were used in this study. Host-cell-free E. chaffeensis was prepared by sonicating a preparation for 8 s at an output setting of 2 with an ultrasonic processor (W-380; Heat Systems, Farmington, N.Y.). After low-speed centrifugation to remove nuclei and unbroken cells, the supernatant was centrifuged at 10,000 × g for 10 min, and the pellet enriched with host-cell-free organisms was used to infect THP-1 cells. Based on pilot experiments, a multiplicity of infection (MOI) (the ratio of the number of bacteria to the number of host cells) of 100 was used in this study, unless indicated otherwise. To prepare heat-treated E. chaffeensis, host-cell-free E. chaffeensis was incubated in a 42 or 60°C water bath for 15 min.
Evaluation of the effects of various compounds on ehrlichial internalization or proliferation.
The following compounds and concentrations were used to evaluate the effects of various compounds on ehrlichial internalization or proliferation: 100 μM genistein (Sigma, St. Louis, Mo.); 50 μM neomycin (BioMol, Plymouth Meeting, Pa.); 10 μM 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione (U-73122) (BioMol); 250 μM MDC (Sigma); 100 μM 8-(diethylamino)octyl-3,4,5-trimethoxybenzoate (TMB-8) (BioMol); 75 μM 2-aminoethoxydiphenyl borate (2-APB) (Calbiochem, San Diego, Calif.); 60 μM 1-{β-[3-(4-methoxyphenyl)propyl]-4-methoxyphenethyl}-1H-imidazole (SKF-96365) (Calbiochem); and 100 μM verapamil (BioMol). To determine the effect on ehrlichial internalization, THP-1 cells (106 cells/well) were incubated in 12-well plates with various compounds for 1 h prior to the addition of host-cell-free E. chaffeensis. At 3 h postinfection (p.i.), the cells were centrifuged to remove extracellular ehrlichiae and treated with pronase E (2 mg/ml) for 3 min to remove bound but uninternalized E. chaffeensis as previously described (40). The THP-1 cells were cultured without inhibitors for an additional 3 days, and then infectivity was determined by counting the number of E. chaffeensis cells in 100 THP-1 cells in triplicate assay wells (41). To determine the effects of compounds on ehrlichial proliferation, the compounds were added at 3 h p.i. to mixtures of THP-1 cells and host-cell-free E. chaffeensis organisms. They were incubated for 3 days, and infectivities were determined.
IP3 assay.
IP3 contents were determined by using the d-myo-IP3-3H assay system as recommended by the manufacturer (Amersham Pharmacia Biotech, Piscataway, N.J.). Briefly, THP-1 cells (1 × 107 cells/ml) were placed into 1.7-ml microcentrifuge tubes and incubated at 37°C for 5 min. Then 0.5-ml portions of prewarmed host-cell-free E. chaffeensis were added to the THP-1 cells at an MOI of 100, and the contents were mixed rapidly by inverting the tubes several times. At the appropriate time, the cells were centrifuged at 2,000 rpm and 4°C for 1 min, and perchloric acid was added to each pellet. The times recorded were the times when the tubes were taken out of the incubator. IP3 extracted in perchloric acid and 3H-labeled IP3 standards were coincubated with IP3-binding protein for 15 min on ice. After unbound [3H]IP3 was washed, the amount of [3H]IP3 released from IP3-binding protein by 0.15 N NaOH was determined with a scintillation counter.
Measurement of [Ca2+]i with a fluorometer.
THP-1 cells were loaded with the acetoxymethyl (AM) ester derivative of fluo-3 (fluo-3/AM) (Molecular Probes, Eugene, Oreg.) at a concentration of 2 μM at 37°C for 30 min on the rotating platform of a water bath. After the cells were washed twice, they were resuspended in RPMI 1640 medium (with 5% FBS but without phenol red) and incubated for 30 min to allow complete hydrolysis of intracellular AM esters. The cells were then stored on ice until they were assayed. A total of 1.5 × 106 fluo-3-preloaded cells were incubated with or without compounds for 1 h at room temperature. After it was warmed to 30°C by using a circulating water bath in the fluorometer, E. chaffeensis was added to the THP-1 cells, the preparation was mixed briefly, and emission at 525 nm after excitation at 488 nm was recorded every 5 s for 10 min at 30°C. The [Ca2+]i was calculated using a calcium calibration buffer kit according to the manufacturer’s instructions (Molecular Probes).
Measurement of [Ca2+]i by ratiometric imaging.
THP-1 cells were loaded with fura-2/AM (Molecular Probes) as described above for fluo-3/AM loading. A total of 5 × 105 preloaded cells were incubated with or without compounds in 1 ml of RMPI 1640 (with 5% FBS but without phenol red) for 1 h. The cells were then added to a poly-l-lysine (Sigma)-coated round coverslip in a chamber, allowed to settle for 10 min, and gently washed twice to remove unattached cells. Changes in [Ca2+]i in individual cells were monitored at room temperature by fluorescence video microscopy by using a PTI ImageMaster system (Photon Technology International, Monmouth Junction, N.J.) attached to an Eclipse TE200 inverted microscope (Nikon, Melville, N.Y.). Alternating excitation wavelengths of 340 and 380 nm were provided by a DeltaRAM monochromator at 1-s intervals. Fields containing approximately 20 cells were selected, and images at an emission wavelength of 520 nm were recorded for at least 5 min. After images were recorded for 30 s to determine the basal level, an equal volume of a host-cell-free E. chaffeensis preparation at an MOI of 100:1 or at an MOI indicated below was added to the chamber slowly, and the contents were mixed by careful pipetting so that image recording was not disturbed. The ratios of fluorescence intensities (emission wavelength, 520 nm) obtained at excitation wavelengths of 340 and 380 nm for individual THP-1 cells were plotted against time.
Immunoprecipitation and Western blotting.
A total of 1 × 107 THP-1 cells that had been pretreated with compounds for 1 h were incubated with host-cell-free E. chaffeensis as described above for the IP3 assay. The cells were lysed in 1 ml of an ice-cold modified radioimmunoprecipitation (RIPA) buffer (50 mM Tris-HCl [pH 7.4], 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) with freshly added protease and phosphatase inhibitors (1 μg of aprotinin per ml, 1 μg of leupeptin per ml, 1 μg of pepstatin A per ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM NaVO3). After incubation on ice for 20 min, the cells were sonicated and centrifuged at 10,000 × g for 10 min to collect the supernatants (cell lysates). For immunoprecipitation, cell lysates were incubated with anti-PLC-γ1 or -γ2 antibodies (0.2 μg/ml; Santa Cruz) overnight, and immunocomplexes were captured by using protein A-agarose beads (Santa Cruz). After the preparations were washed three times with phosphate-buffered saline, the protein A-agarose beads were resuspended in 1× sodium dodecyl sulfate sample buffer and boiled for 5 min to dissociate the immunocomplexes from the beads. Samples were subjected to Western blotting and reacted with anti-phosphotyrosine antibody, anti-PLC-γ1 and -γ2 antibodies, and horseradish peroxidase-conjugated secondary antibodies. Bands were visualized with enhanced chemiluminescence by incubating the membrane with LumiGLO chemiluminescent reagent (Cell Signaling, Beverly, Mass.) and exposing it to X-ray film.
Double-immunofluorescence labeling.
E. chaffeensis-infected THP-1 cells (2 days p.i.) were cytocentrifuged onto glass slides, and then the cells were fixed and permeabilized in Diff-Quik fixative containing methanol for 15 s (32). For antibody labeling, cells were sequentially incubated with primary antibodies (rabbit anti-PLC-γ1 and -γ2 antibodies, mouse anti-phosphotyrosine antibody, or dog anti-E. chaffeensis serum antibody preabsorbed with THP-1 cells) and secondary antibodies (lissamine rhodamine-conjugated anti-rabbit or -mouse antibody, fluorescein isothiocyanate-conjugated anti-dog antibody) in phosphate-buffered saline. The labeled cells were observed with a Nikon microscope coupled to an MRC-1024 confocal laser scanning unit (Bio-Rad, Hercules, Calif.).
Effect of PLC-γ2 antisense oligonucleotides on E. chaffeensis infection.
Morpholino antisense oligonucleotides with complete resistance to nucleases, predictable targeting, and reliable activity in cells (49) were used to determine the effect of PLC-γ2 antisense oligonucleotides on E. chaffeensis infection. PLC-γ2 antisense morpholino oligonucleotides (5′-AATCTACATTGACCGTGGTGGACAT-3′) were made based on the 5′ end 25-nucleotide sequences in the PLC-γ2 open reading frame (Gene Tools LLC, Corvallis, Oreg.). Standard control morpholino oligonucleotides (5′-CCTCTTACCTCAGTTACAATTTATA-3′) having no target and no significant biological activity in THP-1 cells were supplied by Gene Tools. The morpholino oligonucleotides were mixed with ethoxylated polyethylenimine and added to 2 × 106 THP-1 cells in triplicate wells in a 12-well plate. The THP-1 cells were rinsed 3 h later and cultured in fresh medium for 3 days to reduce the level of previously synthesized protein. After the medium was changed, the cells were infected with host-cell-free E. chaffeensis. On 3 day p.i., infectivity was determined as described above, and a Western blot analysis was carried out to determine the protein levels of PLC-γ1 and PLC-γ2.
RESULTS
Effects of various compounds on E. chaffeensis internalization in THP-1 cells.
It is difficult to visually count internalized ehrlichial organisms because they are so small and so few of them are internalized, so various methods, such as flow cytometry of immunofluorescence-labeled organisms and [35S]methionine-labeled N. risticii, were devised previously to study internalization (31, 40, 41). Internalization of the majority of host-cell-free ehrlichiae takes place within 3 h during incubation with host cells, while extracellular ehrlichiae lose infectivity within 3 h (31, 36). In the present study, THP-1 cells were incubated with host-cell-free E. chaffeensis organisms for 3 h in the presence or in the absence of inhibitors. Bound but uninternalized E. chaffeensis organisms were removed by pronase E treatment. The cells were then incubated for 3 days in the absence of compounds, and the ehrlichiae in the THP-1 cells were counted. Since all of the compounds used are reversible inhibitors at the concentrations used and only E. chaffeensis organisms that had been internalized during the initial 3-h incubation period could proliferate, this method provided a simple way to determine the effects of compounds on internalization of E. chaffeensis by amplifying the signal (similar to colony counting for determining the number of viable bacteria). The concentrations of compounds used in this study were determined by dose-response experiments and had no direct toxic effects on host cells or ehrlichiae.
The TGase inhibitor MDC inhibits clustering and internalization of the ligand-receptor complexes in clathrin-coated vesicles (1, 11, 27) and thus prevents internalization of N. risticii in P388D1 cells (31, 40) and internalization of the human granulocytic ehrlichiosis agent in HL-60 cells (55) but not binding of these organisms to the host cells (31, 40). Internalization of N. risticii in P388D1 cells was also inhibited by the PTK inhibitor genistein (56), the Ca2+ channel blocker verapamil, the calmodulin antagonist W-7, and an inhibitor of intracellular Ca2+ mobilization, TMB-8 (41). These inhibitors do not have direct ehrlichiacidal effects and are not toxic to the host cells at the concentrations used (40, 41). Like their effects on internalization of N. risticii in P388D1 cells, MDC, genistein, and verapamil all prevented internalization of E. chaffeensis in THP-1 cells (Table 1). Therefore, internalization or initial establishment of an E. chaffeensis infection in THP-1 cells requires PTK and TGase activities and an increase in the [Ca2+]i. Our results also showed that an inhibitor of PLC, neomycin, prevents ehrlichial internalization (Table 1). Since preincubation of host-cell-free E. chaffeensis with neomycin for 30 min at 37°C did not reduce ehrlichial infectivity compared to the infectivity of E. chaffeensis preincubated with RPMI 1640 medium alone (negative control), neomycin does not seem to have an inhibitory effect directly on E. chaffeensis (data not shown).
TABLE 1.
Effects of various inhibitors on internalization of E. chaffeensisa
| Treatmentb | No. of ehrlichiae per 100 cells | % Infected cells |
|---|---|---|
| None | 3,209 ± 236 | 83.5 ± 6.5 |
| Genistein (100 μM) | 0 | 0 |
| Neomycin (10 μM) | 18 ± 11 | 2.0 ± 0.5 |
| Verapamil (100 μM) | 0 | 0 |
| MDC (250 μM) | 132 ± 17 | 9.0 ± 3.3 |
Reagents were added to THP-1 cells 1 h before infection. At 3 h p.i., the cells were centrifuged and treated with 2 mg of pronase E per ml for 3 min to remove bound uninternalized E. chaffeensis, and then the cells were continuously cultured. The numbers of internalized E. chaffeensis organisms were determined by counting the cells 3 days p.i. The values are means ± standard deviations (n = 3).
Genistein is an inhibitor of PTK; neomycin is an inhibitor of PLC; verapamil is an inhibitor of calcium influx; and MDC is an inhibitor of TGase.
Effects of various compounds on E. chaffeensis proliferation in THP-1 cells.
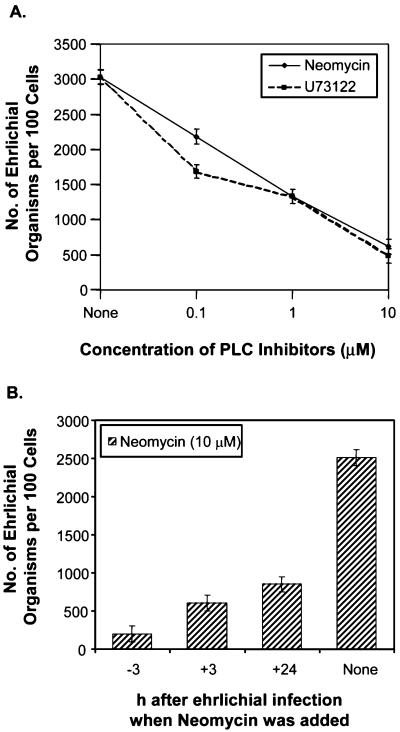
Since most internalization of ehrlichiae takes place during the first 3 h of incubation at 37°C (31, 36), compounds were added at 3 h p.i. to assess their effects on ehrlichial proliferation independent of the inhibitory effects on the initial internalization. Proliferation of N. risticii in P388D1 cells was inhibited by genistein (56), MDC, verapamil, and TMB-8 (41). These compounds also inhibited proliferation of E. chaffeensis in THP-1 cells (Table 2). Additional Ca2+ modulators used in the present study were 2-APB, a cell-permeable inhibitor of IP3-induced Ca2+ release (28), and SKF-96365, an inhibitor of receptor-mediated Ca2+ entry (30). Each of these compounds significantly inhibited proliferation of E. chaffeensis at a concentration that was not toxic to THP-1 cells (Table 2). Two inhibitors of PLC, neomycin and U-73122 (48), also inhibited proliferation (Table 2). The effects of the PLC inhibitors depended on the dose and the time that the inhibitors were added (Fig. 1); the higher the concentration and the earlier the inhibitors were added, the greater the inhibitory effects on ehrlichial proliferation were. These results indicated that the TGase, PTK, and phosphatidylinositol-specific PLC activities and an increase in the [Ca2+]i were required for proliferation of E. chaffeensis in THP-1 cells.
TABLE 2.
Effects of various inhibitors on proliferation of E. chaffeensisa
| Treatmentb | No. of ehrlichiae per 100 cells | % Infected cells |
|---|---|---|
| None | 6,030 ± 400 | 94.1 ± 2.2 |
| Genistein (100 μM) | 90 ± 20 | 7.9 ± 18 |
| Neomycin (10 μM) | 75 ± 25 | 2.5 ± 0.9 |
| U-73122 (10 μM) | 485 ± 105 | 25.7 ± 3.6 |
| TMB-8 (100 μM) | 148 ± 30 | 18.8 ± 2.8 |
| 2-APB (75 μM) | 475 ± 19 | 30.7 ± 4.6 |
| Verapamil (100 μM) | 12 ± 2 | 1.4 ± 0.4 |
| SKF-96365 (60 μM) | 195 ± 60 | 8.6 ± 0.5 |
| MDC (250 μM) | 0 | 0 |
Reagents were added to THP-1 cells at 3 h p.i. and were present throughout the incubation period. The THP-1 cells were infected with host-cell-free E. chaffeensis at an MOI of 100, and infectivities were determined 3 days p.i. The values are means ± standard deviations (n = 3).
Genistein is an inhibitor of PTK; neomycin and U-73122 are inhibitors of PLC; TMB-8 and 2-APB are inhibitors of intracellular calcium release; verapamil and SKF-96365 are inhibitors of calcium influx; and MDC is an inhibitor of TGase.
FIG. 1.
Effects of PLC inhibitors on E. chaffeensis infection. The PLC inhibitors neomycin and U-73122 blocked E. chaffeensis infection in a dose-dependent manner (neomycin and U-73122 were added 1 h prior to ehrlichial infection) (A) or in a time-dependent manner (10 μM neomycin was added to THP-1 cells before or after infection) (B). Infectivity was determined 3 days p.i. as described in Materials and Methods. The values are means ± standard deviations (n = 3) and are representative of the results of more than three independent experiments.
Effects of ehrlichiae and various compounds on production of IP3 by THP-1 cells.
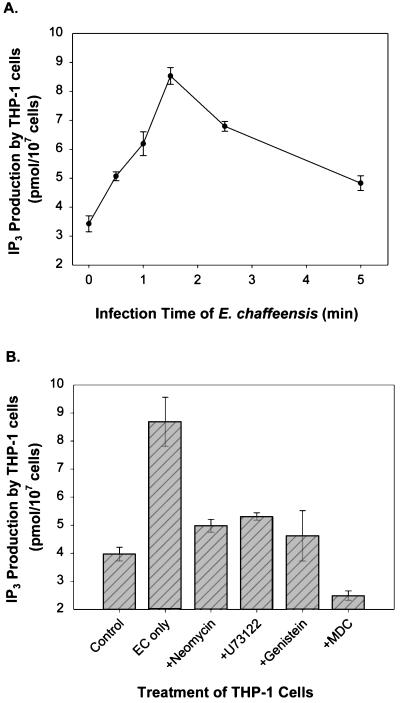
PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate and produces two well-characterized second messengers, IP3 and diacylglycerol. Since ehrlichial entry and proliferation were prevented by PLC inhibitors, the levels of IP3 before and after infection were measured to evaluate PLC activity. Diacylglycerol, an activator of protein kinase C, was not assayed, since our previous data had shown that protein kinase C activity is not required for N. risticii infection of P388D1 cells (41). E. chaffeensis induced a rapid release in the IP3 concentration in THP-1 cells, which reached a peak at 2 min p.i. and then decreased to almost the basal level by 5 min (Fig. 2A). Furthermore, inhibitors of PLC (neomycin and U-73122), PTK (genistein), and TGase (MDC) all blocked the increase in the IP3 level induced by ehrlichiae (Fig. 2B), indicating that IP3 was produced by PLC in a TGase- and PTK-dependent manner. Since activation of PLC-γ isozymes requires tyrosine phosphorylation of the molecules by PTK (34, 46), these results suggest that E. chaffeensis induces the release of IP3 by activating a PLC-γ isozyme through tyrosine phosphorylation.
FIG. 2.
Effects of ehrlichial infection and inhibitors on production of IP3 in THP-1 cells. (A) Rapid release of IP3 induced by ehrlichial infection. The release reached a peak (approximately threefold) at 1.5 min p.i. and decreased to the background level within 5 min. (B) Release of IP3 induced by E. chaffeensis (EC) prevented by pretreatment with inhibitors of PTK, PLC, and TGase. Cells were pretreated with or without inhibitors 1 h before ehrlichial infection, and IP3 samples were extracted with perchloric acid at different times (A) or 1.5 min p.i. (peak release of IP3) (B). The release of IP3 was determined by measuring competitive inhibition of binding of 3H-labeled IP3 to IP3-binding protein. The values are means ± standard deviations (n = 3) and are representative of the results of three independent experiments.
Effects of ehrlichiae and various inhibitors on [Ca2+]i in THP-1 cells.
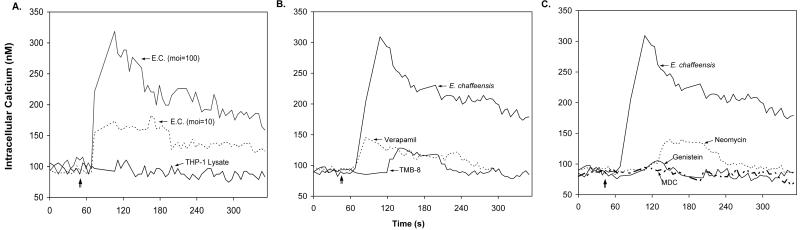
IP3 binds to IP3 receptors located on the endoplasmic reticulum membrane and causes the release of stored Ca2+. Since Ca2+ channel blockers and inhibitors of intracellular Ca2+ mobilization blocked ehrlichial infection, [Ca2+]i was determined in THP-1 cells infected with E. chaffeensis. In fluo-3-loaded THP-1 cells, a rapid increase in [Ca2+]i to 300 nM was detected 30 s after host-cell-free E. chaffeensis was added, and the concentration reached a maximum by 1 min. The increase in [Ca2+]i was greater with a higher MOI (Fig. 3A). As a control, uninfected THP-1 cell lysate did not induce an increase in [Ca2+]i.
FIG. 3.
Effects of ehrlichial infection and inhibitors on [Ca2+]i in a population of cells. [Ca2+]i increased rapidly and transiently in THP-1 cells infected with E. chaffeensis (E.C.) (A), but the increase was prevented by pretreatment with inhibitors of calcium mobilization (verapamil and TMB-8) (B) or inhibitors of PLC (neomycin), PTK (genistein), and TGase (MDC) (C). The intracellular calcium levels in fluo-3-loaded THP-1 cells were determined with a fluorometer as described in Materials and Methods. The inhibitors were added to cells 1 h before the assay, and host-cell-free E. chaffeensis was added to THP-1 cells at the time indicated by the vertical arrow (approximately 50 s p.i.). Assays were carried out at 30°C in a circulating water bath. Fluorescence was measured at 525 nm at 5-s intervals, and Kd was calibrated by using a calcium calibration kit from Molecular Probes. The values are representative of the results of at least four independent experiments. moi, multiplicity of infection.
Increases in [Ca2+]i may result from Ca2+ influx from extracellular space or from Ca2+ released from internal stores. In order to determine the relative contributions of influx and release, [Ca2+]i was measured in THP-1 cells that were preincubated with the Ca2+ channel blocker verapamil, the intracellular calcium release inhibitor TMB-8, or the PLC inhibitor neomycin and stimulated with host-cell-free E. chaffeensis. As shown in Fig. 3B, both TMB-8 and verapamil significantly inhibited an ehrlichia-induced increase in [Ca2+]i. However, TMB-8 and verapamil had different effects on [Ca2+]i. It appeared that the increase in [Ca2+]i occurred in two phases, a rapid elevation phase that occurred within 60 s p.i., followed by a sustained phase. Verapamil inhibited the initial increase and the sustained elevation phase similarly, indicating that Ca2+ influx contributed to both phases (Fig. 3B). In contrast, TMB-8 and neomycin completely blocked the rapid initial increase, but a delayed increase in [Ca2+]i, presumably representing Ca2+ influx, still occurred (Fig. 3B and C). Furthermore, PTK and TGase activities were required for the increase in [Ca2+]i, since genistein and MDC almost completely prevented an increase in [Ca2+]i during E. chaffeensis infection (Fig. 3C). The complete inhibition of an increase in [Ca2+]i by MDC or genistein suggests that these compounds inhibit not only the release of calcium from intracellular calcium stores but also the influx of extracellular Ca2+ through calcium channels.
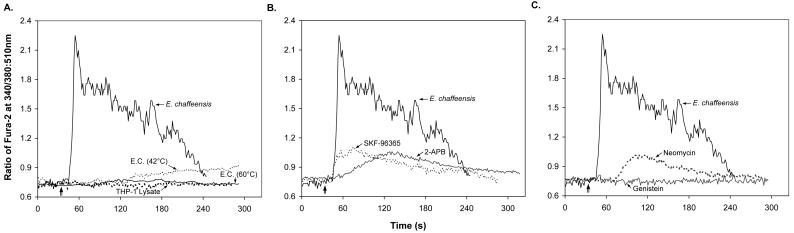
Since [Ca2+]i was measured with a fluorometer by using a population consisting of 106 cells, the kinetics of the changes in [Ca2+]i shown in Fig. 3 reflect the kinetics of the cumulative bacterium-host cell interactions. Therefore, we studied the kinetics of changes in the [Ca2+]i in individual cells by fluorescence microscopy video imaging. As shown in Fig. 4A, the calcium levels in individual cells rose within 20 s after addition of ehrlichia and dropped to the basal level within 4 min. Also, to determine whether active participation of E. chaffeensis is required for the increase in [Ca2+]i, E. chaffeensis was pretreated for 15 min at 42 or 60°C. The results showed that the increase in [Ca2+]i did not take place when E. chaffeensis was heat treated (Fig. 4A). The calcium channel blocker SKF-96365 inhibited the initial peak phase and the sustained phase like verapamil in the fluorometric assay, whereas the cell-permeable IP3 receptor blocker 2-APB and the PLC inhibitor neomycin were more effective in inhibiting the initial rapid increase in [Ca2+]i. Genistein completely prevented an increase in [Ca2+]i caused by E. chaffeensis (Fig. 4B and C). Therefore, the two methods used to measure [Ca2+]i gave similar results. However, the data obtained with the two methods were slightly different. Figure 3A shows that after the increase caused by E. chaffeensis addition, the [Ca2+]i remained higher than the basal level prior to the addition of ehrlichiae even at 6 min p.i. In contrast, Fig. 4A shows that the [Ca2+]i increased more rapidly but decreased to the basal level within 4 min. Because E. chaffeensis may bind to individual host cells at different times, the rate of the initial increase in [Ca2+]i in a population of cells may appear to be lower than that in a single cell due to averaging (Fig. 3A and 4A). Alternatively, an apparent sustained high [Ca2+]i may be due to fluo-3 leakage. The video microscopic method is not affected by leakage of the dye because the region of interest selected for calculating the ratios contains the cell and very little surrounding solution.
FIG. 4.
Effects of ehrlichial infection and inhibitors on [Ca2+]i in an individual cell. The rapid and transient increase in [Ca2+]i (as shown by the ratio of emission by fura-2 at 520 nm under stimulation at 340 nm to emission by fura-2 at 520 nm under stimulation at 380 nm) was induced by E. chaffeensis (E.C.) infection in an individual THP-1 cell (A). The increase in [Ca2+]i required active participation of live E. chaffeensis since heat-treated (42 or 60°C, 15 min) E. chaffeensis did not increase the [Ca2+]i. However, the increase in [Ca2+]i induced by ehrlichial infection was prevented by the calcium mobilization inhibitors SKF-96365 and 2-APB (B) or by the PLC inhibitor neomycin and the PTK inhibitor genistein (C). THP-1 cells were loaded with fura-2/AM and treated with or without inhibitors for 1 h before measurements were obtained. Cells were added to poly-l-lysine-coated coverslip chambers, and images (emission at 520 nm after excitation at 340 and 380 nm) were recorded at 1-s intervals for at least 5 min. Host-cell-free E. chaffeensis was added to the chambers after measurements had been obtained for 30 s (vertical arrow). The values are representative of the results of at least three independent experiments, and similar results were obtained for more than 20 cells in each experiment.
Activation of PLC-γ2 by tyrosine phosphorylation was required for E. chaffeensis infection.
To our knowledge, three PLC isozymes are involved in eukaryotic calcium signaling. Unlike PLC-δ, PLC-β and PLC-γ have been well characterized. The activity of PLC-β is regulated by heterotrimeric G proteins. PLC-γ contains two src-homologous domains, SH2 and SH3, and activation of PLC-γ is mediated by the interaction between the C-terminal SH2 domain and the cognate tyrosine-phosphorylated peptide. The binding of PLC-γ to tyrosine-phosphorylated proteins (or receptors) via the SH2 domain accounts for the initial translocation of the enzyme from cytosolic to particulate fractions and the rapid phosphorylation of PLC-γ by receptor tyrosine kinases or cellular tyrosine kinases coupled to activated receptors. Our results demonstrated that PTK were required for PLC activation by E. chaffeensis infection; therefore, the role of PLC-γ during ehrlichial infection was examined.
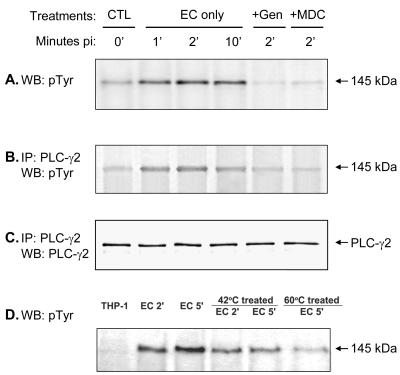
Western blot analysis revealed that THP-1 cells express both PLC-γ1 and -γ2 isotypes, which have the same molecular mass, 145 kDa (data not shown). Western blot analysis in which anti-phosphotyrosine monoclonal antibody was used showed that a 145-kDa band was tyrosine phosphorylated in THP-1 cells as soon as 1 min after addition of E. chaffeensis, and the phosphorylation of this protein was inhibited by genistein and MDC (Fig. 5A), indicating that TGase is required for tyrosine phosphorylation of this protein. In addition, an approximately 200-kDa protein was also tyrosine phosphorylated during E. chaffeensis infection (data not shown).
FIG. 5.
Tyrosine phosphorylation of PLC-γ2 in ehrlichial infection. (A) A 145-kDa protein was rapidly tyrosine phosphorylated in THP-1 cells upon binding of E. chaffeensis (EC). This protein was confirmed to be PLC-γ2 by immunoprecipitation (IP) with anti-PLC-γ2 antibody and by Western blot (WB) analysis with detection with anti-phosphotyrosine (pTyr) antibody (B) or anti-PLC-γ2 antibody (C). Tyrosine phosphorylation of PLC-γ2 was reduced when E. chaffeensis was pretreated at 42 or 60°C for 15 min (D). THP-1 cells were pretreated with genistein (Gen) or MDC for 1 h prior to the addition of E. chaffeensis. At different times, cells were lysed in RIPA buffer. Whole-cell lysates were immunoprecipitated with anti-PLC-γ2 antibody, and the immunoprecipitates or whole-cell lysates were subjected to Western blotting by using anti-phosphotyrosine antibody or anti-PLC-γ2 antibody. The results are representative of the results of more than four independent experiments in which similar results were obtained. CTL, control.
The 145-kDa tyrosine-phosphorylated protein was confirmed to be PLC-γ2 and not PLC-γ1 by immunoprecipitation with an anti-PLC-γ2 antibody and a Western blotting analysis performed with anti-phosphotyrosine antibody (Fig. 5B) or anti-PLC-γ2 antibody (Fig. 5C). The Western blot analysis with anti-phosphotyrosine antibody resulted in no bands from samples immunoprecipitated with anti-PLC-γ1 antibody (data not shown). PLC-γ2 remained tyrosine phosphorylated in E. chaffeensis-infected THP-1 cells even at 2 days p.i., as determined by Western blot analysis. When E. chaffeensis was preincubated at 42 or 60°C for 15 min, the levels of tyrosine phosphorylation of PLC-γ2 were reduced (Fig. 5D), suggesting that viable ehrlichiae or native ehrlichial proteins are required for activation of PLC-γ2.
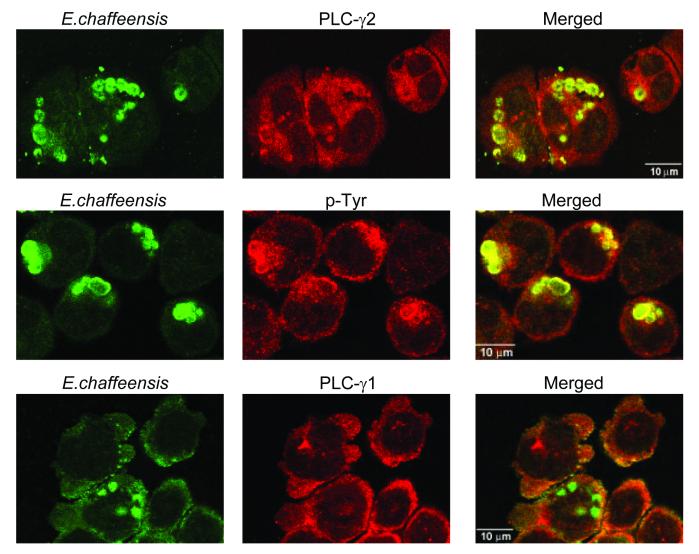
Furthermore, confocal immunofluorescence microscopy revealed colocalization of tyrosine-phosphorylated proteins and PLC-γ2 but not PLC-γ1 with the inclusions of E. chaffeensis (Fig. 6). Tyrosine-phosphorylated proteins and PLC-γ2 were randomly distributed in the cytoplasm of uninfected THP-1 cells (data not shown). Like N. risticii inclusions, most tyrosine-phosphorylated proteins were concentrated in E. chaffeensis inclusions (56). It is likely that PLC-γ2 and other tyrosine-phosphorylated proteins during E. chaffeensis infection, as determined by Western blot analysis, are also concentrated in ehrlichial inclusions.
FIG. 6.
Colocalization of E. chaffeensis inclusions with PLC-γ2 and tyrosine-phosphorylated proteins but not with PLC-γ1. E. chaffeensis-infected THP-1 cells (2 days p.i.) were double labeled as described in the text and observed by confocal microscopy. The following antibodies were labeled: E. chaffeensis (green, left panels) and PLC-γ1 or -γ2 or phosphotyrosine (red, middle panels). The panels on the right are superimposed images viewed with green and red filters. Note the colocalization of E. chaffeensis with PLC-γ2 and phosphotyrosine (yellow). The results are representative of the results of three independent labeling experiments.
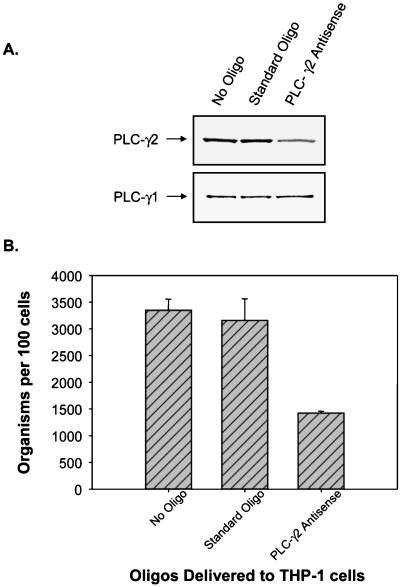
For further confirmation, the requirement for PLC-γ2 for E. chaffeensis infection was tested in THP-1 cells transfected with a PLC-γ2 antisense oligonucleotide. As shown in Fig. 7, delivery of PLC-γ2 antisense oligonucleotides into THP-1 cells significantly decreased the protein level of PLC-γ2 but not the protein level of PLC-γ1 and blocked E. chaffeensis infection. The control oligonucleotides had no effect on the PLC-γ2 protein level or E. chaffeensis infection. These results demonstrate that E. chaffeensis induces the rapid tyrosine phosphorylation of PLC-γ2 required for internalization and proliferation of E. chaffeensis in a TGase-dependent manner. PLC-γ2 catalyzes the formation of IP3 and mediates the increase in [Ca2+]i. Translocation and persistent colocalization of PLC-γ2 and other tyrosine-phosphorylated proteins in E. chaffeensis inclusions suggest that ehrlichial inclusions can actively retain these proteins, which play an important role in maintaining the integrity of inclusions and in facilitating ehrlichial proliferation.
FIG. 7.
Effect of PLC-γ2 antisense oligonucleotides on E. chaffeensis infection. (A) Delivery of PLC-γ2 antisense oligonucleotides (Oligo) into THP-1 cells significantly reduced the level of PLC-γ2 but not the level of PLC-γ1 compared to the levels in untransfected cells or cells transfected with standard control oligonucleotides, as determined by Western blotting. (B) Delivery of PLC-γ2 antisense oligonucleotides into THP-1 cells also significantly reduced E. chaffeensis infection (>50%) (P < 0.05). THP-1 cells were transfected with anti-PLC-γ2 oligonucleotides or standard control oligonucleotides as described in Materials and Methods. Three days after the delivery of antisense oligonucleotides, the cells were infected with host-cell-free E. chaffeensis. On 3 day p.i., infectivity was determined, cells were extracted by using RIPA buffer, and a Western blot analysis was carried out to determine the levels of PLC-γ1 and PLC-γ2. The results are representative of the results of three independent experiments.
DISCUSSION
In this study we demonstrated that E. chaffeensis induces a rapid and modest (300 nM, threefold) increase in [Ca2+]i. The rate of the increase in [Ca2+]i and the maximum level reached may be important for internalization and proliferation of E. chaffeensis in THP-1 cells. In human monocytes incubated with opsonized zymosan particles, which induce superoxide generation in monocytes (37), [Ca2+]i increases from a basal value of 75 ± 11 nM to 676 ± 78 nM by 34 ± 5 s (25). Salmonella induces a slow increase in [Ca2+]i from 100 to 800 nM in the intestinal epithelial cell line Henle-407 over a 30-min incubation period, and this increase is accompanied by membrane ruffling (35). Unlike ehrlichiae, salmonellae induce superoxide generation in monocytes (33, 51). Fc receptor-mediated phagocytosis, which induces rapid superoxide generation (2), is accompanied by an increase in [Ca2+]i to around 700 nM in monocytes (16). The relatively modest increase in [Ca2+]i caused by E. chaffeensis may prevent THP-1 cells from being activated to kill ehrlichiae. As a matter of fact, the Ca2+ ionophore A23187, which causes a substantial increase in [Ca2+]i, kills N. risticii and E. chaffeensis (41; Lin and Rikihisa, unpublished data), whereas Salmonella invades Henle-407 cells more efficiently when it is treated with A23187 (35).
The increase in [Ca2+]i appears to result from both internal stores and the extracellular medium since inhibitors that block calcium mobilization from internal stores and inhibitors that block calcium mobilization from the extracellular space both prevented the increase in [Ca2+]i. Although an increase in [Ca2+]i or a requirement for intracellular Ca2+ for establishment of infection has not been reported for any of the obligately intracellular bacteria, a few facultatively intracellular bacteria, such as EPEC and S. enterica serovar Typhimurium, are known to increase the [Ca2+]i (5, 35). In the case of S. enterica serovar Typhimurium, an increase in [Ca2+]i is required to induce membrane ruffling and internalization (35). However, internalization of Shigella flexeneri in HeLa cells occurs without an increase in [Ca2+]i (10).
Using two different assays, we showed that infection with E. chaffeensis resulted in an increase in [Ca2+]i in THP-1 cells. The PLC-induced increase in [Ca2+]i was due to release of Ca2+ from the internal stores via IP3 receptors and a subsequent influx of extracellular Ca2+ through receptor- or store-operated channels (9). The relative contributions of Ca2+ release and Ca2+ influx to the increase in [Ca2+]i in THP-1 cells were examined by using specific inhibitors of these pathways. Two nonselective Ca2+ channel blockers, verapamil and SKF-96365, significantly inhibited the initial rapid phase and the sustained phase of the increase in [Ca2+]i, suggesting that Ca2+ influx is responsible for the bulk of the increase in [Ca2+]i in response to the infection. These drugs are known to inhibit the activities of voltage-gated Ca2+ channels, as well as receptor- or store-operated channels, including those formed by transient receptor potential proteins (58). Since monocytes do not express voltage-gated Ca2+ channels, it is likely that the influx is mediated by the receptor- or store-operated channels. Because store-operated channels are activated only after depletion of Ca2+ from internal stores, inhibition of intracellular Ca2+ release with TMB-8, 2-APB, and neomycin should also block their activities. Therefore, the delayed increase in [Ca2+]i in the TMB-8-, 2-APB-, or neomycin-treated cells may represent the activity of receptor-operated channels that are not activated by store depletion.
In the present study we showed that the increase in [Ca2+]i was mediated by protein cross-linking by TGase, translocation of PLC-γ2 to the ehrlichial inclusions, activation of PLC-γ2 by tyrosine phosphorylation, and generation of IP3. Parts of these signals are somewhat similar to signals induced by EPEC, Yersinia sp., and Salmonella sp., which internalize by inducing focal assembly of microfilaments at the site of attachment. EPEC inoculates its Tir (translocated intimin receptor) into a host cell by a type III secretion mechanism, and Tir is tyrosine phosphorylated upon insertion into the host cell membrane. Tir is the receptor for an outer membrane protein (intimin) and the focal site of actin polymerization and induces additional signals which subsequently activate PLC-γ1. The cytoskeletal rearrangement by EPEC does not involve the small GTP-binding proteins Rho, Rac, and Cdc42 (13). The effects of EPEC on [Ca2+]i are, however, inconclusive. In a recent report Bain et al. showed that an increase in [Ca2+]i was not required for the lesion caused by EPEC (4). In the case of Yersinia pseudotuberculosis, tyrosine phosphorylation of the focal adhesion protein CAS, subsequent formation of functional CAS-Crk complexes, and activation of the small GTP-binding protein Rac1 led to actin cytoskeleton remodeling and yersinial uptake (53). Tir-mediated uptake of EPEC (43) and invasin-promoted uptake of Yersinia sp. (44), but not S. enterica serovar Typhimurium uptake by HeLa cells, are blocked by PTK inhibitors (45). S. enterica serovar Typhimurium microfilament assembly is directed by a type III secretion system. The proteins delivered include SopE, which induces Rho GTPase activation, and SipA, an actin-binding protein (22, 57), neither of which requires tyrosine phosphorylation. In contrast, an increase in [Ca2+]i and protein tyrosine phosphorylation signals do not lead to polymerization of microfilaments in ehrlichial infections; rather, microfilaments are disassembled during N. risticii infection (39), suggesting that the mechanism is distinct from those described above. Genes homologous to type III secretion machinery genes have not been found in ehrlichiae or in the genome sequence of a related bacterium, Rickettsia prowazekii (3). The identities of ehrlichial components required for induction of these signaling events leading to ehrlichial internalization are not known. However, the present study revealed that viable ehrlichiae or ehrlichial heat-sensitive components (most likely proteins) are required for induction of tyrosine phosphorylation of PLC-γ2 and increases in [Ca2+]i.
It is not clear why Ca2+ channel blockers or inhibitors of intracellular Ca2+ release inhibit not only ehrlichial internalization but also proliferation, since whole-cell [Ca2+]i was not continuously elevated during an ehrlichial infection. The paraphagosomal [Ca2+]i has been reported to be higher than the surrounding cytosol [Ca2+]i in human monocytes, although the whole-cell [Ca2+]i is not higher than the control monocyte [Ca2+]i (25). The local [Ca2+]i around ehrlichial inclusions may be higher than the [Ca2+]i in the remaining cytosol, and this local higher [Ca2+]i may be required for ehrlichial proliferation.
The reasons why an increase in [Ca2+]i is required for ehrlichial internalization and proliferation are not known. Perhaps one reason is that ehrlichial internalization is dependent on a calcium-dependent enzyme, TGase, and microtubules (40). A second reason is that to accommodate proliferating ehrlichial organisms, new membranes should be added and iron and other nutrients should be supplied by fusion of ehrlichial inclusions with transferrin receptor endosomes (7), which are Ca2+-calmodulin- and TGase-dependent processes (19, 23).
A striking finding of the present study was that PLC-γ2 and tyrosine-phosphorylated proteins were rapidly recruited and retained with ehrlichial inclusions even 2 days p.i. or probably during the entire intracellular life span. E. chaffeensis proliferates within membrane-bound vacuoles that display early endosomal markers (7, 32) and do not fuse with lysosomes. The continued colocalization of PLC-γ2 and tyrosine-phosphorylated proteins (the latter are also observed with N. risticii inclusions [56]) may be required to maintain the status of an inclusion. This may explain why inhibitors of PTK and PLC inhibit both ehrlichial entry and proliferation in host cells. Moreover, the spread of E. chaffeensis requires exocytosis of E. chaffeensis or lysis of the host cell and subsequent endocytosis by another cell, which also requires intracellular Ca2+ mobilization and tyrosine phosphorylation; thus, the spread is inhibited by these inhibitors.
The inhibition by MDC of all of the signaling pathways, as well as internalization and proliferation of E. chaffeensis, indicates that activation of TGase may be the most upstream event known and is triggered by the binding of E. chaffeensis, although the mechanism of TGase activation is unclear. In our study, THP-1 cells showed constitutive TGase activity, and the in situ activity rapidly increased as soon as 30 s p.i. when E. chaffeensis was added (Lin and Rikihisa, unpublished data). TGase is required for ligand-receptor complex clustering and endocytosis of various ligands, such as viruses, low-density lipoprotein, α2-macroglobulin, epidermal growth factor, transferrin, and polypeptide hormones like insulin (1, 11, 27, 29, 47). Our results suggest that TGase has a more extensive role in E. chaffeensis in transducing signals for entry, as well as in sustaining proliferation. Which proteins are transglutaminated and how these proteins induce tyrosine phosphorylation of PLC-γ2 remain to be studied. E. chaffeensis inclusions are part of the transferrin and transferrin receptor membrane recycling pathway, as indicated by the uptake of fluorescein isothiocyanate-conjugated iron-saturated transferrin by the inclusions (6). Since the internalization and recycling of transferrin and the transferrin receptor are dependent on TGase (26), this is another site at which MDC can block ehrlichial proliferation in host cells after entry. Transferrin receptors can deliver iron to the cytoplasm through a continual cycle that shuttles the ligand transferrin between the endosomal compartments and the plasma membrane. Iron is essential for ehrlichial survival, since ehrlichiae are extremely sensitive to the intracytoplasmic iron chelator deferoxamine (7).
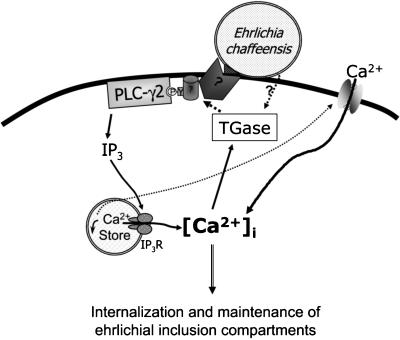
TGase is a calcium-dependent enzyme, and our data showed that it is also able to indirectly regulate [Ca2+]i by activation of a tyrosine kinase-PLC-γ2 pathway. This implies that a positive feedback loop consisting of Ca2+ and TGase may facilitate ehrlichial entry and infection. As shown in Fig. 8, the results of the present study demonstrated that E. chaffeensis activates the following signaling sequences required for internalization: activation of TGase, protein tyrosine phosphorylation, PLC-γ2 activation, production of IP3, and increase in [Ca2+]i.
FIG. 8.
Model of signaling pathway induced by E. chaffeensis infection. Binding of E. chaffeensis to its receptor(s) on host monocytes triggers protein cross-linking by TGase, which activates the receptor or nonreceptor tyrosine kinase. PTK activation mediates the translocation and tyrosine phosphorylation of PLC-γ2, which produces IP3. IP3 in turn induces Ca2+ release from internal stores. The depletion of Ca2+ from internal stores activates store-operated calcium channels on the plasma membrane, and Ca2+ influx from external spaces contributes to the bulk of the increase in [Ca2+]i in response to the infection. Activation of TGase by Ca2+ constitutes a positive feedback loop, and this may facilitate entry of ehrlichiae into host cells or ehrlichial infection. The dotted lines indicate suggested pathways, and the solid lines indicate proven pathways.
Acknowledgments
This work was supported by grants RO1 AI 30010 and RO1 AI 40934 from the National Institutes of Health.
We thank Dennis Jung for assistance with the fluorometer assay.
Editor: J. T. Barbieri
REFERENCES
- 1.Abe, S., K. Yamashita, H. Kohno, and Y. Ohkubo. 2000. Involvement of transglutaminase in the receptor-mediated endocytosis of mouse peritoneal macrophages. Biol. Pharm. Bull. 23:1511–1513. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, C. L., L. Shen, D. M. Eicher, M. D. Wewers, and J. K. Gill. 1990. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J. Exp. Med. 171:1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133–140. [DOI] [PubMed] [Google Scholar]
- 4.Bain, C., R. Keller, G. K. Collington, L. R. Trabulsi, and S. Knutton. 1998. Increased levels of intracellular calcium are not required for the formation of attaching and effacing lesions by enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 66:3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin, T. J., W. Ward, A. Aitken, S. Knutton, and P. H. Williams. 1991. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect. Immun. 59:1599–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron transferrin. Infect. Immun. 62:4804–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnewall, R. E., Y. Rikihisa, and E. H. Lee. 1997. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferring receptor. Infect. Immun. 65:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnewall, R. E., N. Ohashi, and Y. Rikihisa. 1999. Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect. Immun. 67:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge, M. J. 1995. Capacitative calcium entry. Biochem. J. 312:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerc, P. L., B. Berthon, M. Claret and, P. J. Sansonetti. 1989. Internalization of Shigella flexneri into HeLa cells occurs without an increase in cytosolic Ca2+ concentration. Infect. Immun. 57:2919–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, P. J. A., D. R. Davies, A. Levitzki, F. R. Maxfield, P. Milhaud, M. C. Willingham, and I. H. Pastan. 1980. Transglutaminase is essential in receptor-mediated endocytosis of α2-macroglobulin and polypeptide hormones. Nature 283:162–167. [DOI] [PubMed] [Google Scholar]
- 12.Dawson, J. E., B. E. Anderson, D. B. Fishbein, J. L. Sanchez, C. S. Goldsmith, K. H. Wilson, and C. W. Duntley. 1991. Isolation and characterization of an Ehrlichia sp. from a patient with human ehrlichiosis. J. Clin. Microbiol. 29:2741–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVinney, R., D. G. Knoechel, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli: cellular harassment. Curr. Opin. Microbiol. 2:83–88. [DOI] [PubMed] [Google Scholar]
- 14.Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell. Microbiol. 2:365–377. [DOI] [PubMed] [Google Scholar]
- 15.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, description of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol., 51:2145–2165. [DOI] [PubMed] [Google Scholar]
- 16.Edberg, J. C., C. T. Lin, D. Lau, J. C. Unkeless, and R. P. Kimberly. 1995. The Ca2+ dependence of human Fc gamma receptor-initiated phagocytosis. J. Biol. Chem. 270:22301–22307. [DOI] [PubMed] [Google Scholar]
- 17.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718–725. [DOI] [PubMed] [Google Scholar]
- 18.Goebel, W., and M. Kuhn. 2000. Bacterial replication in the host cell cytosol. Curr. Opin. Microbiol. 3:49–53. [DOI] [PubMed] [Google Scholar]
- 19.Grasso, J. A., and M. Bruno. 1990. Calmodulin dependence of transferrin receptor recycling in rat reticulocytes. Biochem. J. 266:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman, C. A., M. Rohde, and K. N. Timmis. 1994. Mechanisms involved in uptake of Bordetella bronchiseptica by mouse dendritic cells. Infect. Immun. 62:5538–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackstadt, T. 2000. Redirection of host vesicle trafficking pathways by intracellular parasites. Traffic 1:93–99. [DOI] [PubMed] [Google Scholar]
- 22.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815–826. [DOI] [PubMed] [Google Scholar]
- 23.Hebbert, D., and E. H. Morgan. 1985. Calmodulin antagonists inhibit and phorbol esters enhance transferrin endocytosis and iron uptake by immature erythroid cells. Blood 65:758–763. [PubMed] [Google Scholar]
- 24.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780–782. [DOI] [PubMed] [Google Scholar]
- 25.Kim, E., R. I. Enelow, G. W. Sullivan, and G. L. Mandell. 1992. Regional and generalized changes in cytosolic free calcium in monocytes during phagocytosis. Infect. Immun. 60:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohno, H., and R. Tokunaga. 1985. Transferrin and iron uptake by rat reticulocytes. J. Biochem. (Tokyo) 97:1181–1188. [DOI] [PubMed] [Google Scholar]
- 27.Levitzki, A., M. Willingham, and I. Pastan. 1980. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 77:2706–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama, T., T. Kanaji, S. Nakade, T. Kanno, and K. Mikoshiba. 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 122:498–505. [DOI] [PubMed] [Google Scholar]
- 29.Maxfield, F. R., M. C. Willingham, P. J. A. Davies, and I. H. Pastan. 1979. Amines inhibit the clustering of α2-macroglobulin and EGF on the fibroblast cell surface. Nature 277:661–663. [DOI] [PubMed] [Google Scholar]
- 30.Merritt, J. E., W. P. Armstrong, C. D. Benham, T. J. Hallam, R. Jacob, A. Jaxa-Chamiec, B. K. Leigh, S. A. McCarthy, K. E. Moores, and T. J. Rink. 1990. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 271:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messick, J. B., and Y. Rikihisa. 1993. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect. Immun. 61:3803–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mott, J., R. E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mott, J., and Y. Rikihisa. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noh, D.-Y., S. H. Shin, and S. G. Rhee. 1995. Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim. Biophys. Acta 1242:99–113. [DOI] [PubMed] [Google Scholar]
- 35.Pace, J., M. J. Hayman, and J. E. Galan. 1993. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell 72:505–514. [DOI] [PubMed] [Google Scholar]
- 36.Park, J., and Y. Rikihisa. 1991. Inhibition of Ehrlichia risticii infection in murine peritoneal macrophages by gamma interferon, a calcium ionophore, and concanavalin A. Infect. Immun. 59:3418–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson, R. D., P. Symes, M. Conboy, A. A. Weiss, and E. L. Hewlett. 1987. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J. Immunol. 139:2749–2754. [PubMed] [Google Scholar]
- 38.Rikihisa, Y., and S. Ito. 1982. Mechanism of entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect. Immun. 38:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rikihisa, Y. 1990. Growth of Ehrlichia risticii in human colonic epithelial cells. Ann. N.Y. Acad. Sci. 590:104–110. [DOI] [PubMed] [Google Scholar]
- 40.Rikihisa, Y., Y. Zhang, and J. Park. 1994. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect. Immun. 62:5126–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rikihisa, Y., Y. Zhang, and J. Park. 1995. Role of Ca2+ and calmodulin in ehrlichial infection in macrophages. Infect. Immun. 63:2310–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rikihisa, Y. 1999. Clinical and biological aspects of infections caused by Ehrlichia chaffeensis. Microb. Infect. 1:367–376. [DOI] [PubMed] [Google Scholar]
- 43.Rosenshine, I., M. S. Donnenberg, J. B. Kaper, and B. B. Finlay. 1992. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 11:3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenshine, I., V. Duronio, and B. B. Finlay. 1992. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect. Immun. 60:2211–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenshine, I., S. Ruschkowski, V. Foubister, and B. B. Finlay. 1994. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect. Immun. 62:4969–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer, W. D., H. A. Brown, and P. C. Sternweis. 1997. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu. Rev. Biochem. 66:475–509. [DOI] [PubMed] [Google Scholar]
- 47.Smethurst, P. A., and M. Griffin. 1996. Measurement of tissue transglutaminase activity in a permeabilized cell system: its regulation by Ca2+ and nucleotides. Biochem. J. 313:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, R. J., L. M. Sam, J. M. Justen, G. L. Bundy, G. A. Bala, and J. E. Bleasdale. 1990. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 253:688–697. [PubMed] [Google Scholar]
- 49.Summerton, J. 1999. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim. Biophys. Acta 1489:141–158. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171–176. [DOI] [PubMed] [Google Scholar]
- 51.Umezawa, K., N. Ohnishi, K. Tanaka, S. Kamiya, Y. Koga, H. Nakazawa, and A. Ozawa. 1995. Granulation in livers of mice infected with Salmonella typhimurium is caused by superoxide released from host phagocytes. Infect. Immun. 63:4402–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velge, P., E. Bottreau, B. Kaeffer, N. Yurdusev, P. Pardon, and N. van Langendonck. 1994. Protein tyrosine kinase inhibitors block the entries of Listeria monocytogenes and Listeria ivanovii into epithelial cells. Microb. Pathog. 17:37–50. [DOI] [PubMed] [Google Scholar]
- 53.Weidow, C. L., D. S. Black, J. B. Bliska, and A. H. Bouton. 2000. CAS/Crk signaling mediates uptake of Yersinia into human epithelial cells. Cell. Microbiol. 2:549–560. [DOI] [PubMed] [Google Scholar]
- 54.Wooldridge, K. G., P. H. Williams, and J. M. Ketley. 1996. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 21:299–305. [DOI] [PubMed] [Google Scholar]
- 55.Yoshiie, K., H.-Y. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, Y., and Y. Rikihisa. 1997. Tyrosine phosphorylation is required for ehrlichial internalization and replication in P388D1 cells. Infect. Immun. 65:2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092–2095. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, X., M. Jiang, and L. Birnbaumer. 1998. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)-293 cells. Evidence for a non-capacitative Ca2+ entry. J. Biol. Chem. 273:133–142. [DOI] [PubMed] [Google Scholar]