Abstract
The accessory gene regulator (agr) and the staphylococcal accessory regulator (sar) are central regulatory elements that control the production of Staphylococcus aureus virulence factors. To date, the functions of these loci have been defined almost exclusively using RN6390, which is representative of the laboratory strain 8325-4. However, RN6390 was recently shown to have a mutation in rsbU that results in a phenotype resembling that of a sigB mutant (I. Kullik et al., J. Bacteriol. 180:4814–4820, 1998). For that reason, it remains unclear whether the regulatory events defined in RN6390 are representative of the events that take place in clinical isolates of S. aureus. To address this issue, we generated mutations in the sarA and agr loci of three laboratory strains (RN6390, Newman, and S6C) and four clinical isolates (UAMS-1, UAMS-601, DB, and SC-1). Mutation of sarA in the cna-positive strains UAMS-1 and UAMS-601 resulted in an increased capacity to bind collagen, while mutation of agr had little impact. Northern blot analysis confirmed that the increase in collagen binding was due to increased cna transcription. Without exception, mutation of sarA resulted in increased production of proteases and a decreased capacity to bind fibronectin. Mutation of agr had the opposite effect. Although mutation of sarA resulted in a slight reduction in fnbA transcription, changes in the ability to bind fibronectin appeared to be more directly correlated with changes in protease activity. Lipase production was reduced in both sarA and agr mutants. While mutation of sarA in RN6390 resulted in reduced hemolytic activity, it had the opposite effect in all other strains. There appeared to be reduced levels of the sarC transcript in RN6390, but there was no difference in the overall pattern of sar transcription or the production of SarA. Although mutation of sarA resulted in decreased RNAIII transcription, this effect was not evident under all growth conditions. Taken together, these results suggest that studies defining the regulatory roles of sarA and agr by using RN6390 are not always representative of the events that occur in clinical isolates of S. aureus.
Staphylococcus aureus is an opportunistic pathogen capable of causing a wide variety of infections. Its pathogenic diversity is due to its ability to produce a diverse array of virulence factors. These factors fall into two groups based on whether they remain associated with the cell surface or are exported into the extracellular milieu. In vitro, these two groups are globally and inversely regulated, with the surface proteins being produced during the exponential growth phase and the exoproteins being produced as cultures enter postexponential growth (33). This is consistent with the fact that the production of S. aureus virulence factors is responsive to a quorum-sensing signal (27). This signal exerts its regulatory effects through a two-component signal transduction system encoded by the accessory gene regulator (agr). Induction of agr results in increased expression of a regulatory RNA known as RNAIII (40). RNAIII modulates the production of S. aureus virulence factors at both the transcriptional and the posttranscriptional levels (37). In general, mutation of agr results in increased production of surface proteins, decreased production of exoproteins, and reduced virulence (1, 10, 20, 44).
RNAIII production is also influenced by a second regulatory locus known as the staphylococcal accessory regulator or sar (9). The sar locus encodes three overlapping transcripts, all of which terminate at a common point downstream of the sarA open reading frame (2). SarA binds to enhancer elements associated with the agr and RNAIII promoters (14, 38, 45). Mutation of sarA therefore results in decreased production of RNAIII (9). However, the observation that sarA agr mutants have reduced virulence even by comparison to agr mutants (6, 10) implies that sarA also has regulatory functions that are independent of its interaction with agr. We confirmed this in studies demonstrating that SarA represses expression of the S. aureus collagen adhesin gene (cna) by binding to cis elements associated with the cna promoter (4). It has also been suggested that SarA controls expression of fnbA in a direct fashion (54); however, in this case, SarA appears to act as an activator since sarA mutants exhibit a concomitant reduction in fnbA transcription and fibronectin binding. SarA also modulates the production of several proteases in an agr-independent manner (8).
The experiments in this study were prompted by the observation that almost all studies examining the sarA and agr regulatory loci have been done in the 8325-4 derivative strain RN6390 (1, 6, 10, 20, 39, 50). However, recent studies have demonstrated that strains derived from 8325 (e.g., BB255, 8325-4, RN4220, and RN6390) carry a deletion in rsbU (17, 19, 30). Because rsbU encodes a positive regulator essential for activation of the stress response sigma factor SigB, this deletion results in a phenotype resembling that of a sigB mutant (30). In addition, one of the sarA promoters is sigB dependent (34), and it was recently demonstrated that SarA levels are reduced in an S. aureus strain COL sigB mutant (18). Taken together, these results suggest that regulatory events defined with RN6390 may not be representative of the events observed in clinical isolates of S. aureus. Indeed, mutation of sarA in the blood isolate DB resulted in increased hemolytic activity while the same mutation had the opposite effect in RN6390 (11, 13). Moreover, our conclusion that sarA is the primary regulatory element controlling transcription of cna was done by introducing cna into RN6390 and its corresponding sarA and agr mutants.
To further evaluate the possibility that the regulatory events observed in RN6390 are not representative of the regulatory events observed in other S. aureus strains, we generated sarA and agr mutations in seven strains of S. aureus, including two cna-positive clinical isolates. Phenotypic comparison of these strains confirmed that the regulatory events observed in RN6390 are not representative of the events that occur in clinical isolates of S. aureus.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Wild-type strains were routinely cultured in tryptic soy broth or on tryptic soy agar (TSA) without antibiotic selection. For verification and routine maintenance, mutants were grown with the appropriate antibiotic selection as follows: 2 μg of minocycline ml−1 for agr::tetM; 3 μg of tetracycline ml−1 for sarA::tet, geh::tet, and sspA::tet; 50 μg of kanamycin and 50 μg of neomycin ml−1 for sarA::kan; and 10 μg of chloramphenicol ml−1 for complementation studies involving pSARA. For phenotypic characterization, all strains were grown without antibiotic selection and under low-aeration conditions (120 rpm at a medium/flask volume ratio of 0.5) as described by Lindsay and Foster (32). Upon completion of the experiments, cultures were diluted and plated on both selective medium and nonselective medium to verify the stability of relevant mutations and/or retention of pSARA.
TABLE 1.
Strains used in this study
| Strain or plasmid | Relevant genotypea | Comments and/or parent strain | Source or reference |
|---|---|---|---|
| UAMS-904 | cna | pSARA transduction donor | 4 |
| RN6911 | cna agr | 8325-4 agr-null transduction donor | 40 |
| PC1839 | cna sarA | 8325-4 sarA::kan transduction donor | 8 |
| UAMS-240 | cna sarA | RN6390 sarA::tet transduction donor | This study |
| UAMS-174 | geh | geh::cna | 21 |
| DU1126 | cna sspA | 8325-4 sspA::tet transduction donor | Tim Foster, Trinity University |
| RN6390 | cna | 8325-4 | 40 |
| UAMS-957 | cna sarA | RN6390 | This study |
| UAMS-982 | cna agr | RN6390 | This study |
| UAMS-981 | cna agr sarA | RN6390 | This study |
| UAMS-979 | cna sarA | UAMS-957 (pSARA) | This study |
| UAMS-980 | cna agr sarA | UAMS-981 (pSARA) | This study |
| DB | cna hlb | Blood isolate | 11 |
| UAMS-931 | cna hlb sarA | DB | This study |
| UAMS-932 | cna hlb agr | DB | This study |
| UAMS-933 | cna hlb agr sarA | DB | This study |
| UAMS-1 | fnbB hlb | Osteomyelitis isolate | 20 |
| UAMS-155 | fnbB hlb agr | UAMS-1 | This study |
| UAMS-929 | fnbB hlb sarA | UAMS-1 | This study |
| UAMS-930 | fnbB hlb agr sarA | UAMS-1 | This study |
| UAMS-969 | fnbB hlb sarA | UAMS-929 (pSARA) | This study |
| UAMS-970 | fnbB hlb agr sarA | UAMS-930 (pSARA) | This study |
| UAMS-962 | fnbB hlb sarA sspA | UAMS-1 | This study |
| UAMS-601 | fnbB hlb | Outbreak isolate | 51 |
| UAMS-949 | fnbB hlb agr | UAMS-601 | This study |
| UAMS-950 | fnbB hlb sarA | UAMS-601 | This study |
| UAMS-951 | fnbB hlb agr sarA | UAMS-601 | This study |
| UAMS-972 | fnbB hlb sarA | UAMS-950 (pSARA) | This study |
| UAMS-990 | fnbB hlb agr sarA | UAMS-951 (pSARA) | This study |
| SC-1 | cna | Outbreak isolate | 22 |
| UAMS-310 | sarA | SC-1 | This study |
| UAMS-311 | agr | SC-1 | This study |
| UAMS-318 | agr sarA | SC-1 | This study |
| UAMS-971 | sarA | UAMS-310 (pSARA) | This study |
| UAMS-989 | agr sarA | UAMS-318 (pSARA) | This study |
| S6C | cna hlb | Laboratory strain | 50 |
| UAMS-985 | cna hlb sarA | S6C | This study |
| UAMS-977 | cna hlb agr | S6C | This study |
| UAMS-987 | cna hlb agr sarA | S6C | This study |
| Newman | cna hlb | Laboratory strain | 54 |
| UAMS-988 | cna hlb sarA | Newman | This study |
| UAMS-974 | cna hlb agr | Newman | This study |
| UAMS-975 | cna hlb agr sarA | Newman | This study |
| pSARA | pLI50sarA | 4 | |
| pAZ106 | Gram-positive suicide vector | 8 | |
| pH4 | pUBS1-sarA::kan | 8 | |
| pDG1513 | Tet cassette | 24 | |
| pTOPOtet | pCRII-tet | This study | |
| pH4tet | pUBS1-sarA::tet | This study | |
| pAZ-SARtet | pAZ106-sarA::tet | This study |
Genotype descriptions are limited to chromosomal genes associated with a negative phenotype due to either mutation (agr, geh, sarA, and sspA), lysogeny (hlb), or the absence of the gene (cna and fnbB).
Generation of regulatory mutants.
Φ11-mediated transduction was used to move the tetM agr-null mutation from RN6911 (21) into the seven target strains. Φ11 was also used to transduce the sarA::kan mutation from PC1839 (8) into RN6390, S6C, Newman, DB, and UAMS-1. To generate sarA agr double mutants in these strains, we transduced the agr-null mutation into the sarA::kan mutants. To construct sarA mutations in UAMS-601 and SC-1, both of which are resistant to multiple antibiotics, including kanamycin, the tetracyline gene from pDG1513 (24) was amplified by PCR and cloned into pCRII to give pTOPOtet. The kanamycin cassette was then removed from pH4 (8) and replaced with the tetracycline marker from pTOPOtet to generate pH4tet. The 3.5-kb sarA::tet fragment was excised from pH4tet by using SalI and BamHI, blunt ended with Klenow fragment, and then subcloned into SmaI-cut pAZ106 to generate pAZ-SARtet. After electroporation into the restriction-deficient S. aureus strain RN4220, transformants were selected on TSA containing tetracycline. Transformants were also resistant to erythromycin, which indicated that pAZ-SARtet was integrated into the chromosome via a single crossover. Transductional outcross was then used to move the sarA::tet mutation into RN6390. The mutation was then transduced into UAMS-601 and SC-1. To generate the UAMS-601 and SC-1 sarA agr double mutants, the agr mutation was transduced into the corresponding sarA mutants with selection for minocycline resistance. After preliminary confirmation by PCR, all mutants were verified by Southern blot analysis (20) with probes for the mutated loci (sarA and agr), as well as four unlinked loci (cna, hlb, mec, and fnbA and/or fnbB). The latter were included to distinguish between the donor and recipient strains used for transduction. For complementation studies, pSARA (Table 1) was introduced into the RN6390, SC-1, UAMS-1, and UAMS-601 sarA and sarA agr mutants by Φ11-mediated transduction. The presence of the plasmid was confirmed by restriction profile. The identity of the recipient strains was confirmed by resistance profile and PCR analysis of the sarA and agr loci (data not shown).
Collagen and fibronectin-binding assays.
Binding assays were done with 125I-labeled type I collagen and 125I-labeled fibronectin (Sigma Chemical Co., St. Louis, Mo.) as previously described (20). However, to ensure that binding was not saturated, we decreased the number of cells and increased the amount of labeled protein used in each assay. Specifically, a volume of culture containing 0.5 optical density units of cells was harvested from mid-exponential-phase cultures (A560 ∼ 0.6 to 1.2), washed with phosphate-buffered saline (PBS), and resuspended in 1.0 ml of PBS. Approximately 2 μg of freshly labeled protein was added, and the relative amount of binding was determined as previously described (20). Binding assays were done in duplicate with each set of assays repeated at least twice.
Northern blot analysis.
Total cellular RNA was isolated as previously described (21). For time course studies, cells were harvested at optical densities (A560) of ca. 0.3, 1.5, and 4.0, which correspond to the mid-exponential, late-exponential, and postexponential growth phases, respectively. Total cellular RNA was also collected from overnight cultures (18 h) and from bacteria grown on TSA. For quantitative comparisons of cna and fnbA transcription, Northern slot blots were done as previously described (53). Conventional Northern blots (20) were used to analyze the temporal pattern of sar and RNAIII transcription. Probes were generated by PCR and labeled with digoxigenin as previously described (20). Densitometric analysis was performed by using a UVP Epi Chemi II gel documentation system equipped with a 12-bit charge-coupled device camera and Labworks software (UVP, Inc., Upland, Calif.).
Protease assays.
Soluble protease assays were performed as previously described (50). Zymogram assays for protease activity were carried out as described by Chan and Foster (8). To prepare samples for analysis, 2 ml of standardized, filter-sterilized culture supernatant harvested from an overnight (18-h) culture was concentrated by centrifugation in a YM-3 Centricon filter (Millipore, Bedford, Mass.) at 7,000 × g in a JA-14 rotor for 20 h. A 5-μl aliquot of concentrated supernatant was then combined with an equal volume of 2× sample buffer without β-mercaptoethanol or dithiothreitol and incubated for 20 min at room temperature. Samples were resolved on 12% acrylamide Zymogram Ready Gels containing casein (Bio-Rad, Hercules, Calif.). The proteolytic activity was then detected by staining undegraded casein with GelCode Blue (Pierce, Rockford, Ill.).
Lipase zymogram.
Fluorescence-based lipase assays were adapted from the method of Diaz et al. (15). Overnight culture supernatants were concentrated as described above, and a 5-μl aliquot was electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with precast 4 to 20% gradient gels (Bio-Rad). Resolved gels were soaked in 2.5% Triton X-100 at room temperature for 30 min, washed for 5 min in 50 mM sodium phosphate buffer (pH 7.0), and incubated in 10 ml of 100 μM 4-methylumbelliferyl butyrate (MUFbutyrate) in 50 mM sodium phosphate (pH 7.0). After a 1-min incubation with the MUF-butyrate, the lipase activity was visualized by using the UVP Epi Chemi II gel documentation system.
Hemolysin assays.
Supernatants from overnight cultures were harvested and filter sterilized with a 0.45-μm (pore-size) acetate syringe filter (CAMEO 25AS; Osmonics, Minnetonka, Minn.). After standardization, culture supernatants were combined with 2% rabbit blood in 10 mM Tris-HCl (pH 7.5)-0.9% NaCl. After 15 min at 37°C, unlysed blood cells were pelleted by centrifugation. For cases in which the parent strain had relatively high hemolytic activity (RN6390, Newman, DB, and S6C), 10 μl of supernatant was typically assayed. For all other strains, 50 μl of supernatant was assayed. The same volume of supernatant used with the parent strain was used with the corresponding regulatory mutants. The hemolytic activity was determined by measuring the optical density (A405) of the cell-free supernatant.
Western blot analysis.
Whole-cell lysates used for SarA Western blots were prepared and analyzed as previously described (4). For Hla Western blots, supernatants from overnight cultures were standardized and concentrated as described above. A 5-μl aliquot was electrophoresed under denaturing conditions on precast 4 to 20% gradient gels. Hla Western blots were developed by using a monoclonal anti-Hla antibody as previously described (50).
RESULTS
Regulation of cna transcription and impact on collagen binding.
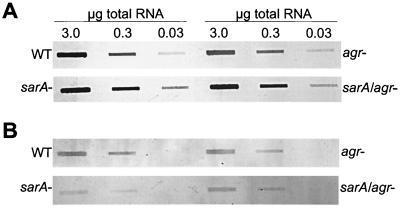
In the cna-positive strains UAMS-1 and UAMS-601, mutation of sarA resulted in a collagen-binding capacity (CBC) that was >2-fold higher than the corresponding parent strain (Table 2). Complementation studies with pSARA confirmed that the increase was a function of the sarA mutation. Mutation of agr resulted in only a slight increase in CBC (Table 2). However, concomitant mutation of sarA and agr appeared to have an additive effect in that the CBC was elevated even by comparison to the sarA mutant. pSARA complementation studies done with sarA agr double mutants restored binding to the level observed in the corresponding agr mutant. These results indicate that mutation of agr has a reproducible effect on the ability to bind collagen; however, we could not demonstrate a reproducible increase in cna transcription in agr mutants (Fig. 1A and Table 2). Northern blot analysis did confirm that the increase in CBC in the sarA and sarA agr mutants was due to increased cna transcription (Fig. 1A and Table 2).
TABLE 2.
Collagen and fibronectin binding in sar and agr mutants
| Parameter and strain | Binding in:
|
|||||
|---|---|---|---|---|---|---|
| Wild typea (dpm) | Mutantb (% binding)
|
|||||
| sar | sar (pSARA) | agr | sar agr | sar agr (pSARA) | ||
| CBC | ||||||
| UAMS-1 | 197,544 ± 36,722 | 217 ± 14c (273 ± 20) | 99 ± 3 | 117 ± 18c (97 ± 10) | 258 ± 15c (255 ± 20) | 1.12 ± 0.03 |
| UAMS-601 | 133,493 ± 22,115 | 235 ± 14c (241 ± 24) | 118 ± 5 | 118 ± 18c (97 ± 1) | 2.58 ± 15c (232 ± 9) | 1.45 ± 0.03 |
| FBC | ||||||
| UAMS-1 | 1,274,323 ± 237,297 | 32 ± 9c (52 ± 3) | 99 ± 17 | 116 ± 20c (82 ± 2) | 38 ± 14c (52 ± 3) | 0.95 ± 0.11 |
| UAMS-601 | 817,297 ± 140,130 | 38 ± 8c (66 ± 3) | 53 ± 8c | 93 ± 18 (107 ± 8) | 23 ± 6c (95 ± 10) | 0.60 ± 0.12c |
| RN6390 | 370,375 ± 77,715 | 74 ± 14c | 101 ± 9 | 347 ± 68c | 151 ± 37 | 2.36 ± 0.19 |
| DB | 1,481,309 ± 188,836 | 34 ± 11c | ND | 106 ± 34 | 42 ± 15c | ND |
| SC-1 | 1,410,816 ± 66,682 | 30 ± 5c | 101 ± 5 | 108 ± 8 | 69 ± 6c | 1.23 ± 0.06 |
| S6C | 2,589,910 ± 56,625 | 48 ± 5c | ND | 110 ± 8 | 76 ± 9 | ND |
| Newman | 295,398 ± 73,212 | 55 ± 2c | ND | 125 ± 15 | 75 ± 10 | ND |
Values for each wild-type strain represent the numbers of counts bound. Results are means ± standard errors of the means and represent the averages of six assays, each of which was done in duplicate.
Values for sarA, agr, and sarA agr mutants are reported as the percent binding relative to the corresponding wild-type strain. Values in parentheses show the percent change in transcription relative to that of the corresponding parent strain based on densitometric analysis of Northern slot blots. Results are means ± standard errors of the means and represent the averages of two Northern blots.
Statistical significance (P < 0.05) as determined by Student’s paired t test to compare each mutant to the corresponding parent strain. In the case of the pSARA-complemented sarA agr mutant, statistical significance was assessed based on comparison to the corresponding agr mutant. ND, not determined.
FIG. 1.
Transcription of cna and fnb genes in UAMS-1 sarA and agr mutants. Northern slot blots were done with probes corresponding to cna (A) or fnbA (B). RNA samples were taken from cultures in the mid- to late-exponential growth phases (A560 ∼ 1.5). Numbers above the lanes in panel A correspond to the total amount of cellular RNA spotted in each well. The same amounts apply to the lanes in panel B. For densitometric analysis, light emission was determined, and the values obtained from all unsaturated lanes were averaged to derive the relative values summarized in Table 2. WT, wild type.
Regulation of fnbA transcription and impact on fibronectin binding.
Because all S. aureus strains encode at least one fnb gene (43), we could assess the impact of sarA and agr on fibronectin binding in all seven strains. Without exception, mutation of sarA resulted in a decreased capacity to bind fibronectin. In RN6390, the decrease was relatively modest in that the sarA mutant bound almost 75% of the fibronectin bound by the wild-type parent strain (Table 2). In other strains, the effect of the sarA mutation was more significant in that the mutants bound 20 to 55% of the fibronectin bound by the corresponding parent strain (Table 2). Conversely, mutation of agr in RN6390 resulted in a 3.5-fold increase in fibronectin-binding capacity (FBC), whereas mutation of agr in each of the other strains had little effect (Table 2).
The amount of fnb mRNA observed in the UAMS sarA mutants was reduced compared to the corresponding parent strain (Fig. 1B and Table 2). The reduction in fnb transcription can be attributed to fnbA because neither UAMS-1 nor UAMS-601 encodes fnbB (data not shown). However, in both strains, the decrease in FBC was greater than the reduction in fnbA transcription (Table 2). The disparity suggests that the reduction in FBC involves factors other than reduced fnbA transcription.
Impact of sarA and agr on proteolytic activity.
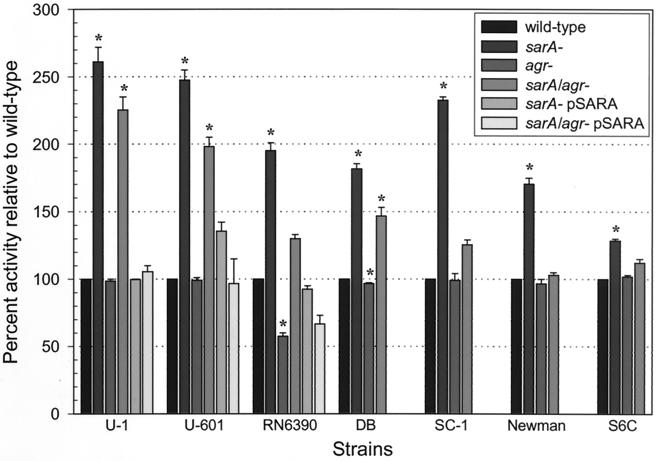
Mutation of sarA resulted in an increase in proteolytic activity ranging from 1.2-fold (Newman) to >2.6-fold (UAMS-1) (Fig. 2). Mutation of agr resulted in reduced proteolytic activity although the effect was obvious only in RN6390. This may be due to the fact that RN6390 supernatants had over twice as much proteolytic activity as those from any other wild-type strain (data not shown). Nevertheless, in all strains, the sarA agr double mutant had a phenotype that was intermediate by comparison to the corresponding sarA and agr mutants (Fig. 2). This indicates that mutation of agr had a negative impact on protease production in all strains.
FIG. 2.
Protease activity in sarA and agr mutants. The proteolytic activity in cell-free culture supernatants was determined by using azocasein. The total protease activity in sarA and agr mutants is shown as the percent activity relative to the corresponding parent strain, which was arbitrarily set at 100%. Values represent the results of at least two independent assays, both of which were done in duplicate. Results are reported as the mean ± the standard error of the mean (SEM). Asterisks denote statistical significance (P < 0.05), as determined by using Student’s paired t test to compare each mutant to the corresponding parent strain. In the case of the pSARA-complemented sarA agr mutant, statistical significance was assessed based on comparison to the corresponding agr mutant. Because the value for all parent strains was set to 100%, this panel does not reflect differences in the amount of protease produced by each parent strain.
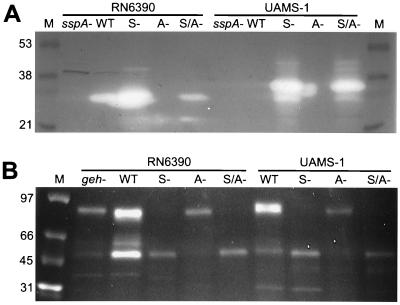
Zymogram analysis indicated that differences in the amount of proteolytic activity observed in the wild-type strains, as well as changes in proteolytic activity observed in the sarA and agr mutants, were due, at least in part, to the production of the staphylococcal serine protease (SspA). More specifically, a single predominant band was detected by zymogram analysis of supernatants harvested from RN6390 (Fig. 3A). Comparison of RN6390 with an RN6390 sspA mutant confirmed that the predominant band was the product of a gene encoded within the ssp operon (48). Zymogram analysis also confirmed that production of this protease was increased in the RN6390 sarA mutant and decreased in the RN6390 agr mutant (Fig. 3A). There was no visible proteolytic band in supernatants harvested from cultures of DB (data not shown), UAMS-601 (data not shown), or UAMS-1 (Fig. 3A). However, a single predominant band was observed in the corresponding sarA and sarA agr mutants. Although this band was slightly larger in the UAMS strains, comparison with a UAMS-1 sspA sarA mutant confirmed that the predominant protease produced by these strains is also encoded within the ssp operon (Fig. 3A). It was not possible to determine whether mutation of agr resulted in decreased production of this protease in DB or the UAMS strains because the zymogram bands were not visible in the wild-type strains or the corresponding agr mutants (Fig. 3A). However, in all three strains, the intensity of the proteolytic band in the sarA agr mutant was decreased compared to the corresponding sarA mutant (Fig. 3A). These results indicate that mutation of agr results in reduced production of ssp-encoded proteases in all strains.
FIG. 3.
Zymogram analysis of protease and lipase production. Cell-free culture supernatants were concentrated by centrifugation and examined by using casein gels (A) or a direct fluorescence assay for lipase activity (B). The results obtained with RN6390 and UAMS-1 are shown for comparison. The results obtained with UAMS-1 were essentially identical to the results observed with UAMS-601 and DB (data not shown). The sspA mutant used as a negative control for RN6390 was generated in the wild-type strain. The sspA mutant used as a negative control for UAMS-1 was generated in the UAMS-1 sarA mutant because the wild-type strain did not produce detectable amounts of protease. The geh mutant used as a control in panel B is in the RN6390 background. WT, wild-type; S−, sarA mutant; A−, agr mutant; S/A−, sarA agr mutant. The lane marked “M” contains molecular size markers.
Impact of sarA and agr on lipase production.
Zymogram analysis of supernatants from RN6390 and UAMS-1 indicated the presence of two prominent lipolytic enzymes (Fig. 3B). As with protease production, lipase production was highest in RN6390, as evidenced by the relative intensity of both bands. This increased intensity was especially apparent with respect to the lower-molecular-weight band (Fig. 3B). Although present in greatly reduced amounts, both bands were also present in an RN6390 glycerol ester hydrolase (geh) mutant, indicating that these bands contain more than one lipase. The larger of the two bands was absent in all sarA and sarA agr mutants, whereas the smaller one was absent in the agr mutants. In all cases, the intensity of the remaining bands was reduced by comparison to the corresponding wild-type strain. In contrast to previous reports (13), these results indicate that lipolytic activity is reduced in both sarA and agr mutants.
Impact of sarA and agr on hemolytic activity.
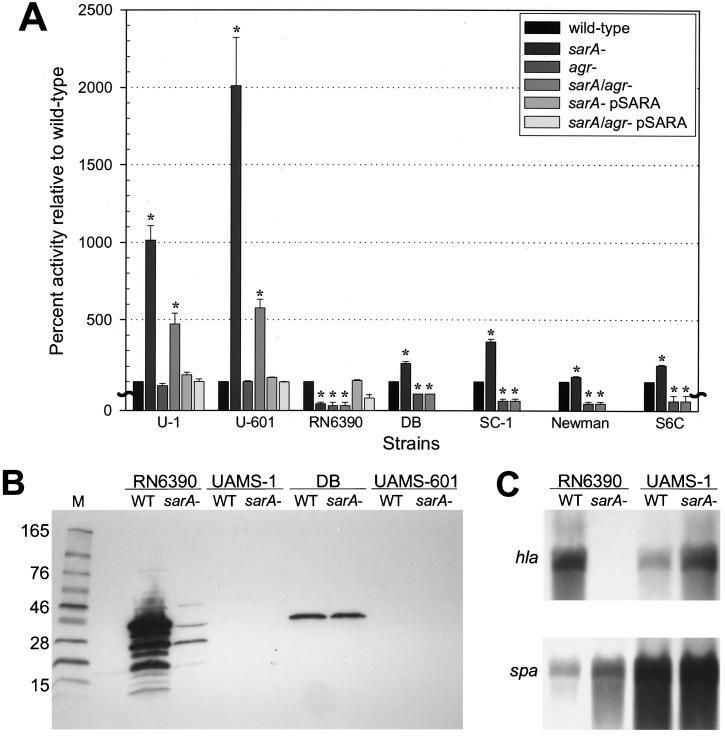
In RN6390, mutation of sarA or agr resulted in reduced hemolytic activity (Fig. 4A). Mutation of agr had the same effect in all other strains, although the decrease was not readily apparent in UAMS-1 or UAMS-601 because the hemolytic activity in these strains was limited. In contrast, mutation of sarA resulted in increased hemolytic activity in all strains other than RN6390 (Fig. 4A). Complementation studies with pSARA confirmed that the sarA mutation was responsible for the increased hemolytic activity in the UAMS strains and the decreased hemolytic activity in RN6390.
FIG. 4.
Hemolytic activity in sarA and agr mutants. (A) The total hemolytic activity in sarA and agr mutants is shown as the percent activity relative to the corresponding parent strain, which was arbitrarily set at 100%. Values represent the results of at least two independent assays, both of which were done in duplicate. Results are reported as the mean ± the SEM. Asterisks denote statistical significance (P < 0.05), as determined by using Student’s paired t test to compare each mutant to the corresponding parent strain. In the case of the pSARA-complemented sarA agr mutant, statistical significance was assessed based on comparison to the corresponding agr mutant. Because the value for all parent strains was set to 100%, this panel does not reflect differences in the amount of hemolytic activity produced by each parent strain. (B) Western blot analysis with an anti-Hla monoclonal antibody. Cell-free culture supernatants were concentrated by centrifugation prior to analysis. (C) Northern blot analysis of RNA isolated from cells in stationary phase with probes corresponding to hla (top) and spa (bottom). Densitometric analysis indicated that the increase in hla transcription in the UAMS-1 sarA mutant was ca. 2.5-fold. We also demonstrated, as a control, that mutation of sarA in RN6390 resulted in increased spa transcription, whereas it had little effect in UAMS-1 (C) or UAMS-601 (data not shown). WT, wild type.
To test whether the changes in hemolytic activity reflect changes in Hla production, we carried out Western blots with an anti-Hla monoclonal antibody. Decreased production of Hla in the RN6390 sarA mutant was apparent (Fig. 4B). In DB, we could detect Hla but could not demonstrate an increase in its production, presumably because there was only a twofold increase in hemolytic activity in the DB sarA mutant (Fig. 4A). We were unable to detect Hla in supernatants from the UAMS-1 and UAMS-601 sarA mutants. This may also be a matter of sensitivity since these strains produced only <10% of the amount of Hla produced by RN6390 (data not shown). We could demonstrate increased transcription of hla in the UAMS-601 (data not shown) and UAMS-1 sarA mutants (Fig. 4C). However, based on densitometric analysis of Northern blots (data not shown), the increase in hla transcription in the UAMS strains was modest (∼2.5-fold) compared to the increase in hemolytic activity (∼10-fold). These results suggest that the increased hemolytic activity observed in UAMS-1 and UAMS-601 may be due, at least in part, to the increased production of a hemolysin other than Hla.
Temporal pattern of sar and RNAIII transcription.
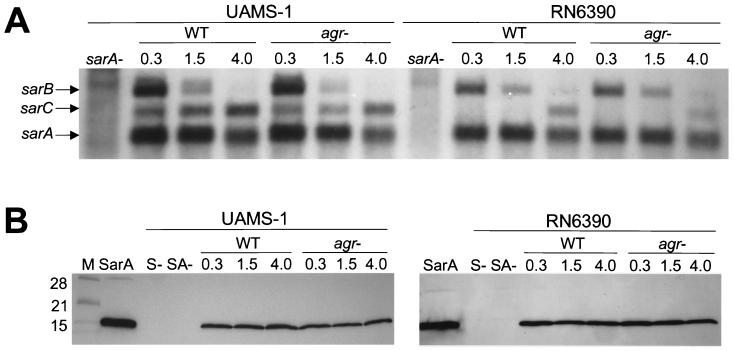
The temporal pattern of sar transcription was identical in all of the strains we examined, including RN6390. More directly, the sarA and sarB transcripts were produced predominately during the exponential growth phase, while the sarC transcript was not produced until cultures entered the postexponential growth phase (Fig. 5A). Although the temporal pattern was consistent, it did appear that the sarC transcript in RN6390 was present in reduced amounts compared to the sarA and sarB transcripts. Because the sarC promoter is SigB dependent (34), it is possible that this reduction is due to the rsbU deletion in RN6390. However, we could not demonstrate a definitive difference in the amount of SarA produced by UAMS-1 and RN6390 (Fig. 5B). Western blots also indicated that SarA is produced constitutively in all strains and that its production is unaffected by mutation of agr (Fig. 5B).
FIG. 5.
Transcription of sar and production of SarA. (A) Northern blots were done by using a probe corresponding to sarA. The identity of the sar transcripts is indicated on the left. (B) Western blot done with polyclonal anti-SarA antibody. Purified SarA (4) was included as a control. In both panels, the numbers above each lane indicate the optical density (A560) of the culture at the time of RNA harvest (in panel A) or preparation of cell lysates (in panel B). WT, wild type; S−, sarA mutant; SA−, sarA agr mutant. The lane marked “M” contains molecular size markers.
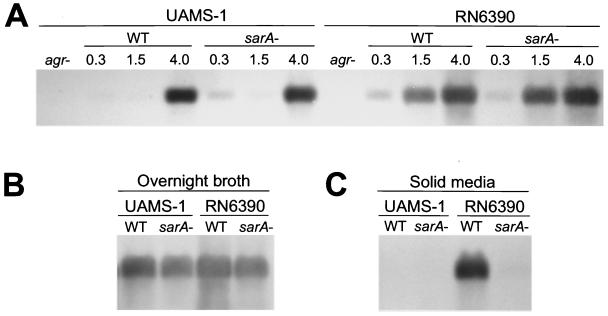
The temporal pattern of RNAIII transcription was similar in all strains to the extent that the amount of RNAIII increased to a maximum as cultures entered postexponential growth phase (Fig. 6A). However, by comparison to RN6390, RNAIII transcription was delayed in both UAMS-1 (Fig. 6A) and UAMS-601 (data not shown). Mutation of sarA had little effect on the temporal pattern of RNAIII production and the overall amount of RNAIII present in post-exponential-phase cultures (Fig. 6A). There was a slight reduction in the amount of RNAIII in UAMS-1 and RN6390 sarA mutants when cells were harvested from overnight, stationary-phase cultures (Fig. 6B). The reduced production of RNAIII in the RN6390 sarA mutant was even more evident when we compared cells harvested from agar plates (Fig. 6C). In UAMS-1, neither the wild-type strain nor the corresponding sarA mutant produced detectable amounts of RNAIII when harvested from plates (Fig. 6C). Again, the characteristics observed in UAMS-1 were also observed in UAMS-601 (data not shown).
FIG. 6.
Transcription of RNAIII. Northern blots were done by using a probe corresponding to RNAIII. (A) Time course with RNA isolated from cells grown in broth culture. The numbers above each lane indicate the optical density (A560) of the culture at the time of RNA harvest. (B) Northern blots done with RNA isolated from overnight (18-h) broth cultures. (C) Northern blots done with RNA harvested from cells grown overnight on TSA. WT, wild type.
DISCUSSION
Several recent studies have emphasized the clonal nature of S. aureus and suggested that a limited number of clones may have particular clinical relevance. For instance, Booth et al. (7) characterized 405 S. aureus isolates by pulsed-field gel electrophoresis and identified 91 distinct profiles, 5 of which were responsible for >65% of all infections. At least 93% of the strains in three of these groups were cna positive. In addition, all strains in four of the five groups encoded fnbA but not fnbB (7). This correlation is significant in light of studies indicating that cna is relatively rare and that most S. aureus strains encode both fnb genes (43, 49). Finally, Papakyriacou et al. (42) found that the two epidemic strains of S. aureus that are collectively responsible for half of all infections in Canada have a unique phenotype defined by the limited production of exotoxins and an enhanced capacity to bind host proteins. It was suggested that this phenotype favors the colonization phase of infection and that it might be directly related to the prevalence of these strains among clinical isolates.
Interestingly, the UAMS strains are clinical isolates that encode cna but not fnbB, produce limited amounts of exoproteins, and have a high binding capacity for host proteins, including collagen and fibronectin. All of these characteristics distinguish the UAMS strains from RN6390. These results suggest that RN6390 may not be representative of the most prominent clinical isolates. In addition, the possibility that RN6390 has a specific attribute (e.g., the rsbU deletion) that impacts on the sarA regulatory pathway suggests that the regulatory circuits defined in RN6390 may be distinct compared to other strains. Indeed, the limited studies that have examined the regulatory roles of sarA in strains other than RN6390 have confirmed the existence of strain-dependent differences (11, 13). Nevertheless, RN6390 has been used for almost all of the studies examining global regulation in S. aureus and its impact on virulence.
To determine whether the phenotype of RN6390 and the impact of the sarA and agr loci are representative of other strains, we carried out a comparison of sarA and agr mutants by using seven strains of S. aureus. With respect to the ability to bind host proteins, mutation of sarA resulted in increased cna transcription and an elevated CBC in both UAMS-1 and UAMS-601. Because the same effect was observed when cna was introduced into the chromosome of RN6390 (21), these results indicate that the rsbU mutation in RN6390 is irrelevant with respect to the regulation of cna transcription.
In all cases, mutation of sarA resulted in a reduced FBC. The mechanism by which sarA modulates the ability to bind fibronectin remains unclear. Wolz et al. (54) recently demonstrated that mutation of sarA in Newman results in reduced transcription of fnbA but not of fnbB. This observation, together with the fact that the UAMS strains encode fnbA but not fnbB, suggests that the reduced FBC in the UAMS strains may be mediated at the transcriptional level. However, in agreement with the findings of Karlsson et al. (28), our Northern blot analysis indicated that mutation of sarA results in only a modest reduction in fnbA transcription. These results suggest that the decrease in fnbA transcription is not entirely responsible for the reduced FBC observed in at least some S. aureus strains.
It has also been demonstrated that the fibronectin-binding proteins (FnBPs) are sensitive to degradation by SspA (V8) protease (35) and that mutation of sarA results in the increased production of several proteases, including SspA (8, 28). We believe our results support the hypothesis that protease degradation is a primary mechanism that modulates the ability to bind fibronectin. For example, there was an inverse relationship between protease production and FBC in all cases, including all but one of the wild-type strains. The same correlation was observed by Rice et al. (47). The only exception we found was Newman, which had a low FBC despite the fact that it produced relatively little protease. Also, in every strain (including Newman) mutation of sarA resulted in increased protease production and a reduced FBC. Although this trend was consistent, RN6390 was unique in that the impact of the sarA mutation on protease production and on FBC was modest compared to all other strains. Similarly, mutation of agr in RN6390 resulted in a dramatic decrease in protease production and a corresponding increase in FBC. In other strains, mutation of agr had little impact on protease production or FBC. Presumably, mutation of agr has a reduced impact on protease production in these strains because they already produce limited amounts. Taken together, these results suggest that the regulatory impact of sarA on FBC is mediated primarily through its impact on the production of proteases. However, they do not allow us to rule out the possibility that sarA modulates FBC at the transcriptional level in at least some strains.
We attempted to definitively address the correlation between protease production and FBC by mutating sspA in the RN6390 and UAMS-1 sarA mutants. The fact that mutation of sspA had no effect on FBC in either strain (data not shown) was somewhat surprising since Karlsson et al. (28) concluded that SspA is the primary protease responsible for the reduced amounts of cell wall-associated FnBP observed in sarA mutants. However, this conclusion was based on release of the protein from the cell surface and not on the ability to bind fibronectin, and Rice et al. (48) found that the binding of soluble fibronectin is not proportional to the amount of cell wall-associated FnBP. More directly, they found that mutation of sspA resulted in a 16-fold increase in surface-associated FnBP but only a twofold increase in FBC. This suggests that our results may not reflect the total increase in surface-associated FnBP associated with mutation of sspA. It is also possible that changes in the production of other proteases compensate for the lack of SspA. Indeed, mutation of sspA in a UAMS-1 sarA mutant had only a slight impact on proteolytic activity, as measured in azocasein assays (data not shown), despite the fact that the effect was readily apparent in zymograms. These results are consistent with the observation that S. aureus clinical isolates have the potential to produce a number of proteases other than SspA (31, 46). It has also been demonstrated that several of these are negatively regulated by sarA (8).
Our results also suggest that the increased protease production observed in sarA mutants may have an impact on other phenotypes. For instance, zymogram analysis indicated the presence of two prominent lipolytic enzymes. The larger of the two bands was absent in sarA mutants and sarA agr double mutants, while the smaller one was absent in agr mutants. There are two possible explanations for this. The first is that these bands represent different lipolytic enzymes, one of which is regulated by sarA, whereas the other is regulated by agr. The alternative is that the two bands are derivatives of the same lipolytic enzyme and that the regulatory effects of sarA and agr are mediated through their impact on the production of proteases. This hypothesis is consistent with the fact that S. aureus lipases are produced as active, 85- to 87-kDa proenzymes that are converted extracellularly to a mature form that ranges between 44 and 46 kDa in size (23). These sizes are consistent with the size of the two most prominent bands observed in our zymograms. The fact that mutation of sarA resulted in elimination of the larger band is consistent with increased proteolytic conversion of the proenzyme to the mature form. Similarly, the fact that mutation of agr resulted in elimination of the smaller band is consistent with decreased processing of the proenzyme. Further support for this hypothesis comes from the observation that RN6390 produces more protease and had a greater proportion of the smaller band than any of the other strains. It should also be noted that the amount of active enzyme present in both sarA and agr mutants was reduced by comparison to the corresponding parent strains. This is in contrast to previous reports indicating that lipase production is increased in both RN6390 and DB sarA mutants (11, 13). While the reduction observed in sarA mutants could be due to degradation rather than to reduced transcription of the corresponding gene, mutation of agr results in reduced proteolytic activity, which means that the reduction in the larger lipase must be due to something other than proteolytic degradation.
It has also been suggested that the increased protease production observed in RN6390 sarA mutants may contribute to reduced hemolytic activity (8). However, in every other strain we examined, mutation of sarA resulted in an increase in hemolytic activity, and it is more difficult to envision how an increased hemolytic activity could be due to an increase in protease production. All strains other than RN6390 and SC-1 are lysogenized with an hlb-converting phage (5) or, in the case of DB, with a defective phage fragment (data not shown). This is consistent with the observation that hemolytic activity was most apparent when assays were done with rabbit blood and suggests that the increased activity reflects the activity of alpha-toxin. However, we could not confirm that Hla production was significantly increased in the UAMS sarA mutants. An alternative explanation is that the increased hemolytic activity observed in the UAMS sarA mutants is due to a hemolysin other than alpha-toxin. Several lines of evidence support this hypothesis. For instance, the amount of hla transcript detected in RN6390 and the UAMS-1 sarA mutant was not significantly different despite the fact that Hla could be detected by Western blot only in RN6390. This suggests that UAMS-1 and its sarA mutant may have a posttranscriptional defect in Hla production. Such defects (e.g., nonsense mutations) have been observed in clinical isolates of S. aureus (5). In any event, the concomitant increase in hemolytic and proteolytic activity in sarA mutants other than RN6390 suggests that the overall increase in the production of this hemolysin may be underestimated by our results.
We found that the temporal pattern of sar transcription was identical in RN6390 and UAMS-1; however, it did appear that the amount of the sarC transcript was reduced in RN6390. Similarly, while the temporal pattern of RNAIII transcription was consistent to the extent that the amount of RNAIII reached a maximum as cultures entered postexponential growth, RNAIII production was detectable earlier in RN6390 than in either of the UAMS strains. This may be due to differences in agr specificity group (26) since sequence analysis of the agrC and agrD variable regions confirmed that both of the UAMS strains belong to agr group III (data not shown). Alternatively, these differences could be related to the rsbU mutation and its impact on sar transcription and/or the production of SarA (3, 18). Although our results did not reveal a significant difference in the amount of SarA produced by any of the strains we examined, it is certainly possible that subtle changes in SarA production can have an impact on phenotype. Indeed, Gertz et al. (18) confirmed that mutation of sigB results in a quantitative reduction in SarA production, and two groups have demonstrated that restoration of rsbU in the 8325 strain BB225 results in decreased hemolytic activity (19) and increased production of the FnBPs (3). Taken together, these results suggest that the differences we observed between RN6390 and the UAMS strains are due to the rsbU mutation in RN6390 and account for why the RN6390 phenotype is not representative of the phenotype observed in clinical isolates of S. aureus.
It is difficult to predict how the differences between RN6390 and the other strains we examined would impact virulence. Although RN6390 is virulent in other models (1, 6, 10, 39), we used a rabbit osteomyelitis model (52), and found this strain to be significantly less virulent than UAMS-1 even after the introduction of cna into the RN6390 chromosome (data not shown). The reasons for this are not completely understood but could be related to the enhanced capacity of UAMS-1 to bind host proteins. This is consistent with our demonstration that mutation of cna in UAMS-1 results in a reduced capacity to cause osteomyelitis (16). Since RN6390 has a limited capacity to bind host proteins, the attenuation of RN6390 sarA mutants (6, 10, 12, 39) might be due to a reduced capacity to make exotoxins. Since RN6390 sarA mutants make less alpha-toxin, this is consistent with the fact that a similar reduction in virulence has been observed with alpha-toxin mutants (29, 36, 41). Whether mutation of sarA would have the same effect in strains other than RN6390 remains unclear. Although other studies found that mutation of sarA in DB attenuated virulence in a rabbit endocarditis model and a murine septic arthritis model (12, 39), DB is a cna-negative strain. This is relevant because the ability to bind collagen contributes to virulence in the same endocarditis model (25). Considering that mutation of sarA in cna-positive strains results in an enhanced capacity to bind collagen and increased hemolytic activity, it is possible that the strain-dependent differences we have observed are clinically relevant. For that reason, it is important to confirm the relevance of studies done with 8325-4 derivatives such as RN6390 by examining the impact of sarA and agr on virulence in other strains. To that end, we are currently evaluating the relative virulence of RN6390 and UAMS-1 sarA mutants by using various animal models of staphylococcal disease.
.
Acknowledgments
We thank Allen Gies for his excellent technical assistance. We also thank Tim Foster, Pan Chan, Simon Foster, and Dan Zeigler for providing strains and plasmids essential to this work.
This work was supported by a grant to M.S.S. from the National Institute of Allergy and Infectious Disease (AI43356).
Editor: E. I. Tuomanen
REFERENCES
- 1.Abdelnour, A., S. Arvidson, T. Bremell, C. Ryden, and A. Tarkowski. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 61:3879–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, M. G., J. H. Heinrichs, and A. L. Cheung. 1996. The molecular architecture of the sar locus in Staphylococcus aureus. J. Bacteriol. 178:4563–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins, J. S., A. F. Gillaspy, T. M. Rechtin, B. K. Hurlburt, and M. S. Smeltzer. 1999. The staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesin gene (cna) in an agr-independent manner. Mol. Microbiol. 33:317–326. [DOI] [PubMed] [Google Scholar]
- 5.Bohach, G. A., and T. J. Foster. 2000. Staphylococcus aureus exotoxins, p.367–378. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 6.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65:1550–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth, M. C., L. M. Pence, P. Mahasreshti, M. C. Callegan, and M. S. Gilmore. 2001. Clonal associations among Staphylococcus aureus isolates from various sites of infection. Infect. Immun. 69:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, P. F., and S. J. Foster. 1998. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J. Bacteriol. 180:6232–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung, A. L., M. G. Bayer, and J. H. Heinrichs. 1997. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J. Bacteriol. 179:3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94:1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 89:6462–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung, A. L., M. R. Yeaman, P. M. Sullam, M. D. Witt, and A. S. Bayer. 1994. Role of the sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect. Immun. 62:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung, A. L., and P. Ying. 1994. Regulation of alpha- and beta-hemolysins by the sar locus of Staphylococcus aureus. J. Bacteriol. 176:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien, Y., and A. L. Cheung. 1998. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J. Biol. Chem. 273:2645–2652. [DOI] [PubMed] [Google Scholar]
- 15.Diaz, P., N. Prim, and F. I. Javier-Pastor. 1999. Direct fluorescence-based lipase activity assay. BioTechniques 27:696–700. [DOI] [PubMed] [Google Scholar]
- 16.Elasri, M. O., J. R. Thomas, R. A. Skinner, J. S. Blevins, K. E. Beenken, C. L. Nelson, and M. S. Smeltzer. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone, in press. [DOI] [PubMed]
- 17.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 1999. Regulation of σB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558–566. [DOI] [PubMed] [Google Scholar]
- 18.Gertz, S., S. Engelmann, R. Schmid, A. K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 182:6983–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillaspy, A. F., S. G. Hickmon, R. A. Skinner, J. R. Thomas, C. L. Nelson, and M. S. Smeltzer. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillaspy, A. F., C. Y. Lee, S. Sau, A. L. Cheung, and M. S. Smeltzer. 1998. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect. Immun. 66:3170–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz, M. B., M. E. Mulligan, R. Kwok, H. O’Brien, C. Caballes, and J. P. Garcia. 1992. Management and epidemiologic analyses of an outbreak due to methicillin-resistant Staphylococcus aureus. Am. J. Med. 92:607–614. [DOI] [PubMed] [Google Scholar]
- 23.Gotz, F., H. M. Verheij, and R. Rosenstein. 1998. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem. Phys. Lipids 93:15–25. [DOI] [PubMed] [Google Scholar]
- 24.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336. [DOI] [PubMed] [Google Scholar]
- 25.Hienz, S. A., T. Schennings, A. Heimdahl, and J. Flock.-I. 1996. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J. Infect. Dis. 174:83–88. [DOI] [PubMed] [Google Scholar]
- 26.Jarraud, S., G. J. Lyon, A. M. Figueiredo, L. Gerard, F. Vandenesch, J. Etienne, T. W. Muir, and R. P. Novick. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182:6517–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji, G., R. C. Beavis, and R. P. Novick. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92:12055–12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson, A., P. Saravia-Otten, K. Tegmark, E. Morfeldt, and S. Arvidson. 2001. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to upregulation of extracellular proteases. Infect. Immun. 69:4742–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kielian, T., A. Cheung, and W. F. Hickey. 2001. Diminished virulence of an alpha-toxin mutant of Staphylococcus aureus in experimental brain abscess. Infect. Immun. 69:6902–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroda, T. M., Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, and J. Liam. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225–1240. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay, J. A., and S. J. Foster. 1999. Interactive regulatory pathways control virulence determinant production and stability in response to environmental conditions in Staphylococcus aureus. Mol. Gen. Genet. 262:323–331. [DOI] [PubMed] [Google Scholar]
- 33.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. [DOI] [PubMed] [Google Scholar]
- 34.Manna, A. C., M. G. Bayer, and A. L. Cheung. 1998. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J. Bacteriol. 180:3828–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGavin, M. J., C. Zahradka, K. Rice, and J. E. Scott. 1997. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect. Immun. 65:2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menzies, B. E., and D. S. Kernodle. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morfeldt, E., D. Taylor, A. von Gabain, and S. Arvidson. 1995. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 14:4569–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morfeldt, E., K. Tegmark, and S. Arvidson. 1996. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol. Microbiol. 21:1227–1237. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson, I. M., T. Bremell, C. Ryden, A. L. Cheung, and A. Tarkowski. 1996. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect. Immun. 64:4438–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Callaghan, R. J., M. C. Callegan, J. M. Moreau, L. C. Green, T. J. Foster, O. M. Hartford, L. E. Engel, and J. M. Hill. 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 65:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papakyriacou, H., D. Vaz, A. Simor, M. Louie, and M. J. McGavin. 2000. Molecular analysis of the accessory gene regulator (agr) locus and balance of virulence factor expression in epidemic methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 181:990–1000. [DOI] [PubMed] [Google Scholar]
- 43.Peacock, S. J., N. P. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23–31. [DOI] [PubMed] [Google Scholar]
- 44.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p.55–81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, N.Y.
- 45.Rechtin, T. M., A. F. Gillaspy, M. A. Schumacher, R. G. Brennan, M. S. Smeltzer, and B. K. Hurlburt. 1999. Characterization of the SarA virulence gene regulator of Staphylococcus aureus. Mol. Microbiol. 33:307–316. [DOI] [PubMed] [Google Scholar]
- 46.Reed, S. B., C. A. Wesson, L. E. Liou, W. R. Trumble, P. M. Schlievert, G. A. Bohach, and K. W. Bayles. 2001. Molecular characterization of a novel Staphylococcus aureus serine protease operon. Infect. Immun. 69:1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice, K., M. Huesca, D. Vaz, and M. J. McGavin. 2001. Variance in fibronectin binding and fnb locus polymorphisms in Staphylococcus aureus: identification of antigenic variation in a fibronectin binding protein adhesin of the epidemic CMRSA-1 strain of methicillin-resistant S. aureus. Infect. Immun. 69:3791–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice, K., R. Peralta, D. Bast, J. de Azavedo, and M. J. McGavin. 2001. Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease. Infect. Immun. 69:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeltzer, M. S., A. F. Gillaspy, F. L. Pratt, M. D. Thames, and J. J. Iandolo. 1997. Prevalence and chromosomal map location of Staphylococcus aureus adhesin genes. Gene 196:249–259. [DOI] [PubMed] [Google Scholar]
- 50.Smeltzer, M. S., M. E. Hart, and J. J. Iandolo. 1993. Phenotypic characterization of xpr, a global regulator of extracellular virulence factors in Staphylococcus aureus. Infect. Immun. 61:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smeltzer, M. S., F. L. Pratt, A. F. Gillaspy, and L. A. Young. 1996. Genomic fingerprinting for epidemiological differentiation of Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 34:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smeltzer, M. S., J. R. Thomas, S. G. Hickmon, R. A. Skinner, C. L. Nelson, D. Griffith, T. R. Parr, Jr., and R. P. Evans. 1997. Characterization of a rabbit model of staphylococcal osteomyelitis. J. Orthop. Res. 15:414–421. [DOI] [PubMed] [Google Scholar]
- 53.Snodgrass, J. L., N. Mohamed, J. M. Ross, S. Sau, C. Y. Lee, and M. S. Smeltzer. 1999. Functional analysis of the Staphylococcus aureus collagen adhesin B domain. Infect. Immun. 67:3952–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolz, C., P. Pohlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36:230–243. [DOI] [PubMed] [Google Scholar]