Abstract
The role of the complement system in host defense against Salmonella infection is poorly defined. Bacterial cell wall O-antigen polysaccharide can activate the alternative pathway in vitro. No studies, however, have elucidated the role of the classical pathway in immunity to Salmonella spp. in vivo. C1q-deficient mice (C1qa−/−) on a 129/Sv genetic background and strain-matched controls were infected intraperitoneally and intravenously with Salmonella enterica serovar Typhimurium and monitored over a 14-day period. After inoculation by either route, the C1qa−/− mice were found to be significantly more susceptible to Salmonella infection. Hepatic and splenic bacterial counts, performed at various time points, showed increased numbers of colonies in complement-deficient mice compared to controls. Analysis of blood clearance showed no difference between the two experimental groups during the first 15 min. However, after 20 min and until 6 h postinfection, numbers of circulating bacteria were significantly higher in complement-deficient mice. In vitro experiments using either resident or thioglycolate-elicited peritoneal macrophages showed a significant increase in the number of bacteria inside C1q-deficient macrophages compared to controls irrespective of the serum used for opsonizing the bacteria. These findings could not be explained either by an increased bacterial uptake, analyzed in vitro and in vivo using green fluorescent protein-tagged salmonellae, or by a defect in the respiratory burst or in NO production. The data presented here suggest the possibility of novel pathways by which C1q may modulate the pathogenesis of infectious diseases caused by intracellular pathogens.
Salmonella infections pose a serious health hazard worldwide, affecting both humans and animals. Natural resistance and acquired immunity to Salmonella spp. may be studied in mice by use of host-adapted salmonellae (Salmonella enterica serovar Typhimurium) which cause systemic infections that mimic the human disease. Salmonella serovar Typhimurium is an intracellular parasite that, after entry into the host, grows rapidly in the mononuclear phagocytic system (MPS) and causes disease (5, 6). Early exponential growth is controlled mainly by the natural resistance-associated macrophage protein 1 (Nramp1) locus (also known as Ity, Lsh, and Bcg), which is expressed by resident peritoneal and splenic macrophages (24) and by Kupffer cells (16). The Nramp1 gene codes for a pH-dependent transporter expressed in the phagosomal membrane (20) and has two allelic forms, r (resistant) and s (susceptible), with Nrampr being the dominant gene (18, 19). In addition, a number of other humoral and cellular effector mechanisms implicated in host resistance to Salmonella infections have been described in the literature.
Complement is an important component of the innate immune system and plays a major role in host defense against bacteria and fungi. The best evidence for this comes from the study of humans with hereditary deficiencies of complement proteins. Patients with deficiencies of complement components often show increased susceptibility to a wide range of bacterial infections. With the advent of gene-targeting technology, it has been possible to engineer genetic deficiencies of complement proteins in mice and to explore in detail the role of complement deficiency in host defense against infection in vivo. The power of this approach has been demonstrated by the work of Wessels and colleagues (42), who studied group B streptococcal infection in mice with targeted gene deletions of C3 and C4. In contrast to the accepted dogma that classical-pathway activation is antibody dependent and alternative-pathway activation is antibody independent, the results of their studies showed a role for antibody-independent classical-pathway activation and antibody-dependent alternative-pathway activation in host defense against this pathogen. In addition, endotoxic-shock models demonstrated an important role for classical-pathway complement activation in the clearance of lipopolysaccharide (LPS) from the circulation, suggesting the presence of natural antibodies that recognize LPS and activate complement (31).
Salmonella strains with differences in the O-antigenic polysaccharide of their LPS preferentially activate complement via the alternative pathway (27, 37-39). The rate of bacterial uptake by macrophages in vitro reflects the differential rate of complement activation (21, 22, 33, 36). In mice, when complement is depleted by pretreatment with cobra venom factor, clearance of the most virulent strain from the blood is unaffected whereas the rapid clearance of the least virulent strain is greatly delayed, suggesting that complement-dependent phagocytosis is an important host defense mechanism in vivo (23). Nevertheless, the role of the complement system in immunity to Salmonella and the importance of the classical pathway in vivo are still unclear.
In the present study we investigated whether the classical pathway contributes to host resistance against Salmonella in vivo. Mice deficient in the first component of the classical pathway, C1q (Clqa−/−), were infected either intraperitoneally (i.p.) or intravenously (i.v.) with the serum-resistant strain Salmonella serovar Typhimurium 12023 and were monitored during the course of infection. Complement-deficient mice showed increased susceptibility to infection with Salmonella serovar Typhimurium compared to strain-matched control animals, suggesting, for the first time, an important role for C1q in protection against Salmonella infection in vivo.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old, weight-matched male mice were used in all experiments. C1qa−/−animals, on a pure 129/Sv (Nrampr) background, were generated as described previously (4). Studies were performed according to institutional guidelines for animal use and care.
Bacterial infection.
The wild-type, serum-resistant strain Salmonella serovar Typhimurium 12023 (kindly provided by David Holden, Imperial College, London, United Kingdom), possessing an O-4,12 polysaccharide side chain, was used in all experiments. Bacteria were grown as stationary overnight cultures in Luria-Bertani (LB) broth. Aliquots were snap-frozen and stored in liquid nitrogen. For each experiment the inoculum was diluted to the appropriate dose in phosphate-buffered saline (PBS) and injected i.p. or i.v. The exact dose was checked by examining growth on LB agar plates.
Enumeration of bacterial counts in the liver and spleen.
At indicated times postinjection, groups of 5 to 10 control and complement-deficient mice were sacrificed. Livers and spleens were aseptically removed, weighed, and homogenized in 5 ml of saline solution. Samples of the homogenate were plated onto LB agar in 10-fold serial dilutions. Viable colonies were assessed by using pour plates of LB agar, and bacterial counts were expressed as CFU per gram of tissue.
Bacterial clearance from the blood.
Control and complement-deficient mice were each infected i.v. with 105 CFU of Salmonella serovar Typhimurium 12023 diluted in 0.2 ml of saline solution. Blood samples (100 μl) were taken at 1, 5, 15, and 30 min and 1, 2, 4, and 6 h postinfection, diluted in 1 ml of saline solution containing 10% EDTA, and plated out onto LB agar in serial dilutions. The number of viable bacteria present in the blood was calculated at each time point.
C3 binding to Salmonella serovar Typhimurium.
Bacteria were grown as overnight cultures in LB. A total of 5 × 108 bacteria were resuspended in 100 μl of either wild-type or C1q-deficient serum and incubated at 37°C for 15 min to allow opsonization to occur. Bacteria were then washed twice in PBS-0.01% Tween. A fluorescein isothiocyanate-conjugated polyclonal goat anti-mouse C3 antibody (ICN, Cappell) was added (at a 1:100 dilution) to the bacteria and kept on ice for 30 min. Bacteria were washed and resuspended in PBS before flow cytometry analysis. Bacteria incubated in C3-deficient serum were used as negative controls.
In vitro assays for Salmonella serovar Typhimurium infection of resident and thioglycolate-elicited peritoneal macrophages.
Resident peritoneal macrophages were recovered by lavage with 5 ml of ice-cold Hanks balanced salt solution-5 mM EDTA. The same procedure was used to isolate thioglycolate-elicited peritoneal macrophages 4 days after i.p. injection with 1 ml of sterile 4% Brewer thioglycolate. Macrophages were cultured overnight in 24-well tissue culture dishes at a density of 106/ml in Dulbecco's modified Eagle medium (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum. Assays of intracellular killing of Salmonella serovar Typhimurium by macrophages were carried out with a 10:1 ratio of bacteria to macrophages by following the method of Lissner et al. (24) with slight modifications. Briefly, adherent macrophages were allowed to ingest Salmonella serovar Typhimurium , which had been preopsonized with 10% control or complement-deficient mouse serum, for 20 min at 37°C. The actual number of serum-suspended bacteria used to infect the macrophages was verified by plate count. To monitor the number of adherent macrophages present at each sample time, macrophages were counted using a hemocytometer eyepiece with a graticule. After a 30-min phagocytosis period, macrophages were washed three times with Hanks medium to remove nonadherent bacteria, and medium supplemented with 100 μg of gentamicin/ml was added to the wells. At specified times (1 and 4 h) macrophages were lysed with 0.5 ml of 1% sodium deoxycholate (Sigma-Aldrich) in distilled water. Each lysate was stirred vigorously with the tip of a sterile pipette and withdrawn from the well for a plate count. Plates were counted to determine the number of viable bacteria inside the macrophages at each time point analyzed.
Uptake of Salmonella serovar Typhimurium by peritoneal macrophages in vitro and in vivo.
Salmonella serovar Typhimurium 12023, constitutively expressing green fluorescent protein (GFP) (8), was kindly provided by David Holden (Imperial College). In vitro , resident or thioglycolate-elicited macrophages from C1qa−/− and wild-type mice were offered GFP-expressing salmonellae (ratio of bacteria to cells, 10:1) for 1 h. Cells were then washed with PBS and fixed with 1% paraformaldehyde in PBS for 15 min on ice before flow cytometry to quantify phagocytosis of bacteria by analysis of GFP-positive green fluorescent cells. In preliminary studies, confocal microscopy was used to confirm that GFP-positive cells contained internalized bacteria. Macrophages offered unlabeled salmonellae were used as negative controls. The percent cells positive for GFP quantified the relative proportion of cells that had phagocytosed salmonellae. The mean fluorescence intensity of these cells provides a measure of the number of salmonellae ingested by each cell. At least 104 cells in all samples were analyzed by a Coulter Epics XL-MCL flow cytometer with System II software. The forward scatter threshold was used to exclude free bacteria from the analysis. In vivo assays were carried out by i.p. injection of C1q-deficient and wild-type mice with 106 CFU each of GFP-expressing or unlabeled salmonellae, followed by collection of peritoneal macrophages by peritoneal lavage after 30 min. Preliminary time course experiments showed that the number of cells harvested from the peritoneum decreased rapidly after 30 min; therefore, subsequent time points were not possible. Peritoneal cells were spun, resuspended in 1 ml of 0.15 M NH4Cl plus 0.001 M KCl for 5 min to lyse contaminating red cells, and then fixed before flow cytometric analysis as above. Analysis was restricted to peritoneal macrophages by identifying this population by use of the monoclonal antibody F4/80 (Caltag, Burlingame, Calif.), a well-characterized macrophage marker. In additional experiments, phagocytosis by thioglycolate-elicited peritoneal macrophages in C1q-deficient and wild-type mice was similarly compared in vivo.
Total nitric oxide assay.
Total nitrite concentrations in supernatants collected from in vitro macrophage experiments were determined by using the Total NO kit (R&D Systems Europe, Abingdon, United Kingdom) as specified by the manufacturer.
Respiratory burst.
Resident and thioglycolate-elicited macrophages were cultured overnight in RPMI medium supplemented with 22 mM HEPES, 1 mM sodium pyruvate, 2 mM l-glutamine, and 10% fetal bovine serum at a density of 3 × 105 cells per well in 96-well Microlite flat-bottom microtiter plates (Dynex Technologies, Durham, N.C.). Nonadherent cells were washed with prewarmed Hanks medium. Salmonella serovar Typhimurium , preopsonized with 10% control or complement-deficient mouse serum, was then added to the macrophages at a 10:1 ratio (bacteria to macrophages) and incubated for 15 min at 37°C. Noningested bacteria were removed by vigorous washing, and 200 μl of prewarmed RPMI medium supplemented with 6 μg of gentamicin/ml and 12.5 μM lucigenin (bis-N-methylacridium) (Sigma-Aldrich) was added as an indicator of superoxide production. As a positive control for the reaction, macrophages were stimulated with 1 μg of phorbol myristate acetate (Sigma-Aldrich)/ml. The intensity of the chemiluminescent response elicited was monitored over 30 min by using a Lucy 1 chemiluminometer (Labtech) with Stingray Software. Counts from noninfected macrophages were used to eliminate background reading.
Histopathology.
Sections were prepared from livers and spleens, fixed by immersion in 10% formalin, and stained with hematoxylin-eosin.
Statistical analysis.
Survival data are presented as Kaplan-Meier plots and were compared by the log rank test. Nonparametric tests were applied throughout unless otherwise stated. Statistics were calculated using GraphPad Prism, version 2.0 (GraphPad Software, San Diego, Calif.). Differences were considered significant at P values of <0.05.
RESULTS
Susceptibility to Salmonella serovar Typhimurium.
Control and complement-deficient mice were infected i.p. or i.v. with 104 and 105 CFU, respectively, of Salmonella serovar Typhimurium strain 12023. The course of the infection was monitored over a 14-day period. After i.p. administration, only 1 death in the control group (n = 16) was observed whereas 17 out of 23 C1q-deficient mice (73%) succumbed within 14 days after infection (P = 0.0001 by the log rank test), with scattered deaths starting on day 6 (Fig. 1a). Similarly, after i.v. inoculation, no mice in the control group (n = 14) died and the complement-deficient mice followed the same pattern seen after i.p. administration, with 8 deaths among 9 mice (88%) recorded over the 14-day period (Fig. 1b) (P < 0.0001 by the log rank test). Similar results were obtained from five separate experiments and from one experiment where heterozygous C1q-deficient littermate mice were used as controls.
FIG. 1.
Survival rates of control and C1q-deficient mice over a 14-day period after infection with Salmonella serovar Typhimurium 12023. (a) Sixteen wild-type and 23 C1q-deficient mice were infected i.p. with 104 CFU/mouse. (b) Fourteen wild-type and nine C1q-deficient mice were infected i.v. with 105 CFU/mouse. Results are expressed as percentages of survivors at the indicated times after infection. Data shown are representative of five separate experiments.
Viable bacterial counts in the spleen and liver.
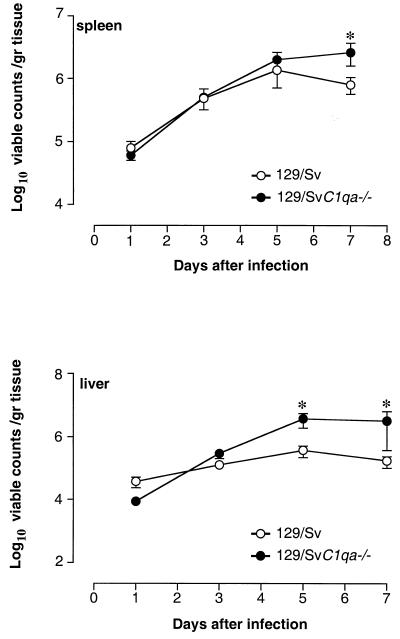
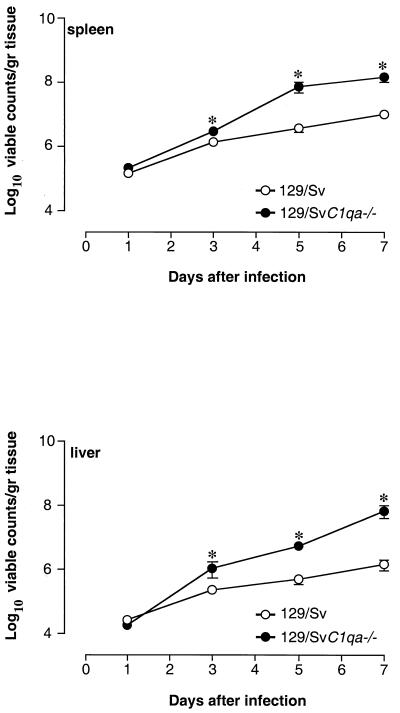
To explore the mechanisms for the accelerated death rates observed in C1qa−/− mice, bacterial counts in the spleen and liver were performed during the course of the infection. Control and complement-deficient mice were injected i.p. or i.v. with the same doses of Salmonella serovar Typhimurium as above, and bacterial counts were determined on days 1, 3, 5, and 7 postinfection. Bacterial counts from C1qa−/− mice that died during the course of infection were not included in the analysis. On days 1 and 3 after i.p. administration, liver and spleen counts were similar in the two experimental groups. On day 5, liver counts were significantly higher in complement-deficient mice than in control animals (P = 0.01 by the Mann-Whitney test), while viable counts in the spleen were similar. On day 7, higher bacterial numbers were found in both spleens and livers of C1qa−/− mice (P < 0.05 by the Mann-Whitney test) (Fig. 2). In these experiments no bacteria were detected in the circulation at any time points analyzed. However, for three C1q-deficient mice that were sacrificed for humane reasons during the in vivo susceptibility study, blood cultures revealed the presence of bacteria in the circulation (516 ± 204 viable counts in 100 μl of blood). After i.v. administration, splenic and hepatic bacterial counts in C1qa−/− mice were slightly higher than those in controls from day 3, with greater differences in bacterial counts appearing from day 5 on (P < 0.05) (Fig. 3). Histological analysis of liver and spleen sections, taken at the same time points during the course of the infection, showed more-pronounced lesions in complement-deficient mice. There was, however, no qualitative difference in the inflammatory morphology of the lesions (data not shown). This was observed in at least three separate experiments.
FIG. 2.
Bacterial counts in the spleens and livers of control and C1q-deficient mice. Mice were infected i.p. with 104 CFU of Salmonella serovar Typhimurium 12023/mouse, and viable counts were performed on days 1, 3, 5, and 7 postchallenge. Results are expressed as mean log10 viable counts per gram of tissue ± standard errors of the means obtained from groups of 5 to 10 mice for each time point. Data are representative of three separate experiments. ∗, P < 0.05.
FIG. 3.
Bacterial counts in the spleens and livers of control and C1q-deficient mice. Mice were infected i.v. with 105 CFU of Salmonella serovar Typhimurium 12023/mouse, and viable counts were performed on days 1, 3, 5, and 7 postchallenge. Results are expressed as log10 viable counts per gram of tissue ± standard errors of the means obtained from groups of 5 to 10 mice for each time point. Data are representative of three separate experiments. ∗, P < 0.05.
Clearance of Salmonella serovar Typhimurium from the blood circulation.
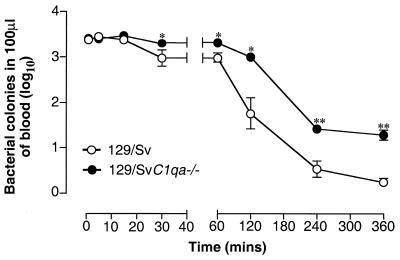
To ascertain whether a difference in initial processing of salmonellae between the two experimental groups may account for their different levels of resistance to this pathogen, C3 opsonization and blood clearance experiments were performed. Binding of C3 to Salmonella serovar Typhimurium was measured in vitro by flow cytometry as described in Materials and Methods. Fluorescence-activated cell sorter analysis showed no difference between the percentages of bacteria opsonized with C3 after incubation in C1q-deficient and wild-type sera (6.13% ± 1.2% and 6.01% ± 1.4%, respectively; P = 0.76 by the Mann-Whitney test). Notably, the percentage of C3-positive bacteria was very low, irrespective of the serum used for opsonization, compared with the level of C3 opsonization observed with other strains of bacteria analyzed at the same time (data not shown). In the blood clearance experiments, no difference was observed between the two groups of mice during the first 15 min after i.v. inoculation. In contrast, at each time point after this 15-min period, complement-deficient mice showed a significant delay in blood clearance (P < 0.05 by the Mann-Whitney test) (Fig. 4). No bacteria were present in the circulation in either group 24 h after inoculation.
FIG. 4.
Clearance of bacteria from the blood of control and C1q-deficient mice infected i.v. with 105 CFU of Salmonella serovar Typhimurium 12023/mouse. Blood samples were collected at 1, 5, 15, and 30 min and 1, 2, 4, and 6 h postchallenge. Results are expressed as log10 viable counts ± standard errors of the means in 100 μl of blood obtained from groups of 5 to 10 mice at each time point. ∗, P < 0.05; ∗∗, P < 0.01.
Salmonella uptake by peritoneal macrophages.
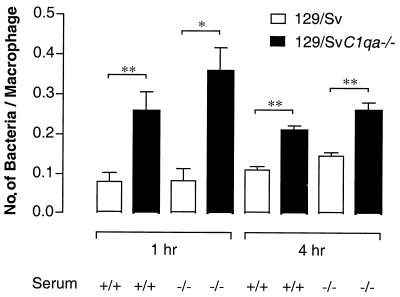
Uptake and inactivation of the bacteria by peritoneal macrophages were initially explored by in vitro assays. Thioglycolate-elicited macrophages were isolated, cultured for 24 h, and infected with salmonellae opsonized with C1q-deficient or -sufficient serum as described in Materials and Methods. Numbers of viable bacteria present inside macrophages were counted at 1 and 4 h after infection. There was no difference in bacterial counts inside cells between salmonellae opsonized with C1q-deficient serum and those opsonized with wild-type serum. The same results were obtained with other mouse complement-deficient sera (C3-, C4-, and factor B-deficient sera) (data not shown). However, in C1q-deficient macrophages, irrespective of the serum used for opsonization, significant increases in the numbers of bacteria per macrophage were detected at the two time points analyzed compared with those in wild-type macrophages (P < 0.05 and P < 0.01 by the Student t test) (Fig. 5). Resident C1q-deficient macrophages gave similar results (data not shown), suggesting the presence of an abnormal phagocytic uptake and/or a defect in intracellular killing of the bacteria. We explored both of these possibilities.
FIG. 5.
Number of bacteria associated inside thioglycolate-elicited control and C1q-deficient macrophages. Bacteria were opsonized in normal mouse serum (+/+) or C1q-deficient serum (−/−) before being added to macrophages at a ratio of 10:1 (bacteria to macrophages). Macrophages were lysed at 1 and 4 h postinfection. Results are expressed as the number of viable cell-associated bacteria divided by the number of macrophages counted. Each bar represents the average from three culture dish wells ± the standard error of the mean. Data are representative of four separate experiments. ∗, P < 0.05 by Student's t test; ∗∗, P < 0.01 by Student's t test.
Uptake by peritoneal macrophages was investigated in vivo and in vitro using GFP-tagged Salmonella serovar Typhimurium and flow cytometric analysis. In both assays, thioglycolate-elicited macrophages from C1q-deficient mice and controls were compared. There was no difference either in the percentage of macrophages that phagocytosed salmonellae or in the quantity of bacteria that were ingested by each macrophage (Table 1). Resident macrophages gave similar results (data not shown).
TABLE 1.
Uptake of GFP-tagged salmonellae in vivo and in vitro by thioglycolate-elicited macrophagesa
| Mice (no.) | % Macrophages positive for GFP
|
Relative mean fluorescence of GFP-positive macrophages
|
||
|---|---|---|---|---|
| In vivo | In vitro | In vivo | In vitro | |
| Control (3) | 69.2 ± 5.9 | 29.4 ± 6.9 | 8.25 ± 1.0 | 8.2 ± 0.23 |
| Clqa−/− (3) | 49.5 ± 9.6 (P = 0.15) | 32.4 ± 0.2 (P = 0.6) | 12.9 ± 2.7 (P = 0.18) | 8.6 ± 0.22 (P = 0.33) |
Data are expressed as means ± standard errors of the means and are representative of three independent experiments.
Production of reactive oxygen and nitrogen species in response to Salmonella.
Assays measuring the respiratory burst and production of NO were used to compare the antimicrobial activities of C1q-deficient and wild-type peritoneal macrophages. Total nitrite levels (an index of NO production) in supernatants collected from in vitro macrophage assays were found to be similar (Table 2).
TABLE 2.
Nitrite concentrations in culture supernatants from thioglycolate-elicited macrophages
| Genotype | Nitrite concn (μmol/liter)a at:
|
||
|---|---|---|---|
| Macrophages | Serum | 1 h | 4 h |
| +/+ | +/+ | 17.7 ± 0.5 | 19.1 ± 0.4 |
| +/+ | −/− | 18.3 ± 0.5 | 23.1 ± 6.3 |
| −/− | +/+ | 19.0 ± 0.6 | 18.5 ± 0.9 |
| −/− | −/− | 17.7 ± 0.8 | 20.7 ± 0.8 |
Data are expressed as means ± standard errors of the means and are representative of three independent experiments.
Production of reactive oxygen species was quantified over a 30-min period after Salmonella inoculation by chemiluminescence using lucigenin as an indicator of superoxide production. As with NO production, C1q-deficient macrophages showed a response comparable to that of control macrophages, irrespective of the serum used for preopsonizing the bacteria (data not shown), suggesting no defects in the production of the oxidative antimicrobial mediators in vitro.
DISCUSSION
In the present work we studied the role of C1q in the host defense against Salmonella infection. Unexpectedly, we found that C1q-deficient mice have an enhanced susceptibility to Salmonella infection administered by two different routes. Wild-type mice injected with salmonellae survived throughout the period of observation, while C1qa−/− mice started to succumb to infection from approximately day 6, suggesting a defect in their ability to control the growth of the bacteria in the MPS. The increased numbers of viable bacterial colonies in the spleens and livers of C1q-deficient mice confirmed this.
It is well known from previous studies that the O-antigenic polysaccharide part of the cell wall LPS is important in the virulence of Salmonella. Differences in the carbohydrate side chain determine the rate of complement activation and C3 deposition by the alternative pathway, and this has been shown to determine the virulence of the strains (27, 37, 38). In our study we used Salmonella serovar Typhimurium strain 12023, a serum-resistant strain with an O side chain of O-4,12.
To fully explore the role of the classical pathway in immunity to Salmonella infection in vivo, it would have been ideal to use both C4- and C3-deficient mice in experiments identical to those that we performed with C1q-deficient mice. Unfortunately, C3- and C4- deficient mice are not available on the 129/Sv genetic background, which is Nrampr, but only on the C57BL/6 genetic background, which is Nramps. Even at very low doses, C57BL/6 mice infected with the virulent Salmonella serovar Typhimurium strain that we used (12023) succumbed very rapidly, within the first 3 days. Hence, sublethal-infection experiments could not be performed in vivo with the C57BL/6 animals. However, we were able to investigate whether there were significant differences in C3 binding to Salmonella serovar Typhimurium 12023 between C1q-deficient and C1q-sufficient serum. In vitro analysis of C3 deposited on the bacteria after incubation with wild-type serum showed a very low grade of C3 binding, with no significant differences between C1q-deficient and -sufficient serum. These findings indicated that C3 binding to the bacterial cell wall is unlikely to play a major role in immunity against this pathogen. Antibody-independent binding of C1q to bacteria is unusual but not unprecedented. It has been reported for Listeria monocytogenes (2), Legionella pneumophila (29), Klebsiella pneumoniae (1), Escherichia coli (3), group B streptococci (9), and Salmonella enterica serovar Minnesota (7, 34). Our observations would suggest that C1q binding might play an important role in host defense against infections.
During the course of an infection caused by invasive salmonellae, bacteria are rapidly cleared from the circulation, and they localize and grow in the mononuclear cells of the MPS. Previous studies (23) have shown that in mice treated with cobra venom factor, which causes depletion of alternative-pathway components and C3, removal of the O-4,12 bacteria during the first 5 min is complement independent. Our study demonstrated that the bacteria were removed from the bloodstream less rapidly in complement-deficient mice than in control mice. This delay, however, was seen only after the first 15 min, and by 24 h no bacteria were detected in the circulation in either experimental group. These data would suggest that, even if the initial rapid blood clearance of a serum-resistant strain is not dependent on activation of the classical pathway, nevertheless C1q may affect the subsequent rate of ingestion of the bacteria by the MPS.
There are several molecular mechanisms by which intracellular pathogens interact with host cells. In some cases, phagocytosis takes place by direct binding and specific recognition between molecules on the microorganism surface and the complementary structures on the phagocyte surface. However, in most cases specific molecules act as bridges between the surface of the pathogen and specific receptors on the phagocytes. C1q and C1q receptors (10) may represent one of the receptor-mediated mechanisms in the recognition of Salmonella by phagocytes. A role for C1q in the phagocytosis of pathogens has already been suggested for Salmonella serovar Minnesota (11, 12, 32). In our study, using GFP-tagged salmonellae, there was no evidence of a defect in bacterial uptake by C1q-deficient peritoneal macrophages analyzed in vivo and in vitro. However, in in vitro assays, both unstimulated and thioglycolate-elicited macrophages from C1q-deficient mice showed a significant increase in intracellular bacterial counts over those in wild-type cells. This significant increase, more prominent at 4 h postinoculation, was independent of the C1q-deficient or -sufficient serum used for preopsonizing the bacteria. Previous studies have shown that peritoneal macrophages can synthesize C1q (25, 30) and that endogenous C1q is expressed in the membranes of macrophages. It has also been suggested that this endogenous C1q can function as an Fc-like receptor on macrophages during secretion (26, 17), and it may be involved in the binding of gram-negative bacteria. Notably, the impairment in the intracellular growth of the bacteria inside C1q-deficient macrophages could not be corrected by opsonizing the bacteria in C1q-sufficient mouse serum. From our data it is tempting to speculate that the interaction between C1q on macrophages and salmonellae might modulate the pathways of the intracellular killing of the bacteria.
Survival of Salmonella-infected mice requires suppression of bacterial growth in the tissues and establishment of a plateau phase until clearance is eventually accomplished. The host defense mechanisms that operate in the plateau phase are complex and as yet not fully understood. The respiratory burst and NO are known to make distinct contributions to the anti-Salmonella activity of macrophages. In vitro, NADPH oxidase-dependent killing is confined to the first few hours following phagocytosis, while inducible nitric oxide synthase contributes to later phases of antimicrobial activity (40). This also applies to the in vivo situation, where the oxidative burst is needed for initial bacterial killing and for control of bacterial growth in the first few days of the infection, whereas inducible nitric oxide synthase-mediated mechanisms play a role in host resistance later in the course of the disease (28). There is evidence that C1q, when presented in a multivalent fashion, can trigger the generation of toxic oxygen radicals by neutrophils (15, 35). While the C1q-mediated stimulation of neutrophils shares some parameters with other neutrophil activation systems, there are several quite distinct parameters that indicate an initial signaling pathway different from those previously described for NADPH oxidase. The first is that while C1q triggers superoxide production, it does not induce degranulation of primary or secondary granules (14). Other characteristics that are distinct from those of most (not all) previously described activators include the lack of pertussis toxin inhibition and the lack of a requirement for stable adherence to a surface for activation of the oxidase (13). We investigated the activity of the C1q-deficient macrophages against salmonellae by measuring the respiratory burst and the production of total NO. In vitro biochemical analysis of both pathways did not demonstrate a defect in C1q-deficient macrophages compared to control macrophages. Our study, however, cannot exclude the possibility that the lack of C1q may have an effect on the intracellular localization rather than the activation of the phagocyte NADPH oxidase following infection of macrophages with salmonellae. Interference with NADPH oxidase trafficking has been shown to contribute to the virulence of Salmonella (41).
In conclusion, the capacity to survive within the macrophage is an absolute requirement for Salmonella virulence in vivo, and our findings indicate that C1q may interfere with the effector mechanisms by which the mononuclear phagocytes combat this intracellular pathogen. Further studies will be required to elucidate these mechanisms at the molecular level.
Acknowledgments
This work was supported by Wellcome Trust grant 049658.
We thank all of the staff in the animal facility for technical assistance. We are grateful to H. T. Cook and Margarita Lewis for help in the histopathology analysis.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alberti, S., G. Marques, S. Camprubi, S. Merino, J. M. Tomas, F. Vivanco, and V. J. Benedi. 1993. C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect. Immun. 61:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez, C., E. Carrasco-Marin, and F. Leyva-Cobian. 1993. Role of complement component C1q in phagocytosis of Listeria monocytogenes by murine macrophage-like cell lines. Infect. Immun. 61:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, B., S. Chesne, G. J. Arlaud, and M. G. Colomb. 1985. Antibody-independent interaction between the first component of human complement, C1, and the outer membrane of Escherichia coli D31 m4. Biochem. J. 232:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botto, M., C. Dell'Agnola, A. E. Bygrave, E. M. Thompson, H. T. Cook, F. Petry, M. Loos, P. P. Pandolfi, and M. J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56-59. [DOI] [PubMed] [Google Scholar]
- 5.Carter, P. B., and F. M. Collins. 1974. Growth of typhoid and paratyphoid bacilli in intravenously infected mice. Infect. Immun. 10:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clas, F., and M. Loos. 1981. Antibody-independent binding of the first component of complement (C1) and its subcomponent C1q to the S and R forms of Salmonella minnesota. Infect. Immun. 31:1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 9.Eads, M. E., N. J. Levy, D. L. Kasper, C. J. Baker, and A. Nicholson-Weller. 1982. Antibody-independent activation of C1 by type Ia group B streptococci. J. Infect. Dis. 146:665-672. [DOI] [PubMed] [Google Scholar]
- 10.Eggleton, P., A. J. Tenner, and K. B. Reid. 2000. C1q receptors. Clin. Exp. Immunol. 120:406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Euteneuer, B., S. Storkel, and M. Loos. 1986. Contributions of C1q, bacterial lipopolysaccharide, and porins during attachment and ingestion phases of phagocytosis by murine macrophages. Infect. Immun. 51:807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Euteneuer, B., S. Storkel, and M. Loos. 1985. Differences in attachment and phagocytosis of Salmonella minnesota strains (S form, Re mutant) by mouse peritoneal macrophages: participation of endogenous C1q and bacterial surface components (LPS, porins). Curr. Top. Microbiol. Immunol. 121:85-97. [DOI] [PubMed] [Google Scholar]
- 13.Goodman, E. B., D. C. Anderson, and A. J. Tenner. 1995. C1q triggers neutrophil superoxide production by a unique CD18-dependent mechanism. J. Leukoc. Biol. 58:168-176. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, E. B., and A. J. Tenner. 1992. Signal transduction mechanisms of C1q-mediated superoxide production. Evidence for the involvement of temporally distinct staurosporine-insensitive and -sensitive pathways. J. Immunol. 148:3920-3928. [PubMed] [Google Scholar]
- 15.Hamada, A., J. Young, R. A. Chmielewski, and B. M. Greene. 1988. C1q enhancement of antibody-dependent granulocyte-mediated killing of nonphagocytosable targets in vitro. J. Clin. Investig. 82:945-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington, K. A., and C. E. Hormaeche. 1986. Expression of the innate resistance gene Ity in mouse Kupffer cells infected with Salmonella typhimurium in vitro. Microb. Pathog. 1:269-274. [DOI] [PubMed] [Google Scholar]
- 17.Heinz, H. P., H. Dlugonska, E. Rude, and M. Loos. 1984. Monoclonal anti-mouse macrophage antibodies recognize the globular portions of C1q, a subcomponent of the first component of complement. J. Immunol. 133:400-404. [PubMed] [Google Scholar]
- 18.Hormaeche, C. E. 1979. Genetics of natural resistance to salmonellae in mice. Immunology 37:319-327. [PMC free article] [PubMed] [Google Scholar]
- 19.Hormaeche, C. E. 1979. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology 37:311-318. [PMC free article] [PubMed] [Google Scholar]
- 20.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang-Takasaki, C. J., N. Grossman, and L. Leive. 1983. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J. Immunol. 130:1867-1870. [PubMed] [Google Scholar]
- 22.Liang-Takasaki, C. J., P. H. Makela, and L. Leive. 1982. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J. Immunol. 128:1229-1235. [PubMed] [Google Scholar]
- 23.Liang-Takasaki, C. J., H. Saxen, P. H. Makela, and L. Leive. 1983. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect. Immun. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lissner, C. R., R. N. Swanson, and A. D. O'Brien. 1983. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J. Immunol. 131:3006-3013. [PubMed] [Google Scholar]
- 25.Loos, M. 1983. Biosynthesis of the collagen-like C1q molecule and its receptor functions for Fc and polyanionic molecules on macrophages. Curr. Top. Microbiol. Immunol. 102:1-56. [DOI] [PubMed] [Google Scholar]
- 26.Loos, M., W. Muller, G. Boltz-Nitulescu, and O. Forster. 1980. Evidence that C1q, a subcomponent of the first component of complement, is an Fc receptor of peritoneal and alveolar macrophages. Immunobiology 157:54-61. [DOI] [PubMed] [Google Scholar]
- 27.Makela, P. H., V. V. Valtonen, and M. Valtonen. 1973. Role of O-antigen (lipopolysaccharide) factors in the virulence of Salmonella. J. Infect. Dis. 128(Suppl.):81-85. [DOI] [PubMed] [Google Scholar]
- 28.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mintz, C. S., P. I. Arnold, W. Johnson, and D. R. Schultz. 1995. Antibody-independent binding of complement component C1q by Legionella pneumophila. Infect. Immun. 63:4939-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller, W., H. Hanauske-Abel, and M. Loos. 1978. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J. Immunol. 121:1578-1584. [PubMed] [Google Scholar]
- 31.Reid, R. R., A. P. Prodeus, W. Khan, T. Hsu, F. S. Rosen, and M. C. Carroll. 1997. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J. Immunol. 159:970-975. [PubMed] [Google Scholar]
- 32.Ryan, U. S., D. R. Schultz, J. D. Goodwin, J. M. Vann, M. P. Selvaraj, and M. A. Hart. 1989. Role of C1q in phagocytosis of Salmonella minnesota by pulmonary endothelial cells. Infect. Immun. 57:1356-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxen, H., I. Reima, and P. H. Makela. 1987. Alternative complement pathway activation by Salmonella O polysaccharide as a virulence determinant in the mouse. Microb. Pathog. 2:15-28. [DOI] [PubMed] [Google Scholar]
- 34.Stemmer, F., and M. Loos. 1985. Evidence for direct binding of the first component of complement, C1, to outer membrane proteins from Salmonella minnesota. Curr. Top. Microbiol. Immunol. 121:73-84. [DOI] [PubMed] [Google Scholar]
- 35.Tenner, A. J., and N. R. Cooper. 1982. Stimulation of a human polymorphonuclear leukocyte oxidative response by the C1q subunit of the first complement component. J. Immunol. 128:2547-2552. [PubMed] [Google Scholar]
- 36.Valtonen, M. V. 1977. Role of phagocytosis in mouse virulence of Salmonella typhimurium recombinants with O antigen 6,7 or 4,12. Infect. Immun. 18:574-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valtonen, M. V., M. Plosila, V. V. Valtonen, and P. H. Makela. 1975. Effect of the quality of the lipopolysaccharide on mouse virulence of Salmonella enteritidis. Infect. Immun. 12:828-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valtonen, V. V. 1970. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J. Gen. Microbiol. 64:255-268. [DOI] [PubMed] [Google Scholar]
- 39.Valtonen, V. V., and P. H. Makela. 1971. The effect of lipopolysaccharide modification--antigenic factors 1,5,12 2 and 27--on the virulence of salmonella strains for mice. J. Gen. Microbiol. 69:107-115. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 42.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]