Abstract
To clarify the invasive process of Vibrio parahaemolyticus, an invasion assay was performed using cells expressing dominant negative small GTPases of the Rho family. This assay showed that the dominant negative host phenotype facilitates bacterial invasion, suggesting that the mechanism of V. parahaemolyticus invasion differs from that reported for other invasive bacteria.
Vibrio parahaemolyticus, which causes acute gastroenteritis after the consumption of raw seafood, produces thermostable direct hemolysin (TDH), TDH-related hemolysin (TRH), or both. Research into the pathogenesis of this bacterium has been particularly directed towards these toxins (12). Other virulence factors of V. parahaemolyticus have also been identified and studied (11, 16, 17, 18, 19), but the overall mechanism of V. parahaemolyticus pathogenesis has not been elucidated. Some cases of infection result in primary septicemia and wound infection, as with Vibrio vulnificus infections (6, 9).
In our previous study, we showed that some strains of V. parahaemolyticus have an invasive phenotype. These invasive isolates can rearrange the cellular cytoskeleton and disturb tyrosine kinase-mediated signal transduction during the invasion process (2). The organization of the host cellular cytoskeleton is mainly regulated by Rho family small GTPases, including Rho, Rac, and Cdc42 (20). Invasion of bacterial cells is frequently the result of bacterial manipulation of the host cell cytoskeleton at the level of these small GTPases, the activity of which is associated with particular membrane and cytoskeleton rearrangements, such as lamellipodia (Rac), filopodia (Cdc42), and stress fibers (Rho).
Recent studies have revealed that invasive bacteria such as Shigella flexneri, Salmonella enterica serovar Typhimurium, Neisseria gonorrhoeae, and Pseudomonas aeruginosa induce cytoskeletal rearrangements associated with the small GTPases Rho, Rac, and Cdc42 (1, 5, 7, 8, 10, 22). Rho family small GTPases are essential for the infection of epithelial cells by Shigella flexneri (1, 22), and SopE produced by Salmonella enterica serovar Typhimurium activates Rac-1 and Cdc42 involved with membrane ruffling (8). SptP induces the cytoskeletal actin changes induced by S. enterica serovar Typhimurium by functioning as a GTPase-activating protein (GAP) for Rac-1 and Cdc42 (7). The invasive process of N. gonorrhoeae requires an Src-like tyrosine kinase and Rac-dependent host signaling (10). Therefore, in this study we used an invasion assay in Caco-2 cells, which express the dominant wild-type or mutant Rho, Rac, and Cdc42 to confirm whether the invasion process of V. parahaemolyticus involves signaling by small GTPases of the Rho family.
The invasion assay was performed as previously described (2). In brief, Caco-2 cells were grown at 37°C under 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Invitrogen, Groningen, The Netherlands). The cells were infected with an invasive strain of V. parahaemolyticus, AQ4023 (serotype O3:K6), for 3 h. After infection, the Caco-2 cells were washed thoroughly and lysed with 0.1% Triton X-100 in phosphate-buffered saline. This suspension was plated onto LB agar containing 3% NaCl. The invasion rate was calculated as the number of colonies formed divided by the number of bacteria inoculated.
To observe the effect of the dominant negative forms of the small GTPases, the plasmids pMEPyori F-RhoN19, pMEPyori F-RacN17, and pMEPyori F-42N17 were constructed. These plasmids encode the dominant negative small GTPases RhoN19, RacN17, and Cdc42N17, respectively (15), fused to the FLAG epitope (Sigma, St. Louis, Mo.). The plasmids were transfected into Caco-2 cells using Lipofectamine Plus (Invitrogen) according to the manufacturer’s instructions.
Vectors encoding wild-type small GTPases were constructed from pMEPyori18Sf (13), into which the genes for the small GTPases RhoA, Rac-1, and Cdc42Hs were ligated between the SalI and XbaI sites. To create the dominant negative genotype, the oligonucleotides for RhoN19 (5′-GGAGATGGAGCATGCGGCAAGAATTGCTTGCTCATTGTCTTTTAGC-3′ and 5′-GCTAAAGACCAATGAGCAAGCAATTCTTGCCGCATGCTCCATCTCC-3′), RacN17 (5′-GTGGTGGGAGACGGCGCCGTAGGTAAAAATTGCCTACTGATC-3′ and 5′-GATCAGTAGGCAATTTTTACCTACGGCGCCGTCTCCCACCAC-3′), and Cdc42N17 (5′-GCTGTTGGTAAAAATTGTCTCCTGATCTCCTACACAACAAAC-3′ and 5′-GTTTGTTGTGTAGGAGATCAGGAGACAATTTTTACCAACAGC-3′) were used with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). The efficiency of transient expression was assayed by immunofluorescence microscopy with an anti-FLAG monoclonal antibody (Sigma) and Alexa 350-labeled anti-mouse immunoglobulin G (IgG) (Molecular Probes, Eugene, Oreg.).
To assess the dominant mutant phenotype, Caco-2 cells expressing the dominant mutant were stained with rhodamine-phalloidin (Molecular Probes), and the cytoskeletal change was confirmed (15) (see Fig. 3B). In this study, transfection into Caco-2 cells was transient, and transfected cultures consisted of a mixture of transfected and untransfected cells. However, we had no difficulty comparing the effects of small GTPase mutations on the invasiveness of these bacteria, as all assays using transfected Caco-2 cells had a constant efficiency rate of approximately 40%.
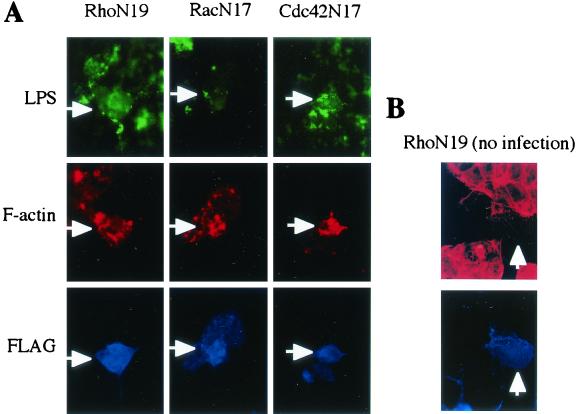
FIG. 3.
Immunofluorescent micrographs of V. parahaemolyticus-infected Caco-2 cells expressing dominant negative Rho family GTPases. Dominant negative Rho (RhoN19), Rac (RacN17), and Cdc42 (Cdc42N17) fused to the Flag epitope were expressed in Caco-2 cells as described in the text. V. parahaemolyticus was stained with anti-LPS antibody (green). F-actin was stained with rhodamine-phalloidin (red). Expression of dominant negative Rho family GTPases was detected by staining with anti-Flag antibody (blue). Arrows indicate Caco-2 cells expressing dominant mutant Rho family GTPases with V. parahaemolyticus infection (A) and without infection as a control (B). Magnification, ×1,000.
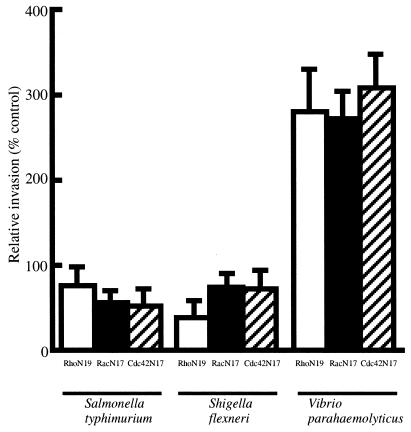
The invasion assay showed that V. parahaemolyticus AQ4023 invaded Caco-2 cells expressing dominant negative GTPases much more readily than it invaded mock-transfected cells (Fig. 1). On the other hand, the invasion rates in the positive controls, S. flexneri and S. enterica serovar Typhimurium, decreased in cells expressing the inactive form of the Rho family GTPases, as previously reported (1, 8, 22). In the study using botulinum C3 exoenzyme, which inactivates Rho by ADP-ribosylation (3), we found that AQ4023 can more readily invade Caco-2 cells treated with C3 exoenzyme (10 μg/ml; Wako Pure Chemicals, Osaka, Japan) (218.4 ± 42.8%) than untreated cells (100.0 ± 14.7%), as indicated by the relative ratio of invasion. These results indicate that the invasive process of V. parahaemolyticus may be different from the processes of other well-known invasive bacteria and suggest that the mechanism of the V. parahaemolyticus invasion process competes with downstream effectors of the Rho family and bacterial factors.
FIG. 1.
Effect of dominant negative Rho, Rac, and Cdc42 on bacterial invasion of Caco-2 cells. Invasion efficiency into the mock-transfected control was taken as 100%. Invasiveness was determined as described in the text, and relative invasiveness was calculated as a percentage of the mock-transfected control. Results are given as means ± standard deviations of three independent assays, each done in duplicate. Dominant negative Rho, Rac, and Cdc42 are denoted RhoN19, RacN17, and Cdc42N17, respectively.
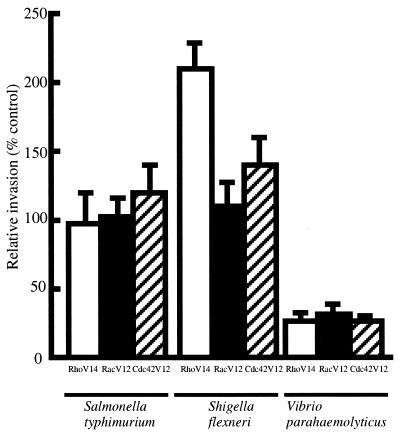
Under this assumption, epithelial cells expressing the dominant active form of Rho family proteins may prevent the invasion process by V. parahaemolyticus. Expression of the dominant active form of the Rho family induced constitutive activation (not temporary) of the cytoskeletal rearrangement (15), and the effect of the dominant active form on the invasion process was readily observed. Therefore, we constructed plasmids expressing the dominant active form of these proteins, as described above. Oligonucleotides for RhoV14 (5′-CTGGTGATTGTTGGAGATGTAGCATGCGGCAAGACTTGC-3′ and 5′-GCAAGTCTTGCCGCATGCTACATCTCCAACAATCACCAG-3′), for RacV12 (5′-GTGTGTGGTGGTGGGAGACGTCGCTGTAGGTAAAACTTGC-3′ and 5′-GCAAGTTTTACCTACAGCCACGTCTCCCACCACCACACAC-3′), and for Cdc42V12 (5′-GTTGTGGGCGATGTTGCTGTTGGTAAAACATGTCTCCTGATCTCC-3′ and 5′-GGAGATCAGGAGACATGTTTTACCAACAGCAACATCGCCCACAAC-3′) were used in site-directed mutagenesis, and generated plasmids pMEPyori F-RhoV14, pMEPyori F-RacV12, and pMEPyori F-42V12, respectively. These were transfected into Caco-2 cells. The invasion assay using Caco-2 cells expressing the dominant active form of Rho, Rac, and Cdc42 showed that the invasion rate of V. parahaemolyticus was decreased compared with mock-transfected controls (Fig. 2). The invasion rates of S. flexneri and S. enterica serovar Typhimurium increased in this assay (Fig. 2).
FIG. 2.
Effect of dominant active Rho, Rac, and Cdc42 on bacterial invasion of Caco-2 cells. Invasion efficiency of the mock-transfected control was taken as 100%. Invasiveness was determined as described in the text, and relative invasiveness was calculated as a percentage of the mock-transfected control. Results are given as means ± standard deviations of three independent assays, each done in duplicate. Dominant active Rho, Rac, and Cdc42 are denoted as RhoV14, RacV12, and Cdc42V12, respectively.
The bacterial invasion process is involved with cytoskeletal rearrangement. Actin accumulation inhibitors, such as cytochalasin D, block the entry of invasive bacteria, including V. parahaemolyticus (2). Immunofluorescence microscopy was used to confirm whether the actin accumulation caused by V. parahaemolyticus occurs in Caco-2 cells expressing the dominant negative small GTPases. F-actin was stained with rhodamine-phalloidin, and Caco-2 cells expressing small GTPases fused to the Flag epitope were stained with anti-Flag antibody and Alexa 350-labeled anti-mouse IgG (Molecular Probes). Bacterial cells were stained with anti-V. parahaemolyticus lipopolysaccharide (LPS) antibody (anti-O3 antibody; Denka Seiken, Tokyo, Japan) and Alexa 488-labeled anti-rabbit IgG.
F-actin accumulation was observed in infected Caco-2 cells expressing RhoN19, RacN17, and Cdc42N17 fused to the FLAG epitope, as shown in Fig. 3A. In the control study, F-actin stress fibers disappeared in Caco-2 cells expressing the dominant negative Rho GTPases compared with nontransfected cells (Fig. 3B). Similarly, the dominant mutant forms of Rac and Cdc42 inhibited cytoskeletal actin formation (data not shown). These immunofluorescent microscopic images show that the invasive process of V. parahaemolyticus is exerted via the regulation of actin rearrangements downstream from the Rho family GTPases.
Taken together, these results indicate that the invasive process of V. parahaemolyticus mainly affects the downstream signaling cascade of the Rho family GTPases. Recent studies have revealed that many pathogenic bacteria can invade eukaryotic cells via the various processes involved with cytoskeletal rearrangement. Several studies purporting to clarify the mechanism of bacterial invasion have investigated the small GTPases, Rho, Rac, and Cdc42, as these are thought to regulate the distinct structures making up the actin cytoskeleton (20). To the best of our knowledge, this is the first report demonstrating that inactivation of small GTPases increases bacterial entry into cultured cells.
Other actin rearrangements of the host cell, such as the actin pedestal formation induced by enteropathogenic E. coli (EPEC), suggest some relationship between V. parahaemolyticus invasion and the pedestal formation by EPEC, which elongates the actin pedestal in HeLa cells expressing dominant negative Rho, Rac, and Cdc42 (4). EPEC-induced actin pedestal formation requires Wiskott-Aldrich syndrome protein (WASP) or N-WASP (14), which is a downstream effector of Cdc42 (21). In this study, using the invasion assay and Caco-2 cells expressing the dominant mutant small GTPases, we showed that RhoN19, RacN17, and Cdc42N17 activate V. parahaemolyticus invasion. This suggests that V. parahaemolyticus invasion does not stimulate the Rho family small GTPases directly, but rearranges the cytoskeletal organization. The cytoskeleton is mainly regulated by the Rho family, and effector molecules activated by Rho family proteins are necessary for cellular functions. This study suggests there may be competition between virulence factors produced by V. parahaemolyticus and effectors located downstream from the Rho family. However, it is unclear whether common effectors downstream from each Rho family GTPase adjust the cytoskeleton.
In another interpretation of the invasive process of V. parahaemolyticus, a signaling cascade not involving the Rho family GTPases may function in cytoskeletal regulation. However, recent research has not revealed any novel regulation of cytoskeletal rearrangements. A detailed study is required to resolve whether host cellular effector molecules are involved in V. parahaemolyticus invasion. It is possible that unknown factors produced by V. parahaemolyticus modulate cytoskeletal activation. Moreover, cytoskeletal arrangements are induced by various effectors downstream from the Rho family GTPase signaling cascade, and the activation of the c-Jun N-terminal kinase (JNK) signaling cascade transduced to the transcription factors for the inflammation. Therefore, in future studies, not only cytoskeletal rearrangements but also cytokine expression via JNK activation should constitute the main focus for research into V. parahaemolyticus pathogenesis.
Acknowledgments
This study was supported by a grant-in-aid from the Research for the Future program of the Japan Society for the Promotion of Science (JSPS-RFTF97L00704).
Editor: D. L. Burns
REFERENCES
- 1.Adams, T., M. Giry, P. Boquet, and P. Sansonetti. 1996. Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J. 15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 2.Akeda, Y., K. Nagayama, K. Yamamoto, and T. Honda. 1997. Invasive phenotype of Vibrio parahaemolyticus. J. Infect. Dis. 176:822–824. [DOI] [PubMed] [Google Scholar]
- 3.Aktories, K., U. Braun, S. Rosener, I. Just, and A. Hall. 1989. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem. Biophys. Res. Commun. 158:209–213. [DOI] [PubMed] [Google Scholar]
- 4.Ben-ami, G., V. Ozeri, E. Hanski, F. Hofmann, K. Aktories, K. M. Hahn, G. Bokoch, and I. Rosenshine. 1998. Agents that inhibit Rho, Rac, and Cdc42 do not block formation of actin pedestals in HeLa cells infected with enteropathogenic Escherichia coli. Infect. Immun. 66:1755–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, L. M., S. Hobbie, and J. E. Galan. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274:2115–2118. [DOI] [PubMed] [Google Scholar]
- 6.Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. M. Hammond, S. Thompson, S. Wilson, N. H. Bean, P. M. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Infect. Dis. 181:1661–1666. [DOI] [PubMed] [Google Scholar]
- 7.Fu, Y., and J. E. Galan. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293–297. [DOI] [PubMed] [Google Scholar]
- 8.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPase that induces membrane ruffling and nuclear responses in host cells. Cell 93:815–826. [DOI] [PubMed] [Google Scholar]
- 9.Hardy, W. G., and Klontz, K. C. 1996. The epidemiology of Vibrio infections in Florida, 1981–1993. J. Infect. Dis. 173:1176–1183. [DOI] [PubMed] [Google Scholar]
- 10.Hauck, C. R., T. F. Meyer, F. Lang, and E. Gulbins. 1998. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires an Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda, T., M. Arita, E. Ayala, and T. Miwatani. 1988. Production of pili on Vibrio parahaemolyticus. Can. J. Microbiol. 34:1279–1281. [DOI] [PubMed] [Google Scholar]
- 12.Honda, T., and T. Iida. 1993. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Med. Microbiol. 4:106–113. [Google Scholar]
- 13.Horiguchi, Y., N. Inoue, M. Masuda, T. Kashimoto, J. Katahira, N. Sugimoto, and M. Matsuda. 1997. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl. Acad. Sci. USA 94:11623–11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalman, D., O. D. Weiner, D. L. Goosney, J. W. Sedat, B. B. Finlay, A. Abo, and J. M. Bishop. 1999. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell Biol. 1:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moorman, J. P., D. Luu, J. Wickham, D. A. Bobak, and C. S. Hahn. 1999. A balance of signaling by Rho family small GTPase RhoA, Rac1 and Cdc42 coordinates cytoskeletal morphology but not cell survival. Oncogene 18:47–57. [DOI] [PubMed] [Google Scholar]
- 16.Nagayama, K., T. Oguchi, M. Arita, and T. Honda. 1994. Correlation between cell-associated mannose-sensitive hemagglutination by Vibrio parahaemolyticus and adherence to a human colonic cell line Caco-2. FEMS Microbiol. Lett. 120:207–210. [DOI] [PubMed] [Google Scholar]
- 17.Nagayama, K., T. Oguchi, M. Arita, and T. Honda. 1995. Purification and characterization of a cell-associated hemagglutinin of Vibrio parahaemolyticus. Infect. Immun. 63:1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakasone, N., and M. Iwanaga. 1990. Pili of a Vibrio parahaemolyticus strain as a possible colonization factor. Infect. Immun. 58:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakasone, N., and M. Iwanaga. 1992. The role of pili in colonization of the rabbit intestine by Vibrio parahaemolyticus Na2. Microbiol. Immunol. 36:123–130. [DOI] [PubMed] [Google Scholar]
- 20.Nobes, C. D., and A. Hall. 1995. Rho, Rac, Cdc42 GTPase regulates the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62. [DOI] [PubMed] [Google Scholar]
- 21.Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, and M. W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97:221–231. [DOI] [PubMed] [Google Scholar]
- 22.Watarai, M., Y. Kamata, S. Kozaki, and C. Sasakawa. 1997. rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J. Exp. Med. 185:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]