Abstract
Internalization of group A streptococcus by human epithelial cells has been extensively studied during the past 6 years. It is now clear that multiple mechanisms are involved in this process. We have previously demonstrated that the CsrR global regulator controls the internalization of an invasive M type 3 strain through regulation of the has (hyaluronic acid synthesis) operon, as well as another, unknown gene(s). Recently, it was reported that the CsrR-regulated cysteine protease (SpeB) is also involved in bacterial uptake. In this study we have examined the roles of CsrR, hyaluronic acid capsule, and SpeB in streptococcal internalization. We have constructed isogenic mutants of the M3 serotype deficient in the csrR, hasA, and speB genes and tested their ability to be internalized by HEp-2 epithelial cells. Inactivation of csrR abolished internalization, while inactivation of either hasA or speB increased the internalization efficiency. Mutation in csrR derepressed hasA transcription and lowered the activity of SpeB, while no effect on speB transcription was observed. The speB mutant expressed smaller amounts of capsule, while the hasA mutant transcribed more csrR and speB mRNAs. Thus, it seems that complex interactions between CsrR, SpeB, and capsule are involved in modulation of group A streptococcus internalization.
Streptococcus pyogenes (group A streptococcus [GAS]) is a major human pathogen capable of causing a wide range of infections ranging from mild, superficial pharyngitis and impetigo to severe, invasive myositis, necrotizing fasciitis, and toxic shock-like syndrome (11, 37). In addition, it is responsible for the poststreptococcal sequelae of acute rheumatic fever and acute glomerulonephritis (31). The last 15 years have witnessed a worldwide increase in the incidence of severe GAS invasive infections (37). While, several serotypes have been implicated in severe invasive infections, M1 and M3 are among the most common serotypes isolated (11, 24, 37).
Although GAS has been considered an extracellular pathogen, several studies have provided evidence that many serotypes are able to enter and persist within human epithelial and endothelial cell lines (16, 21, 26, 30). Moreover, the presence of intracellular GAS has also been documented, ex vivo, in tonsils of asymptomatic carriers (32). GAS strains enter epithelial cell lines with variable efficiency. It has been documented that strains derived from invasive infections are internalized poorly, while strains derived from mild pharyngitis are internalized at higher rates (29). Thus, evasion of internalization might represent an important biological feature associated with GAS virulence.
GAS produces several surface-associated and secreted components that have been implicated in internalization. M protein and hyaluronic acid (HA) capsule are two major virulence factors of GAS that inhibit phagocytosis and enhance virulence in animal models (11). Both components, as well as several fibronectin-binding proteins, were shown to mediate adhesion of GAS to human epithelial cells (11). Genetic and immunologic studies revealed that both M type 6 and F1 proteins are also involved in promoting efficient internalization by respiratory epithelial cells (3, 21, 30). There are contrasting reports regarding the role of HA capsule in GAS internalization. In one study the presence of capsule did not affect internalization (1), while other studies reported both augmentation (8) and inhibition (17, 20, 35; this study) of bacterial entry.
Streptococcal pyrogenic exotoxin B (SpeB) is an extracellular cysteine protease encoded by a chromosomal gene (speB) that exists in all GAS strains (42). The role of SpeB in internalization is also controversial. Inactivation of speB enhanced the internalization of GAS serotypes M49, M2, and M3 into human umbilical vein endothelial cells and Chinese hamster ovary cells (CHO-K1) (5, 7), while others have reported a decreased internalization rate of a speB mutant of serotype M49 (39).
Recently, a global two-component regulatory system (csr) that regulates expression of the has (HA synthesis) operon was identified in GAS (4, 28). Further studies have shown that csr also regulates ska (encoding streptokinase), sagA (encoding streptolysin S), speMF (encoding mitogenic factor), and speB (15, 18). Therefore, it was suggested that this system be renamed cov (control of virulence factors) (15).
In a previous study we found that a Tn916 insertion in the putative promoter of csr abolishes internalization of an invasive M3 serotype into epithelial cells (20). Generation of isogenic mutants deficient in either csrR (the regulatory gene of csr), hasA (the first gene in the HA capsule synthesis operon, encoding hyaluronan synthase) (14), or both revealed that csrR affects internalization through regulation of both capsule synthesis and other, yet-unknown genes (20). It has been previously reported that the speB gene is also associated with GAS internalization (39). Recently, Heath et al. reported that speB is regulated by csr (18), while Federle et al. found no such correlation (15). Thus, we hypothesized that csrR might modulate GAS internalization by regulating both capsule and SpeB expression. In the present study, we employed various isogenic mutants of an invasive M type 3 serotype strain, deficient in csrR, hasA, speB, csrR and speB, and speB and hasA, to elucidate the role of each of the genes in GAS internalization.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
SP268 is an invasive GAS strain of the M3 serotype (20). GAS strains were grown overnight in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.2% yeast extract (THY) in standing cultures at 37°C, unless otherwise mentioned. Escherichia coli strain DH5αMCR (Life Technologies, Paisely, United Kingdom) was grown in Luria-Bertani broth with agitation at 37°C and used for cloning purposes. When appropriate, antibiotics were added at the following concentrations: for GAS, streptomycin at 1 mg/ml, erythromycin at 1 μg/ml, and kanamycin at 0.5 mg/ml; for E. coli, erythromycin at 0.5 mg/ml and kanamycin at 25 μg/ml. Plasmid pJRS233 (kindly provided by J. Scott, Emory University, Atlanta, Ga.) is a temperature-sensitive E. coli gram-positive shuttle vector that replicates in gram-positive bacteria at 30°C but not at 37°C. Plasmid pBR322 carrying the aphA3 gene was kindly provided by M. Caparon (Washington University, St. Louis, Mo.).
DNA techniques.
Purification of GAS chromosomal DNA and transformation of GAS with plasmid DNA by electroporation were performed as previously described (6). Restriction enzymes, T4 DNA ligase, and Taq polymerase were used according to the instructions of the manufacturer (Fermentas Inc., Hanover, Md.). Purification of plasmids and of PCR-amplified fragments was performed using a Plasmid Midi kit (Qiagen Inc., Santa Clarita, Calif.) and a High Pure PCR product purification kit (Boehringer Mannheim, Indianapolis, Ind.), respectively.
Construction of hasA, speB, csrR speB, and speB hasA isogenic mutants.
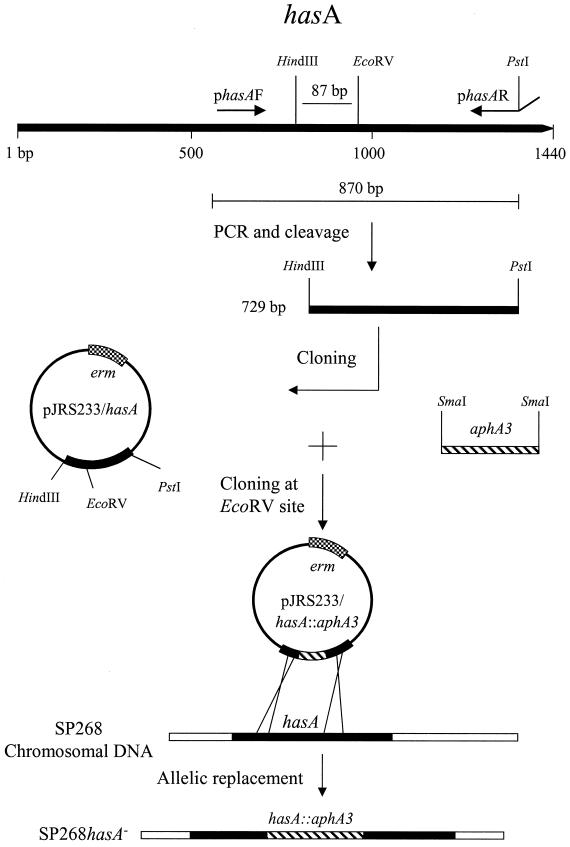
Insertional inactivation of hasA was performed as illustrated in Fig. 1. An internal 870-bp DNA fragment of hasA was amplified from SP268 chromosomal DNA using primers phasAF and phasAR (Table 1). The PCR conditions used were as follows: 94°C for 3 min, followed by 32 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 1 min. The amplified fragment was cut with HindIII and PstI, and the resulting hasA fragment was ligated to pJRS233 cut with the same enzymes. The hasA DNA fragment contains an EcoRV site located 87 bp downstream from its 5′ end. Plasmid pBR322 carrying the aphA3 gene was cut with SmaI to release the aphA3 gene. The aphA3 gene was then ligated to EcoRV-cut pJRS233/hasA to form plasmid pJRS233/hasA::aphA3. The plasmid was introduced into strain SP268 by electroporation, and transformants were selected on THY agar plates containing erythromycin and kanamycin at 30°C. Following a temperature shift to 39°C (nonpermissive temperature), nonmucoid integrants were selected on THY agar plates containing erythromycin and kanamycin. To achieve allelic replacement of the native hasA gene with the insertionally inactivated one (hasA::aphA3), integrants were serially passaged in THY broth containing kanamycin at the permissive temperature of 30°C. Insertionally inactivated hasA mutants (SP268 hasA) were selected by their capacity to grow on THY plates containing kanamycin but not erythromycin. Allelic replacement was verified by PCR amplification of the bacterial chromosome with phasAF and phasAR primers and sequence analysis of the amplified DNA fragment.
FIG. 1.
Construction of hasA mutant. An internal fragment of hasA was amplified using primers phasAF and phasAR (Table 1) and digested with HindIII and PstI. The resulting fragment was cloned into pJRS233 previously cleaved with the same enzymes to generate plasmid pJRS233/hasA. A SmaI-aphA3 fragment was cloned into the EcoRV site of pJRS233/hasA. The resulting plasmid, pJRS233/hasA::aphA3, was introduced into strain SP268 by electroporation, and allelic replacement of the wild-type hasA allele was achieved by homologous recombination.
TABLE 1.
Primers used in this study
| Primer | Sequence | Location (reference) |
|---|---|---|
| phasAF | ACGTTATCGTTCACCGTTCCC | 5′ region of hasA (19) |
| phasAR | GGGGCTGCAGAGTGACCTTTTTACGTGCCCC | 3′ region of hasA (19) |
| pspeBF | GATCAAAACTTTGCTCGTAACG | 5′ region of speB (39) |
| pspeBR | CTAAGGTTTGATGCCTACAACAG | 3′ region of speB (39) |
| JL2F | TTGCAAGGGTTGTTTGATG | 5′ region upstream from csrR (this study) |
| JL20R | GGGAATCAGTGTAAAGGCAG | 3′ region downstream from csrR (this study) |
| prspLF | GAATGTAGATGCCTACAATTAACCA | 5′ region of rspL (15) |
| prspLR | TTTACGACTCATTTCTCTCTTTATCCC | 3′ region of rspL (15) |
| ulpucF | GTAAAACGACGGCCAGTG | 5′ region upstream from multiple cloning site of pJRS233 |
For inactivation of speB gene, we PCR amplified a 1,115-bp chromosomal internal fragment by using the pspeBF and pspeBR primers under the following conditions: 94°C for 3 min, followed by 34 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 1 min, and extension at 72°C for 1 min. The amplified DNA fragment was cut with HindIII and PstI, and the 662-bp fragment was cloned into pJRS233. The recombinant plasmid pJRS233/speB was then introduced into strains SP268, SP268 Δcsr (20), and SP268 hasA by electroporation. Transformants and integrants were selected, essentially, as described previously, except that only erythromycin was used to select the SP268 hasA integrant mutant. To verify integration of the recombinant plasmid pJRS233/speB into the chromosomes of the various strains, chromosomal DNA of each integrant was subjected to PCR using the pUC18 universal primer (site located near the cloning site of pJRS233) (Table 1) and the pspeBR primer (site located on the GAS chromosome). The SP268 mutants deficient in speB, speB and csrR, and speB and hasA were designated SP268 speB, SP268 speB csrR, and SP268 speB hasA, respectively.
Protease assay.
Protease activity was detected qualitatively by growing GAS on Trypticase soy agar plates supplemented with 3% skim milk (Sigma, St. Louis, Mo.). The appearance of a clear zone around a colony after incubation for 24 h at 37°C indicated casein hydrolysis. The quantitative azocasein assay was performed as described by Tsai et al. (39). Briefly, 200 μl of 18-h-growth supernatant of GAS was added to 400 μl of a prewarmed reaction mixture (2.7 mg of azocasein [ICN Biochemicals Inc., Costa Mesa, Calif.] per ml in 50 mM Tris-HCl [pH 8]), and the mixture was incubated at 37°C for 20 min. The reaction was stopped by addition of 100 μl of 15% ice-cold trichloroacetic acid and holding on ice for 15 min. After centrifugation, an equal volume of 0.5 M NaOH was added to the supernatant and the absorbance was measured at 450 nm.
HA determination.
GAS was grown to early logarithmic phase (optical density at 600 nm of 0.15), and the HA capsule was extracted and quantified as described previously (20) by the Stain-All method (35). HA from Streptococcus zooepidermicus (General Biotechnology, Rehovot, Israel) was used as a standard.
Internalization assay.
Internalization of GAS into HEp-2 epithelial cells was tested as previously described (21). Briefly, HEp-2 cells were grown in Dulbecco modified Eagle medium in a 24-well plate, and the confluent monolayer was infected with ≈106 CFU of an overnight GAS culture per well for 2 h at 37°C. Nonattached bacteria were washed with warm Dulbecco modified Eagle medium, and the cell monolayer was incubated with fresh medium containing gentamicin (100 μg/ml) and penicillin (5 μg/ml) for an additional 2 h to kill extracellular adherent bacteria. HEp-2 cells were then washed to remove the antibiotics and lysed with ice-cold water. Serial dilutions of the lysate were spread on Trypticase soy agar plates, and the CFU of the surviving intracellular bacteria grown at 37°C for 24 to 48 h were counted. Each experiment was repeated three to four times, and results from a representative experiment are shown. In some experiments GAS strains were grown in the presence of 10 μM cysteine protease inhibitor E64 (Sigma) or incubated with the inhibitor for 1 h at 37°C prior to infection. In these experiments E64 was present throughout the 2-h infection period. To check for possible multiplication of GAS during the infection period, the CFU were also determined at 2 h postinfection. No significant variations were noted (data not shown). The internalization efficiency was calculated by dividing the number of CFU that survived antibiotic treatment by the number of CFU at 2 h postinfection. Data are presented as relative internalization (percent), where the internalization of the parent strain (SP268) is referred to as 100%.
RNA hybridization analysis.
Strains were grown in 10 ml of THY broth to mid-log phase (optical density at 600 nm of 0.6) and to stationary phase (18 h). Three milliliters of bacterial culture was centrifuged, and the pellets were immediately frozen in a dry ice-acetone solution. The pellets were thawed on ice and resuspended in 100 μl of Tris-HCl (pH 8). Five microliters of 10% sodium dodecyl sulfate-Tween lysing solution (1 ml of Tween 20, 2.5 ml of 1 M Tris-HCl, 12.5 ml of 0.2 M EDTA, and 34 ml of double-distilled water) was added, mixed, and incubated at 65°C for 15 min to lyse the bacteria. Total RNA was purified following the addition of 1 ml of TRI reagent (Molecular Research Center, Inc., Cincinnati, Ohio) as recommended by the manufacturer.
DNA probes were generated by PCR amplification of internal DNA fragments derived from hasA and speB (Table 1). The csrR probe (1,095 bp) was amplified from the SP268 chromosome with primers JL2F and JL20R (Table 1). The housekeeping gene rspL served as an internal control to assess the amounts of total mRNA in the various samples, as described before (15). Amplification of the rspL probe was achieved using the prspLF and prspLR primers (Table 1). DNA probes were labeled with [α-32P]dCTP by using the Klenow fragment with the Rediprime DNA labeling system I (Amersham-Pharmacia Biotech Inc., Piscataway, N.J.) according to the manufacturer’s instructions.
For Northern blot hybridization, 5 μg of total RNA was loaded and run on a formaldehyde gel as described previously (34). The gel was rinsed twice with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature and transferred to Hybond-N (Amersham-Pharmacia) membranes by capillary action using the same solution. RNA was cross-linked to the membranes by UV and prehybridized for 1 h with Rapid hybridization solution (Amersham-Pharmacia) at 42°C. Hybridization was carried out at 57°C for 2 h. Finally, the blots were washed twice with 2× SSC containing 0.1% sodium dodecyl sulfate for 20 min at 42°C and exposed to Kodak X-Omat AR film. To assess the changes in mRNA quantity, the radioactive intensities of the hybridized bands were quantified using Kodak one-dimensional image analysis software (Eastman Kodak Co., Rochester, N.Y.) and compared to the fluorescence intensity of the 23S rRNA in each lane. The intensities of the hasA- and csrR-hybridized bands, in the log phase, were also compared to the intensity of the rpsL-hybridized (control) band in each lane. The two quantification methods yielded similar results.
Nucleotide sequence accession number.
The sequence of the internal speB fragment of SP268 has been deposited in GenBank under accession no. AY035886.
RESULTS
Quantification of HA capsule in SP268 and its isogenic mutants.
In order to confirm the expected phenotype of each mutant (SP268 hasA, SP268 speB hasA, SP268 csrR, and SP268 csrR speB), the amounts of HA in the parent strain and in each of the mutants were determined (Table 2). As expected, the csrR mutants SP268 csrR and SP268 csrR speB produced the largest amounts of HA. Interestingly, the speB-deficient mutant SP268 speB produced less HA capsule than the parent strain. Consistent with this finding, the double mutant SP268 csrR speB produced approximately 4 times less HA capsule than SP268 csrR. These findings suggest that the presence of SpeB is essential for maximal expression of the HA capsule.
TABLE 2.
Cell-associated HA capsule and protease activity of SP268 and its isogenic mutants
| Strain | HA, fg/CFU (mean ± SD) | Protease activitya (mean ± SD)
|
|
|---|---|---|---|
| Without hyaluronidase treatment | With hyaluronidase treatmentb | ||
| SP268 | 2 ± 0.1 | 0.242 ± 0.02 | 0.22 ± 0.02 |
| SP268 csrR | 44 ± 0.5 | 0.092 ± 0.01 | 0.1 ± 0.01 |
| SP268 hasA | <1 | 0.474 ± 0.05 | 0.448 ± 0.03 |
| SP268 speB | <1 | 0 | |
| SP268 csrR speB | 12 ± 0.3 | 0 | |
| SP268 speB hasA | <1 | 0 | |
Units of optical density at 450 nm.
GAS strains were grown for 18 h in the presence of hyaluronidase (10 U/ml) prior to the determination of protease activity.
Characterization of cysteine protease activity in SP268 and its isogenic mutants.
To verify the lack of protease activity in speB mutants, all strains were tested by the azocasein assay (Table 2). Interestingly, inactivation of csrR reduced protease activity in strain SP268 csrR by 62% compared to the parent strain. In contrast, inactivation of hasA increased cysteine protease activity in strain SP268 hasA by 100% (Table 2). These results suggest that the presence of capsule might interfere with SpeB activity. To test this possibility, strains SP268 and SP268 csrR were grown in the presence of hyaluronidase and the protease activity was determined. While hyaluronidase completely degraded HA (data not shown), it did not affect protease activity (Table 2). These results suggest that the presence of cell-associated HA capsule is not involved in the inhibition of protease activity. To verify that the protease activity measured was indeed due to cysteine protease activity, the effect of the specific cysteine protease inhibitor E64 on the protease activity was tested. It was found that E64 completely inhibited the protease activity (data not shown).
Transcription of csrR, hasA, and speB in the various mutants.
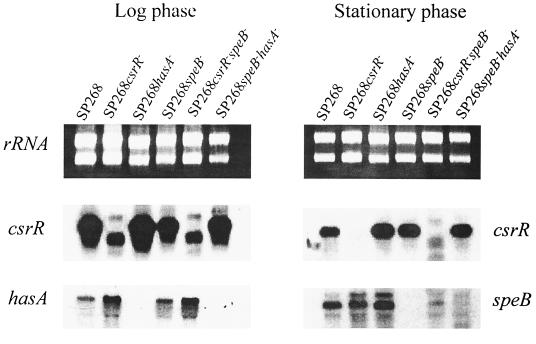
To examine possible interactions between csrR, speB, and hasA at the transcriptional level, total RNA from each strain was isolated from mid-log-phase as well as from stationary-phase bacteria and used for Northern blot analysis. In agreement with previous studies, csrR mRNA was detected during both growth phases (15), whereas hasA and speB mRNAs were detected only in log phase (9, 15, 40) and stationary phase (40), respectively (Fig. 2). Analysis of hasA transcript levels in the different mutants revealed that the SP268 csrR mutant produced threefold more hasA mRNA than the parent strain (Fig. 2; Table3). The hasA transcript levels in the wild type and the SP268 speB mutant were similar. Likewise, the hasA transcript levels in strains SP268 csrR speB and SP268 csrR were similar. These results suggest that speB is not involved in the transcriptional regulation of the hasA gene. Analysis of the speB transcript level revealed that the parent strain and the SP268 csrR mutant produced similar amounts of speB mRNA (Fig. 2; Table 3). However, the SP268 hasA mutant produced 53% more speB mRNA than the parent strain did. Interestingly, inactivation of hasA also resulted in 26 and 50% increases in the levels of csrR mRNA at the log and stationary phases of growth, respectively (Fig. 2; Table 3). These results suggest that CsrR is not involved in transcriptional regulation of speB. However, inactivation of hasA transcription augmented the transcription of both the csrR and speB genes.
FIG. 2.
Northern blot analysis. Total RNAs isolated from SP268 strain and its isogenic mutants at the mid-log and stationary phases of growth were separated by formaldehyde gel electrophoresis and visualized by ethidium bromide staining. RNA was transferred to nylon membranes and hybridized to csrR, hasA, or speB mRNA probes labeled with [α-32P]dCTP. The results shown are representative of those from two independent experiments.
TABLE 3.
Relative concentrations of csrR, hasA, and speB mRNA transcripts in strain SP268 and its isogenic mutantsa
| Strain | Log phase
|
Stationary phase
|
||
|---|---|---|---|---|
| csrR/rRNA ratio | hasA/rRNA ratio | csrR/rRNA ratio | speB/rRNA ratio | |
| SP268 | 51 | 5 | 18 | 13 |
| SP268 csrR | 0 | 14 | 0 | 11 |
| SP268 hasA | 63 | 0 | 28 | 20 |
| SP268 speB | 42 | 6 | 22 | 0 |
| SP268 csrR speB | 0 | 14 | 0 | 0 |
| SP268 hasA speB | 51 | 0 | 27 | 0 |
Data were from Fig. 2. Ratios of the band intensities of the hybridized csrR, hasA, and speB mRNA transcripts to the band intensities of the corresponding 23S rRNAs in SP268 and its isogenic mutants are shown. Band intensity was determined using Kodak one-dimensional image analysis software.
Effect of CsrR, HA capsule, and cysteine protease on internalization.
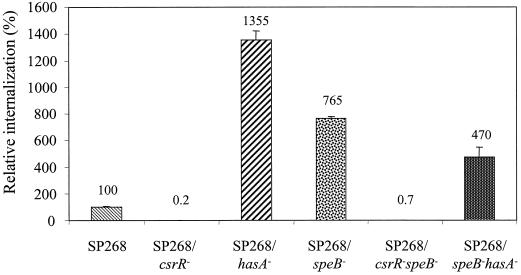
In a previous study we have found that inactivation of csrR decreased the internalization efficiency of strain SP268 by 330-fold. The effect was associated mainly with overexpression of HA capsule. However, inactivation of both csrR and hasA resulted in a lower internalization rate than in the hasA mutant (20), suggesting that CsrR affects internalization by regulating an additional gene(s) (20). Since CsrR was reported to repress transcription of speB (18), it was possible that CsrR affects internalization by down-regulating SpeB expression. To test this possibility, we examined the internalization efficiencies of the hasA-, speB-, csrR-, hasA- and speB-, and csrR- and speB-deficient mutants (Fig. 3). Both csrR mutants, SP268 csrR and SP268 csrR speB were internalized poorly by HEp-2 cells. However, inactivation of hasA, speB, or both significantly increased the internalization capacities of the SP268 hasA, SP268 speB, and SP268 speB hasA mutants, by 13.6-, 7.6-, and 4.7-fold compared to the parent strain, respectively (Fig. 3). It is possible that the mutants might affect the integrity of the epithelial cell membranes, either from outside or from inside the cells, thereby affecting the internalization results due to cell death or to a change in antibiotic permeability. To exclude this possibility, we have determined membrane integrity and cell death by the trypan blue exclusion assay. At 2 and 4 h postinfection, HEp-2 cells infected with SP268 were stained with trypan blue (0.25% [wt/vol] in phosphate-buffered saline). It was found that only 10% (±2%) of the cells were stained, suggesting that the membranes of these cells were damaged. Similar data were obtained for cells infected with the various isogenic mutants (data not shown), indicating that none of the tested strains exerted a unique cytotoxic effect upon HEp-2 cells under the experimental conditions used in this study.
FIG. 3.
Internalization efficiencies of strain SP268 and its isogenic mutants. The internalization efficiency of the parent strain SP268 was normalized to 100. Internalization efficiencies of the various mutants are expressed as relative percentages of that of the wild-type strain. Data are from a representative experiment and are expressed as averages ± standard deviations.
Effect of E64 on internalization.
The finding that mutation in speB resulted in substantial augmentation of GAS internalization might imply that the cysteine protease activity of SpeB might have a role in GAS internalization. SpeB has been found to cleave extracellular matrix proteins such as fibronectin and vitronectin (22), as well as bacterial surface proteins, including M1, protein H, and C5a peptidase (2). Therefore, SpeB could potentially affect internalization by cleaving surface proteins of either GAS or HEp-2 cells and, consequently, could affect bacterial entry into mammalian cells. To test this hypothesis, we examined the internalization efficiency of the parent strain (SP268) and the SpeB mutant (SP268 speB) in the presence of cysteine protease inhibitor (E64). As shown in Table 4, preincubation of E64 for 1 h with GAS had no effect on the internalization efficiency of the parent strain. However, incubation of E64 during bacterial growth (18 h) increased the internalization rate by 2.6-fold. Still, the elevated internalization capacity was significantly lower than that of the SP268 speB mutant (Fig. 3). In control experiments, E64 was found to have no effect on the viability of GAS during the various incubation periods (data not shown).
TABLE 4.
Effect of the cysteine protease-specific inhibitor E64 on internalization of strain SP268
| Strain | Relative internalization (%)a
|
||
|---|---|---|---|
| Control | With E64
|
||
| 1 h | 18 h | ||
| SP268 | 100 ± 20 | 130b ± 30 | 260c ± 60 |
| SP268 speB | 1,220c ± 390 | NDd | ND |
Results are presented as the mean percentage of the value for the control (SP268 without inhibitor) ± standard deviation.
No significant difference compared to control (Student’s t test).
Statistically significant difference compared to control (P < 0.001 by Student’s t test).
ND, not determined.
Sequence analysis of speB.
It has been reported that most GAS strains associated with severe invasive infections express a SpeB variant that binds integrins through an RGD motif (38). It was suggested that SpeB might directly participate in processes leading to internalization. To test whether the speB allele in strain SP268 contains the RGD motif, the internal speB fragment was amplified by PCR with primers pspeBF and pspeBR. The resulting DNA fragment was sequenced by multiple reactions using both strands as templates. The putative amino acid sequence was found to carry an RSD motif (Fig. 4), which is characteristic of the speB1 and speB3 alleles (38).
FIG. 4.
Partial amino acid sequence of the deduced SpeB protein of strain SP268. The RSD motif at positions 307 to 309 and the alanine at position 317 are in boldface. Amino acids are numbered according to the translated speB gene (GenBank accession number L26125).
DISCUSSION
In a previous study we have shown that the two-component regulatory system csr modulates the internalization of strain SP268 by controlling capsule synthesis as well as by regulating an additional, unknown, gene(s) (20). In this study we further characterized the involvement of csr in the uptake of strain SP268 by HEp-2 cells. Recent studies demonstrated that CsrR is a global negative regulator that represses, in addition to the has operon (4, 28), the transcription of ska, sagA, and speMF (15) and of speB (18). It is possible that one or more of these genes might also be involved in the internalization process. Indeed, the speB gene was found to affect GAS entry into epithelial cells, although different studies have reported contrasting outcomes (5, , 39). To address the possibility that CsrR affects internalization by regulating both capsule and SpeB expression, we constructed isogenic mutants of strain SP268 deficient in hasA, csrR, speB, csrR and speB, and speB and hasA and examined their internalization capacities.
In accordance with our previous results (20), inactivation of csrR abolished the internalization, while inactivation of hasA significantly augmented the entry of strain SP268 into HEp-2 cells (Fig. 3). The csrR-deficient mutant transcribed more hasA mRNA than the parent strain and consequently expressed excessive amounts of HA capsule (Fig. 2; Table 3). This finding is in agreement with previous reports regarding the effect of csrR on HA synthesis (4, 15, 18, 20, 28). The internalization efficiency of the unencapsulated mutant was increased by more than one order of magnitude compared to that of the parent strain. Several studies have shown that the HA capsule impedes GAS adhesion (12, 17, 20) as well as internalization in various cell models (17, 20, 23, 35). The inhibitory effect of HA capsule was attributed to masking of bacterial surface molecules required for interactions with host cells (23, 35). Nonetheless, a recent study has shown that HA might also act as an adhesin that mediates attachment of GAS to human keratinocytes, through interaction with the mammalian HA receptor, CD44 (36).
Inactivation of the speB gene resulted in a substantial increase (sevenfold) in the internalization efficiency (Fig. 3), suggesting that intact SpeB directly or indirectly represses GAS internalization. The inhibitory effect, however, was much lower in a genetic background devoid of csrR (csrR speB). Our findings are consistent with those of Burns et al. (5) and Chaussee et al. (7) and support the hypothesis that csrR modulates internalization not only by regulating has transcription but also by modulating SpeB expression.
Inactivation of the speB gene caused significant decreases in the amounts of HA of both the parent strain and the csrR mutant (Table 2). This finding might explain, at least partially, the effect of speB mutation on GAS internalization. However, the finding that the double mutant deficient in both the hasA and speB genes was internalized by HEp-2 cells less efficiently than the hasA mutant suggests that SpeB affects internalization via multiple mechanisms.
SpeB seems to have a major role in GAS virulence. Studies employing various murine models have revealed that the SpeB protease is responsible for inhibition of phagocytosis, dissemination in organs, tissue injury, and death (11). SpeB might also contribute to virulence due to its wide spectrum of biological functions. It degrades human vitronectin, cleaves fibronectin, releases active kinins from kininogen, and activates human interleukin 1β and a 66-kDa human matrix metalloprotease (11) and induces apoptosis (25). Furthermore, it has been reported that SpeB could cleave several bacterial surface proteins, including M1 protein (2), which is known to promote GAS internalization (10, 13). Thus, SpeB might affect GAS internalization by processing both GAS and epithelial surface proteins required for this process. Indeed, Chaussee et al. (7) have reported that inactivation of SpeB specifically enhanced fibronectin-mediated internalization of strain NZ131 (M49) into CHO-K1 cells. In concurrence with this theory, we found that a cysteine protease inhibitor substantially increased GAS internalization (Table 4). Thus, it is possible that SpeB affects internalization by processing GAS proteins which are required for bacterial uptake. A possible target for SpeB activity might be the M3 protein, which was shown to be involved in GAS entry into HEp-2 cells (3). However, inhibition of cysteine protease activity did not increase the internalization efficiency of the parent strain to that of the speB mutant (Table 4). Hence, SpeB apparently possesses additional activities that affect internalization.
It has been reported that most GAS strains associated with severe invasive infections carry the speB2 allele, which encodes an RGD motif (38). Thus, it is possible that SpeB2 also acts as an adhesin that binds integrin receptors expressed on the host’s epithelial cells. However, sequence analysis revealed that strain SP268 carries an RSD and not an RGD motif, followed by alanine in position 317 (Fig. 4), which is characteristic of the speB1 allele (38).
Interestingly, inactivation of speB in a hasA background has resulted in decreased internalization efficiency (Fig. 3). This effect is reminiscent of the result reported by Tsai et al. regarding a speB mutant of strain NZ131 (39). Thus, it is possible that the contrasting results obtained in the various studies (5, 7, 39; this study) pertain to variations in the amount of HA expressed by each strain, in addition to other strain- and cell line-specific factors.
Interactions between SpeB and HA in several GAS serotypes have recently been reported (27, 33, 41). Woischnik et al. (41) have demonstrated that inactivation of speB caused a serotype-dependent decrease in HA expression. Although both speB-inactivated serotypes (M3 and M49) transcribed less has mRNA, the amount of HA decreased only in the M3 serotype (41). Leonard et al. (27) found that small-colony variants of an M49 serotype, produced during prolonged incubation at stationary phase, transcribed less has and speB mRNAs than typical large colonies. In contrast, Raeder et al. (33) found that large-colony variants of an M64 serotype produced large amounts of HA but lacked SpeB activity. In our study, lower expression of HA in the speB mutants was not associated with down-regulation of hasA transcripts (Table 3), and the link between capsule expression and SpeB remains unclear.
Inactivation of hasA caused a significant increase in SpeB protease activity (100%) (Table 2), due to up-regulation of speB transcription (Table 3). This is in agreement with the results of Woischnik et al., who found that has mutants of an M3 serotype secreted 20 to 30% more SpeB than the parent strain (41). Augmentation of SpeB activity could not be related to the presence of capsule, because exogenous hyaluronidase did not affect SpeB expression (Table 2). Since HA and SpeB are expressed at different stages of growth (9, 15, 40), a direct influence of one molecule on the other seems unlikely. Therefore, the mechanism underlying the interactions between the two genes remains unclear.
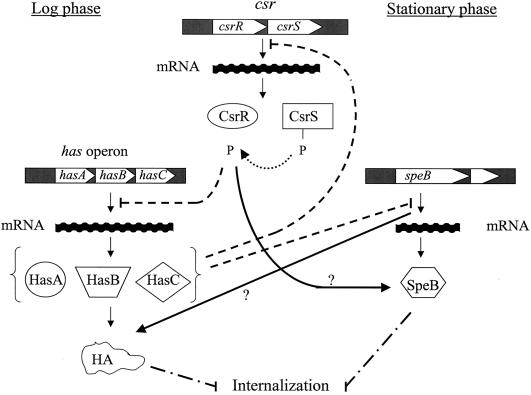
The complex interactions between the csrR, hasA, and speB genes in strain SP268 and their effects upon GAS internalization are summarized in Fig. 5. The global regulator CsrR controls capsule expression by repressing hasA transcription. It also enhances SpeB activity, by an unknown mechanism, at a posttranscriptional level. On the other hand, hasA negatively controls transcription of both the csrR and speB genes, while an intact SpeB is required for capsule expression. Finally, expression of either capsule or SpeB inhibits the internalization capacity of strain SP268. Thus, the internalization capacity of this strain seems to reflect a temporal virulence phenotype, with the highest internalization rate presented by the avirulent phenotype. This finding is in agreement with the notion that evasion of internalization might represent an important biological feature of virulent GAS strains (35).
FIG. 5.
A simplified model depicting the relationship between csr, has, speB, and GAS internalization. Dashed lines represent negative regulation. Solid lines represent positive regulation. The line with dashes and dots represents inhibition. The dotted line represents activation by phosphorylation. P, phosphate. CsrR is a response regulator that becomes activated upon phosphorylation (5). Activation of CsrR results in negative regulation of has operon transcription and positive regulation of speB activity at the log and stationary phases of growth, respectively. Expression of has represses transcription of the csrR and speB genes, while expression of speB augments the amount of cell wall-associated HA. Expression of each of the virulence factors, HA or SpeB, negatively affects GAS internalization. A question mark indicates that the mechanism underlying the interaction is not clear.
Acknowledgments
This study was supported by the Israel Science Foundation, founded by the Israel Academy of Sciences and Humanities, and by a grant from the Chief Scientist’s Office of the Ministry of Health, Israel.
We thank M. Wessels for his advice regarding quantitative determination of HA capsule and June Scott for providing plasmid pJRS233.
Editor: E. I. Tuomanen
REFERENCES
- 1.Bennett-Wood, V. R., J. R. Carapetis, and R. M. Robins-Browne. 1998. Ability of clinical isolates of group A streptococci to adhere to and invade HEp-2 epithelial cells. J. Med. Microbiol. 47:899–906. [DOI] [PubMed] [Google Scholar]
- 2.Berge, A., and L. Bjorck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862–9867. [DOI] [PubMed] [Google Scholar]
- 3.Berkower, C., M. Ravins, A. E. Moses, and E. Hanski. 1999. Expression of different group A streptococcal M proteins in an isogenic background demonstrates diversity in adherence to and invasion of eukaryotic cells. Mol. Microbiol. 31:1463–1475. [DOI] [PubMed] [Google Scholar]
- 4.Bernish, B., and I. van de Rijn. 1999. Characterization of a two-component system in Streptococcus pyogenes, which is involved in regulation of hyaluronic acid production. J. Biol. Chem. 274:4786–4793. [DOI] [PubMed] [Google Scholar]
- 5.Burns, E. H. J., S. Lukomski, J. Rurangirwa, A. Podbielski, and J. M. Musser. 1998. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb. Pathog. 24:333–339. [DOI] [PubMed] [Google Scholar]
- 6.Caparon, M. G., and J. R. Scott. 1991. Genetic manipulation of pathogenic streptococci. Methods Enzymol. 204:556–586. [DOI] [PubMed] [Google Scholar]
- 7.Chaussee, M. S., R. L. Cole, and J. P. van Putten. 2000. Streptococcal erythrogenic toxin B abrogates fibronectin-dependent internalization of Streptococcus pyogenes by cultured mammalian cells. Infect. Immun. 68:3226–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary, P. P., L. McLandsborough, L. Ikeda, D. Cue, J. Krawczak, and H. Lam. 1998. High-frequency intracellular infection and erythrogenic toxin A expression undergo phase variation in M1 group A streptococci. Mol. Microbiol. 28:157–167. [DOI] [PubMed] [Google Scholar]
- 9.Crater, D. L., and I. van de Rijn. 1995. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J. Biol. Chem. 270:18452–18458. [DOI] [PubMed] [Google Scholar]
- 10.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darmstadt, G. L., L. Mentele, A. Podbielski, and C. E. Rubens. 2000. Role of group A streptococcal virulence factors in adherence to keratinocytes. Infect. Immun. 68:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859–870. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty, B. A., and I. van de Rijn. 1994. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J. Biol. Chem. 269:169–175. [PubMed] [Google Scholar]
- 15.Federle, M. J., K. S. McIver, and J. R. Scott. 1999. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 181:3649–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greco, R., L. De Martino, G. Donnarumma, M. P. Conte, L. Seganti, and P. Valenti. 1995. Invasion of cultured human cells by Streptococcus pyogenes. Res. Microbiol. 146:551–560. [DOI] [PubMed] [Google Scholar]
- 17.Hagman, M. M., J. B. Dale, and D. L. Stevens. 1999. Comparison of adherence to and penetration of a human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol. Med. Microbiol. 23:195–204. [DOI] [PubMed] [Google Scholar]
- 18.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husmann, L. K., D. L. Yung, S. K. Hollingshead, and J. R. Scott. 1997. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect. Immun. 65:1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadoun, J., and S. Sela. 2000. Mutation in csrR global regulator reduces streptococcus pyogenes internalization. Microb. Pathog. 29:311–317. [DOI] [PubMed] [Google Scholar]
- 21.Jadoun, J., V. Ozeri, E. Burstein, E. Skutelsky, E. Hanski, and S. Sela. 1998. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J. Infect. Dis. 178:147–158. [DOI] [PubMed] [Google Scholar]
- 22.Kapur, V., S. Topouzis, M. W. Majesky, L. L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327–346. [DOI] [PubMed] [Google Scholar]
- 23.Kawabata, S., H. Kuwata, I. Nakagawa, S. Morimatsu, K. Sano, and S. Hamada. 1999. Capsular hyaluronic acid of group A streptococci hampers their invasion into human pharyngeal epithelial cells. Microb. Pathog. 27:71–80. [DOI] [PubMed] [Google Scholar]
- 24.Kehoe, M. A., V. Kapur, A. M. Whatmore, and J. M. Musser. 1996. Horizontal gene transfer among group A streptococci: implications for pathogenesis and epidemiology. Trends Microbiol. 4:436–443. [DOI] [PubMed] [Google Scholar]
- 25.Kuo, C. F., J. J. Wu, P. J. Tsai, F. J. Kao, H. Y. Lei, M. T. Lin, and Y. S. Lin. 1999. Streptococcal pyrogenic exotoxin B induces apoptosis and reduces phagocytic activity in U937 cells. Infect. Immun. 67:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaPenta, D., C. Rubens, E. Chi, and P. P. Cleary. 1994. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. USA 91:12115–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard, B. A., M. Woischnik, and A. Podbielski. 1998. Production of stabilized virulence factor-negative variants by group A streptococci during stationary phase. Infect. Immun. 66:3841–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30:209–219. [DOI] [PubMed] [Google Scholar]
- 29.Molinari, G., and G. S. Chhatwal. 1998. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J. Infect. Dis. 177:1600–1607. [DOI] [PubMed] [Google Scholar]
- 30.Molinari, G., S. R. Talay, P. Valentin-Weigand, M. Rohde, and G. S. Chhatwal. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect. Immun. 65:1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier, C. 2000. Rheumatic fever—is it still a problem? J. Antimicrob. Chemother. 45(Suppl):13–21. [DOI] [PubMed]
- 32.Osterlund, A., R. Popa, T. Nikkila, A. Scheynius, and L. Engstrand. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107:640–647. [DOI] [PubMed] [Google Scholar]
- 33.Raeder, R., E. Harokopakis, S. Hollingshead, and M. D. Boyle. 2000. Absence of SpeB production in virulent large capsular forms of group A streptococcal strain 64. Infect. Immun. 68:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrager, H. M., S. Alberti, C. Cywes, G. J. Dougherty, and M. R. Wessels. 1998. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Investig. 101:1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens, D. L. 2000. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu. Rev. Med. 51:271–288. [DOI] [PubMed] [Google Scholar]
- 38.Stockbauer, K. E., L. Magoun, M. Liu, E. H. Burns, Jr., S. Gubba, S. Renish, X. Pan, S. C. Bodary, E. Baker, J. Coburn, J. M. Leong, and J. M. Musser. 1999. A natural variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc. Natl. Acad. Sci. USA 96:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai, P. J., C. F. Kuo, K. Y. Lin, Y. S. Lin, H. Y. Lei, F. F. Chen, J. R. Wang, and J. J. Wu. 1998. Effect of group A streptococcal cysteine protease on invasion of epithelial cells. Infect. Immun. 66:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unnikrishnan, M., J. Cohen, and S. Sriskandan. 1999. Growth-phase-dependent expression of virulence factors in an M1T1 clinical isolate of Streptococcus pyogenes. Infect. Immun. 67:5495–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woischnik, M., B. A. Buttaro, and A. Podbielski. 2000. Inactivation of the cysteine protease SpeB affects hyaluronic acid capsule expression in group A streptococci. Microb. Pathog. 28:221–226. [DOI] [PubMed] [Google Scholar]
- 42.Yu, C. E., and J. J. Ferretti. 1991. Frequency of the erythrogenic toxin B and C genes (speB and speC) among clinical isolates of group A streptococci. Infect. Immun. 59:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]