Abstract
Papain (from papaya latex; EC 3.4.22.2) and Pronase (from Streptomyces griseus; EC 3.4.24.31) caused optimum depolymerization of chitosan at pH 3.5 and 37 °C, resulting in LMMC (low molecular mass chitosan) and chito-oligomeric–monomeric mixture. The yield of the latter was 14–16% and 14–19% respectively for papain- and Pronase-catalysed reactions, depending on the reaction time (1–5 h). HPLC revealed the presence of monomer(s) and oligomers of DP (degree of polymerization) 2–6, which was also confirmed by matrix-assisted laser-desorption ionization–time-of-flight MS. Along with the chito-oligomers, the appearance of only GlcNAc (N-acetylglucosamine) in Pronase-catalysed chitosanolysis was indicative of its different action pattern compared with papain. Fourier-transform infrared, liquid-state 13C-NMR spectra and CD analyses of chito-oligomeric–monomeric mixture indicated the release of GlcNAc/GlcNAc-rich oligomers. The monomeric sequence at the non-reducing ends of chito-oligomers was elucidated using N-acetylglucosaminidase. The chito-oligomeric–monomeric mixture showed better growth inhibitory activity towards Bacillus cereus and Escherichia coli compared with native chitosan. Optimum growth inhibition was observed with chito-oligomers of higher DP having low degree of acetylation. The latter caused pore formation and permeabilization of the cell wall of B. cereus, whereas blockage of nutrient flow due to the aggregation of chito-oligomers–monomers was responsible for the growth inhibition and lysis of E. coli, which were evidenced by scanning electron microscopy analysis. The spillage of cytoplasmic enzymes and native PAGE of the cell-free supernatant of B. cereus treated with chito-oligomeric–monomeric mixture further confirmed bactericidal activity of the latter. Use of papain and Pronase, which are inexpensive and easily available, for chitosanolysis, is of commercial importance, as the products released are of considerable biomedical value.
Keywords: bactericidal activity, chitosan, chitosanolysis, oligomer, papain, Pronase
Abbreviations: BHI, brain–heart infusion; CFU, colony-forming unit; DA, degree of acetylation; DP, degree of polymerization; FTIR, Fourier-transform infrared; GPC, gel-permeation chromatography; LMMC, low molecular mass chitosan; MALDI–TOF-MS, matrix-assisted laser-desorption ionization–time-of-flight MS; RP, reverse phase; SEM, scanning electron microscopy
INTRODUCTION
Chitosan is a natural, non-toxic, co-polymer of GlcN (glucosamine) and GlcNAc (N-acetylglucosamine) obtained after partial de-N-acetylation of chitin, which, in turn, is a major component of the shells of crustaceans and found commercially in the offal of marine food processing industry [1]. In spite of its abundance and various biofunctionalities, utilization of chitosan is restricted, owing to its high molecular mass, high viscosity and, thus, low absorption for in vivo applications [2]. Recent studies on chitosan depolymerization have drawn considerable attention, since the products obtained are easily water-soluble and also possess versatile biofunctional properties such as antitumour [3–5], immuno-stimulating [2] and antimicrobial activities [6,7] and are being used for alleviating problems due to osteoarthritis–gastritis [8].
Chitosanolysis could be achieved by physical, chemical or enzymatic means, of which enzymatic depolymerization is preferred, since reaction and thus product formation could be controlled by means of pH, temperature and reaction time [9]. Since chitosanase, the enzyme for chitosanolysis, is expensive and unavailable in bulk, there was a need to identify an alternative and inexpensive way for obtaining chitosan depolymerization products. During search for the same, susceptibility of chitosan to various non-specific enzymes such as cellulase, pectinase, pepsin, papain and wheatgerm lipase was reported, although the enzyme purity was in doubt [10,11].
Recently, we have shown that a homogeneous isoenzyme of Aspergillus niger pectinase with polygalacturonase activity, as well as highly purified preparations of pepsin, papain and Pronase, could depolymerize chitosan quantitatively, yielding LMMC (low molecular mass chitosan), chito-oligomers and monomers [12–14]. In the present study, emphasis was given for isolation and biophysical characterization of chito-oligomers and monomers obtained using papain and Pronase and to identify the monomeric sequence of the oligomers at the non-reducing end by enzymatic means. In addition, the growth inhibitory activity of chito-oligomeric–monomeric mixture, as well as the individual oligomers towards both Gram-positive (Bacillus cereus) and Gram-negative (Escherichia coli) bacteria, and their probable mode of action are reported.
EXPERIMENTAL
Materials
Papain (from papaya latex; EC 3.4.22.2), Pronase (from Streptomyces griseus; EC 3.4.24.31), Sephadex G15, GlcN, GlcNAc, chito-oligomers [DP (degree of polymerization) 2–4] and malto-oligomers were obtained from Sigma (St. Louis, MO, U.S.A.). Biogel P2 and P30 were from Bio-Rad Laboratories (Hercules, CA, U.S.A.). N-Acetyl-β-glucosaminidase (from bovine kidney) was obtained from Boehringer Mannheim (Mannheim, Germany). Other chemicals used were of the highest purity available.
Chitosan, obtained by heterogeneous N-deacetylation of shrimp chitin [15], was purified by dissolving in 1% acetic acid, filtering through a plug of glass wool to remove suspended particles and finally precipitating with 2% (v/v) Na2CO3. The precipitate was thoroughly washed with water, freeze-dried and stored at cold temperature.
Enzyme purity
GPC (gel-permeation chromatography; Biogel P2 column), SDS/PAGE and capillary zone electrophoresis were performed as described elsewhere [13,14]. Furthermore, enzyme purity was ascertained by RP-HPLC (reverse phase HPLC) and N-terminal sequence analysis as described below.
RP-HPLC
Mobile phase used were 0.1% trifluoroacetic acid (solvent I) and acetonitrile/water (70:30, v/v) containing 0.05% trifluoroacetic acid (solvent II). Papain/Pronase was dissolved in triple distilled water and analysed by RP-HPLC on the Shimadzu LC-8A system. Then, 20 μl (10 mg·ml−1) of the enzyme solution was loaded on to a Shimpak octadecylsilane column (250 mm×4.5 mm) previously equilibrated with the mobile phase (solvent I). After injecting enzyme, the column was washed with solvent I (10 min), followed by gradient run consisting of 0% solvent II traversing to 100% in 90 min at a flow rate of 1.0 ml·min−1. The elution was monitored at 230 and 280 nm.
N-terminal amino acid analysis (automated gas-phase protein sequencing)
This was performed on Applied Biosystems protein sequenator (Model 789, Applied Biosystems, Foster City, CA, U.S.A.), which carries out Edman degradation by supplying gaseous reagents for the coupling and cleavage reactions.
Prefractionation of chito-oligomers/monomers
Chitosan solution (1%; dissolved in 1% acetic acid, pH adjusted to 3.5) was separately treated with enzymes (i.e. papain and Pronase) in the ratio 100:1 (w/w), incubated for different periods (1–5 h) at optimum temperature (37 °C), followed by arresting the reaction by heat-denaturing the enzyme (100 °C, 5 min) and adjusting the pH to 7.0 using 2 M NaOH. The supernatant separated by centrifugation (1000 g and 10 min) was concentrated by freeze-drying and passed through a Sephadex G15 column (80 cm×1.0 cm), and the fractions (1 ml; eluted with water) containing reducing equivalents (i.e. oligomers/monomers) were pooled and freeze-dried.
SEM (scanning electron microscopy)
Samples were spread on a double-sided conducting adhesive tape pasted on to a metallic stub, subjected to gold covering and observed under a scanning electron microscope (LEO 435 VP; LEO Electron Microscopy, Cambridge, U.K.) at 20 kV.
FTIR (Fourier-transform infrared) spectroscopy
IR spectral studies were performed in a PerkinElmer 2000 spectrometer under dry air at ambient temperature using KBr pellets. Samples in quantities of 4 mg each were mixed thoroughly with 200 mg of KBr, and 40 mg of the mixture was pelletized. Reproducibility of the spectra was verified on two preparations and the DA (degree of acetylation) was determined using the formula (A1655 cm−1/A3450 cm−1)×100/1.33, where A is the absorbance at these wavelengths, calculated from the baseline [16].
CD
CD spectra of samples (5 mg·ml−1 in 0.1 M HClO4) were recorded in a Jasco J-810 automatic recording spectropolarimeter (Japan Spectroscopic, Tokyo, Japan) continuously purged with N2 before and during the experiment. Slits were programmed to yield 10 Å (1 Å=10−10 m) bandwidth at each wavelength so that the resolution was more or less constant. The spectra were recorded between 200 and 240 nm and baseline was obtained using 0.1 M HClO4. After accumulation of scans, the spectra were standardized to mean residual ellipticity expressed as θ in degree·cm2 per residue using the mean residual mass of GlcNAc [17].
Liquid state 13C-NMR spectroscopy
Chito-oligomeric–monomeric mixture was dissolved in 1 ml of 2H2O (for chitosan, 0.98 ml of 2H2O+0.02 ml of deuterium chloride was used). After ensuring complete dissolution, the spectra were recorded with a Bruker AMX 400 spectrometer at 100 mHz and 27 °C. The DA was calculated using the equation, ICH3/(IC1+IC2+IC3+IC4+IC5+IC6), where I is the intensity of methyl as well as C1–C6 ring carbon atoms [18].
Determination of DP of chito-oligomers
HPLC
Freeze-dried sample was subjected to HPLC on an aminopropyl column (3.9 mm×300 mm; Waters Associates, Waters Technologies Ireland, IDA Business Park, Drinagh, Wexford, Ireland). Acetonitrile/water (70:30) mixture was the mobile phase used at a flow rate of 1.0 ml·min−1. Detection was performed using refractive index detector and the column was precalibrated with chito-oligomers, malto-oligomers and monomers.
MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight MS)
This was done on Kompact analytical SEQ MALDI–TOF mass spectrometer (Kratos, Manchester, U.K.) at an accelerated voltage of 30 kV using 2,5-dihydroxybenzoic acid as the matrix.
Identification of the non-reducing end residues of the oligomers
Individual chito-oligomers were isolated using Biogel P2 column (100 cm×0.3 cm) equilibrated with water and precalibrated with chito-oligomers and monomers [19]. The oligomeric mixture as well as the individual oligomers (up to 500 μg of reducing equivalents) were treated with N-acetyl-β-glucosaminidase (0.5 unit), incubated at ambient temperature for 1 h, followed by the reducing equivalent assay according to the modified Schales method [20].
Indicator bacteria and inoculum preparation
Strains of B. cereus F4810, E. coli MTCC 118, Listeria monocytogenes Scott A, Yersinia enterocolitica MTCC 859, Staphylococcus aureus FRI 722 and B. licheniformis CFR 1621 (indicator bacterial cultures) were obtained from the culture collection maintained in the Food Microbiology Department of this Institute. The cultures were maintained at 6 °C on BHI (brain–heart infusion) agar slants (HiMedia, Mumbai, India) and subcultured at 15 day intervals. Before use, the culture was successively propagated twice in BHI broth at 37 °C. Cell suspensions of the culture, individually, were prepared from 20 h-old BHI culture broth with appropriate dilution in 0.85% saline, giving individual counts of 102–106 CFU·ml−1 (where CFU stands for colony-forming unit).
Bacterial growth inhibitory activity
Antibacterial activity of chito-oligomeric–monomeric mixture as well as the individual oligomers was studied against indicator bacterial strains, individually, in nutrient broth following the method of Chen et al. [21]. To 10 ml aliquots of nutrient broth (HiMedia) supplemented with 0.5% dextrose, a cell suspension of specific bacterial strain (102–106 CFU/tube) containing 0.1–0.5 mg% levels of chito-oligomeric–monomeric mixture and 0.05–0.1 mg% of individual chito-oligomers separated on Biogel P2 column was added. Native chitosan solubilized in 1% acetic acid and pH adjusted to 6.0 was taken as the control. The tube contents were mixed and incubated at 37 °C for approx. 20 h. From these, 1 ml aliquots were transferred into fresh 9 ml nutrient broth tubes, whereas the remaining broth aliquots were pour-plated with BHI agar and incubated for 24 h at 37 °C. The plates were observed for bacterial colonies and the tubes were observed for turbidity. Bactericidal activity was calculated using [(C−T)/C]×100, wherein C and T are colony numbers in the control and chitosan sample plates respectively [7]. After 24 h of incubation, 0.5 ml aliquots (both from control and tube containing 106 CFU+chitosan or chito-oligomeric–monomeric mixture or individual mono-oligomer/chito-oligomer) were transferred to microcentrifuge tubes followed by centrifugation. The pellets obtained were treated with 0.3 M phosphate buffer (pH 7.0), fixed with glutaraldehyde (1%) for 1 h at 4 °C and further treated with 10% (v/v) absolute alcohol in a sequential manner. The dried samples were subjected to SEM.
RESULTS AND DISCUSSION
Enzyme activity
Earlier, we reported that PAGE of commercial papain gives two bands, of which one was the major, whereas Pronase showed a single band [13,14]. The major band of papain, which was associated with both proteolytic and chitosanolytic activities, was isolated by GPC on a Biogel P30 column. SDS/PAGE and capillary zone electrophoresis of the latter, as well as Pronase, also showed a single band/peak, indicating their homogeneity [13,14]. Furthermore, the appearance of a single peak on RP-HPLC confirmed their purity beyond ambiguity (Figure 1). In addition, N-terminal sequences of papain and Pronase were found to be IPEYVDYREK and SQGSVYXXPYAD respectively. BLAST analysis of the former was corresponding to the papain from papaya latex [22], whereas that of Pronase did not show any homology, since the N-terminal sequence of Pronase, i.e. type XXI protease from S. griseus, is yet to be determined. Preincubation of the purified papain and Pronase with PMSF (Sigma), an inhibitor of serine proteases, resulted in complete inhibition of both chitosanolytic and proteolytic activities, whereas treatment with chito-oligomers (inhibitors of chitosanases) before the assay did not show any such reduction in chitosanolytic activities of papain and Pronase.
Figure 1. RP-HPLC profiles of (A) papain and (B) Pronase.
The purified papain and Pronase showed optimum chitosanolytic activity at pH 3.5 and 37 °C, with a specific activity of 1.78 and 1.16 units·mg of protein−1 respectively. Papain showed continuous depolymerization even after 12 h of incubation with a negligible increment in the release of reducing equivalents beyond 4–5 h, whereas, for Pronase, there was a decrease in the activity after 4 h.
SEM and molecular mass
Native chitosan had a molecular mass of approx. 71±2 kDa as determined by viscometry and GPC (Sepharose CL 4B column, 200 cm×0.9 cm). SEM of chitosan showed a particle-like structure as a result of aggregation of individual chains by inter- and intramolecular hydrogen-bonding network, whereas that of the chito-oligomeric–monomeric mixture showed sparsely distributed spongy-type morphology and required a much higher magnification (×2000) compared with native chitosan (×500), in support of their decreased molecular mass (Figure 2).
Figure 2. SEM of (A) native chitosan (×500), and chito-oligomeric–monomeric mixture (×2000) obtained using papain (B) and (C) Pronase.
Prefractionation of chito-oligomeric–monomeric mixture
Upon centrifugation of the reaction mixture (1000 g and 10 min), the major chitosanolytic product, LMMC, got sedimented out (yield, 71–86%), whereas the percentage yield of the chito-oligomeric–monomeric mixture was 14–17% and 14–19% for papain- and Pronase-catalysed reactions respectively depending on the reaction time (1–5 h). Unless stated otherwise, further biophysical characterizations were performed for 5 h samples.
FTIR
Native chitosan showed a DA of approx. 26%, whereas the chito-oligomeric–monomeric mixture had a DA of 35.2 and 37.8% respectively for papain- and Pronase-catalysed reactions (Figure 3). This increase in DA, although surprising, was contributed by LMMC, which had a DA of 17 and 14% respectively for papain- and Pronase-catalysed reactions [13,14], and the average DA of LMMC+chito-oligomeric–monomeric mixture was nevertheless equal to that of native chitosan [i.e. (17+35.2)/2=26.1 for papain and (14+37.8)/2=25.9 for Pronase]. Thus it could be concluded that both papain and Pronase cleave only the glycosidic bonds leaving the N-acetyl groups intact, thus confirming their depolymerizing action rather than a de-N-acetylating effect.
Figure 3. FTIR spectra of (A) native chitosan, and chito-oligomeric–monomeric mixture obtained using papain (B) and (C) Pronase.
In the IR spectra (Figure 3), a peak at 1320 cm−1 corresponds to acetyl groups [23]. An increase in the intensity of this peak in chito-oligomeric–monomeric mixture compared with native chitosan further confirmed increase in DA, and from these it is evident that both papain and Pronase result in the release of GlcNAc/GlcNAc-rich oligomers. A slight shift in the peak around 3370 cm−1 was indicative of orderliness of the products, probably due to increase in DA [24]. The region between 1420 and 1435 cm−1 is considered to be conformation-sensitive for polysaccharides, which depends on the orientation of the primary hydroxy group, and its shift in the chito-oligomeric–monomeric mixture compared with native chitosan indicates a change in the secondary structural environment [25].
CD
In the CD spectra (Figure 4), Domard [17] correlated the peak near 211 nm due to η→π* transition of GlcNAc and thus acetyl content, which is independent of chain length, conformation, ionic strength and pH. The chito-oligomeric–monomeric mixture obtained showed an increase in this peak height, indicating an increase in the acetyl content, and between the two samples, the Pronase-catalysed product showed slightly more DA, in agreement with IR observations.
Figure 4. CD spectra of (■) native chitosan, and chito-oligomeric–monomeric mixture obtained using papain (●) and (▲) Pronase.
Liquid state 13C-NMR
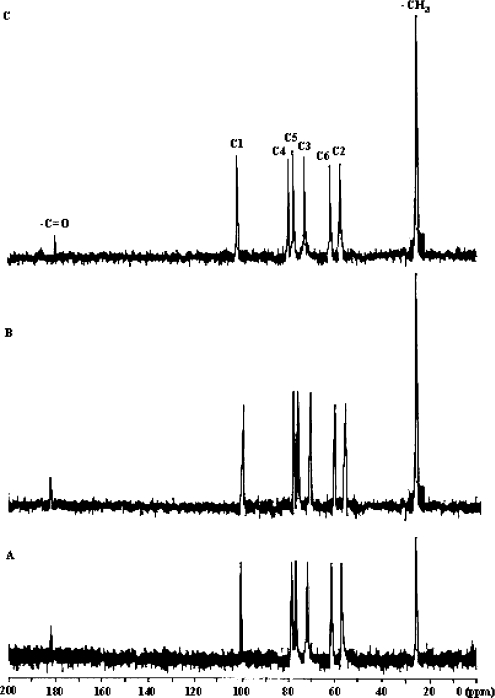
The DA values of 35.4 and 37.2%, deduced from NMR, respectively for chito-oligomeric–monomeric mixture obtained using papain and Pronase were in good agreement with those calculated by IR spectra. An increase in the peak height (Figure 5) corresponding to –CH3 (≈25.0 p.p.m.) and –C=O (near 176.0 p.p.m.) of chito-oligomeric–monomeric mixture in comparison with those of native chitosan further confirmed increased DA of these products. Considerable change in the chemical-shift (δ) values (downfield shift, Table 1) indicated an altered conformation.
Figure 5. Liquid-state 13C-NMR spectra of (A) native chitosan, and chito-oligomeric–monomeric mixture obtained using papain (B) and (C) Pronase.
Table 1. Liquid-state 13C-NMR chemical-shift (δ) values (p.p.m.) of native chitosan and chito-oligomeric/monomeric mixture obtained using papain and Pronase.
| δ (p.p.m.) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | –C=O | C1 | C4 | C5 | C3 | C6 | C2 | –CH3 |
| Chitosan | 181.21 | 98.19 | 77.21 | 75.42 | 70.65 | 60.69 | 58.47 | 24.58 |
| Chito-oligomeric/monomeric mixture | ||||||||
| Papain | 177.15 | 98.14 | 77.26 | 75.41 | 70.71 | 60.78 | 56.46 | 23.01 |
| Pronase | 178.48 | 102.1 | 79.26 | 77.84 | 72.64 | 62.18 | 58.98 | 24.00 |
Identification of monomers and determination of DP of oligomers
HPLC profiles of the chito-oligomeric–monomeric mixture are depicted in Figure 6. A sudden decrease in the viscosity of chitosan solution after the addition of enzymes was indicative of their exo-action, whereas appearance of oligomers of DP 2–6 as well as monomer(s) revealed simultaneous endo-action of proteases, confirming their non-specific activities during depolymerization of chitosan. The yield of chito-oligomers was maximal with Pronase-catalysed chitosanolysis, whereas papain resulted in more of monomers (GlcN and GlcNAc). Their DP calculated by GPC on Biogel P2 (results not shown) and HPLC profiles were in good agreement with each other. Papain caused release of dimer to hexamer along with both the monomers, whereas Pronase formed only GlcNAc along with dimer to hexamer. The appearance of only GlcNAc in the latter was indicative of its different action pattern in comparison with papain. The percentage yield of individual oligomers as determined by HPLC (Table 2) was comparable with that obtained from chitosanase from the strains of Penicillium [26].
Figure 6. HPLC profiles of chito-oligomeric–monomeric mixture obtained after depolymerization (5 h) of chitosan using (A) papain and (B) Pronase.
Table 2. Percentage yield of individual chito-oligomers.
| Chito-oligomer yield (%)* | |||||
|---|---|---|---|---|---|
| Enzyme | Dimer | Trimer | Tetramer | Pentamer | Hexamer |
| Papain | 2.9 | 2.6 | 2.4 | 2.2 | 1.9 |
| Pronase | 6.1 | 5.0 | 1.8 | 1.3 | 0.7 |
*Based on HPLC separation.
MALDI–TOF-MS
The structures of these oligomers were deduced by subjecting them to MALDI–TOF-MS (Figure 7). Papain gave rise to oligomers of DP 2 (376.6), DP 3 (530.8 and 561.4), DP 4 (760.5), DP 5 (954.8), DP 6 (1083.2 and 1114.6) and to a minor extent DP 7 (1396.5) and DP 8 (1490.6), which were not observed in HPLC, probably due to the lack of sensitivity. Similarly, the major oligomers obtained after chitosanolysis using Pronase corresponded to DP 2 (374.4 and 396.8), DP 3 (526.3 and 558.4), DP 4 (716.8), DP 5 (922.6) and DP 6 (1030.5 and 1053.2), whereas DP 7 (1287.4) and DP 9 (1620.2) were the minor oligomeric products. A slight increase in the m/z values of some of the oligomers could be due to their sodiated forms.
Figure 7. MALDI–TOF-MS of the chito-oligomers obtained after depolymerization of chitosan using (A) papain and (B) Pronase.
Identification of the non-reducing-end residues of the oligomers
Treatment of the chito-oligomeric mixture obtained from papain with N-acetyl-β-glucosaminidase, an enzyme specific for the release of GlcNAc from the non-reducing end, gave an increase in reducing equivalents, suggesting the enzyme action on -GlcN-GlcNAc- or -GlcNAc-GlcNAc-, resulting in the products with GlcNAc at the non-reducing end, which was also supported by the presence of GlcNAc along with chito-oligomers. On the contrary, upon the addition of N-acetyl-β-glucosaminidase to the chito-oligomeric mixture derived from Pronase, there was no change in the reducing equivalents, indicating Pronase action on -GlcNAc-GlcN-type linkage, which gives oligomers having GlcNAc at the intermediate position or at the reducing end. The data indicated a preference of GlcNAc at the non-reducing end for chitosanolysis by Pronase. In agreement with this, treatment of individual chito-oligomers (isolated using a Biogel P2 column, bed volume 90 ml, elution with water) with N-acetyl-β-glucosaminidase showed no change in reducing equivalents, whereas some of the oligomers from the papain-catalysed reaction did show an increment in reducing equivalents (for trimer and tetramer, 2-fold increase in the reducing equivalent; for hexamer, 1.7-fold increase), in support of the fact that GlcNAc is at the non-reducing ends of those oligomers. Put together, the possible monomeric sequence of the oligosaccharides could be deduced (Table 3).
Table 3. Probable sequences of monomers in the derived chito-oligomers.
| Oligomer | m/z | GlcN:GlcNAc | Probable sequence |
|---|---|---|---|
| Chitosanolysis by papain | |||
| Dimer | 376.6 | 2:0 | GlcN-GlcN |
| Trimer | 530.8 | 2:1 | GlcNAc-(GlcN)2 |
| 561.4 | 1:2 | GlcNAc-GlcN-GlcNAc | |
| Tetramer | 760.5 | 2:2 | GlcNAc-(GlcN)2-GlcNAc or GlcNAc-GlcN-GlcNAc-GlcN |
| Pentamer | 954.8 | 2:3 | GlcN-(GlcNAc)3-GlcN or (GlcN)2-(GlcNAc)3 or GlcN-GlcNAc-GlcN-(GlcNAc)2 |
| Hexamer | 1083.2 | 3:3 | GlcNAc-(GlcN)3-(GlcNAc)2 |
| 1114.6 | 2:4 | (GlcN)3-(GlcNAc)3 | |
| Chitosanolysis by Pronase | |||
| Dimer | 374.4 | 2:0 | GlcN-GlcN |
| 396.8 | 1:1 | GlcN-GlcNAc | |
| Trimer | 526.3 | 2:1 | (GlcN)2-GlcNAc or GlcN-GlcNAc-GlcN |
| 558.4 | 1:2 | GlcN-(GlcNAc)2 | |
| Tetramer | 716.8 | 3:1 | (GlcN)3-GlcNAc/GlcN-GlcNAc-(GlcN)2 or (GlcN)2-GlcNAc-GlcN |
| Pentamer | 922.6 | 3:2 | (GlcN)3-(GlcNAc)2 or GlcN-(GlcNAc)2-(GlcN)2 or GlcN-GlcNAc-GlcN-GlcNAc-GlcN |
| Hexamer | 1030.5 | 5:1 | (GlcN)5-GlcNAc or GlcN-GlcNAc-(GlcN)4 |
| 1053.2 | 4:2 | (GlcN)4-(GlcNAc)2 or GlcN-(GlcNAc)2-(GlcN)3 | |
Bacterial growth inhibitory activity
Compared with native chitosan, chito-oligomeric–monomeric mixture showed better growth inhibitory effect towards both Gram-positive and -negative bacteria such as B. cereus, E. coli, Y. enterocolitica and B. licheniformis. However, for detailed study, only strains of B. cereus F4810 (Gram-positive) and E. coli D21 (Gram-negative) were selected (Table 4). As the percentage yield of chito-oligomers/monomers was dependent on reaction time, 5 h samples containing 14% chito-oligomers/monomers showed better growth inhibitory activity compared with 1 and 3 h samples, which contained 17–19% of chito-oligomeric–monomeric mixture. Like LMMC [13], an increase in the concentration of chito-oligomeric–monomeric mixture did not show linear inhibitory effect and unlike LMMC, 100% inhibition was not obtained at any concentration. Relatively, papain-derived chito-oligomeric–monomeric mixture showed better growth inhibitory activity.
Table 4. Growth inhibitory effect of native chitosan and chito-oligomeric/monomeric mixture towards B. cereus (106 CFU·ml−1) and E. coli (104 CFU·ml−1).
| Inhibition (%) | ||||
|---|---|---|---|---|
| Concn. | Indicator | |||
| Compound | (mg%) | bacterium… | B. cereus F4810 | E. coli D21 |
| Native chitosan | 0.01 | 60 | 0 | |
| 0.03 | 50 | 0 | ||
| 0.05 | 10 | 0 | ||
| Chito-oligomers/monomers | ||||
| Chitosanoysis by papain | 0.1 | 67 | 40 | |
| 0.2 | 72 | 62 | ||
| 0.3 | 80 | 52 | ||
| 0.4 | 58 | 34 | ||
| 0.5 | 44 | 18 | ||
| Chitosanolysis by Pronase | 0.1 | 48 | 38 | |
| 0.2 | 66 | 60 | ||
| 0.3 | 74 | 46 | ||
| 0.4 | 52 | 30 | ||
| 0.5 | 38 | 14 | ||
The growth inhibitory effect of the purified mono- and chito-oligomers at two different concentrations (50 and 100 μg) was investigated (Figure 8). Of the two monomers used, GlcN showed 5–10% inhibition depending on the concentration, which was surprising, whereas GlcNAc did not show any such activity, in support of the fact that the antibacterial effect of chitosan/chito-oligomers is essentially due to free-NH3+ groups, which are responsible for the binding of negative charges on the bacterial cell surface to bring about antibacterial activity [21]. A close examination of Figure 8 indicates that percentage growth inhibition by GlcN and trimer obtained using both papain and Pronase is similar, which is due to their structural similarities, whereas the other oligomers obtained using Pronase showed relatively a higher growth inhibition, probably due to their GlcN/GlcNAc ratio. For example, the tetramer obtained using papain had GlcN/GlcNAc ratio of 2:2, whereas that of Pronase was 3:1. Similarly, between trimer and hexamer, the latter showed better growth inhibitory effect. This explains a role played by DP and DA in the antibacterial activity of oligomers. In conclusion, it is likely that higher the DP of oligomers rich in free GlcN residues, the higher is their growth inhibitory activity. At this point, it has to be noted that, although the chito-oligomeric–monomeric mixture obtained using Pronase showed lesser growth inhibitory effect compared with that of the papain-depolymerized product, individual oligomers obtained using the former showed better bactericidal activity and this is due to the presence of more of GlcNAc residues (see the FTIR subsection, Table 2 and Figure 6, where DA is more, presence of 6.1% GlcNAc-GlcNAc and release of only GlcNAc).
Figure 8. Growth inhibitory effect of individual sugars towards B. cereus (106 CFU·ml−1) and E. coli (104 CFU·ml−1).
(A) and (B) represent chitosanolytic products of papain- and Pronase-catalysed reactions respectively.
The precise mechanism of growth inhibitory activity of chitosan is yet to be elucidated [1] and no information is available so far on the activity of GlcN/GlcNAc and chito-oligomers of DP 2–6. In the present study, SEM data revealed pore formation and thus permeabilization of the cytoplasmic contents of B. cereus, and irregularities on the cell surface of E. coli, after treatment with chito-oligomeric–monomeric mixture (Figure 9), indicating their different action patterns. When the B. cereus culture suspended in saline was treated (24 h at 37 °C) with chito-oligomeric–monomeric mixture, followed by analyses of carbohydrate and protein contents in the cell-free supernatant, there was 25–30-fold increase compared with control culture supernatants, confirming permeabilizing action of the test samples. However, similar results were not obtained with E. coli in the initial hours, although, after approx. 72 h of incubation, a slight increase in the carbohydrate–protein content was observed. To confirm this, both B. cereus and E. coli were suspended in saline and divided into three parts, and the first part was subjected to sonication, the second part was taken as control and the third was treated with chito-oligomeric–monomeric mixture. After approx. 20 h of incubation, all the three were centrifuged (1000 g and 15 min), and the supernatants were dialysed to remove the saline, concentrated and subjected to native PAGE [27]. As depicted in Figure 10, sonicated samples showed the presence of proteins, whereas the control samples did not. Similar to sonicated samples, the chito-oligomeric–monomeric mixture-treated B. cereus showed the presence of protein, whereas that of E. coli did not show any protein, in support of permeabilization of the cytoplasmic contents of the former. Furthermore, the identification of cytoplasmic enzymes such as maltase, lactase and protease in the cell-free supernatants of sonicated and chito-oligomeric–monomeric mixture-treated B. cereus supported spillage of cytoplasmic contents. The amino acid sequence of the protein separated on native PAGE (electrophoretic mobility, RF=0.15) from B. cereus was found to be -AGKTFPDV-, and this was identified as a fragment of the surface-layer (S-layer) protein of B. cereus (-XGKTFPDV). S-layer protein is a p-crystalline mono-layered assembly of proteins, which coat the cell surface of B. cereus, and its presence in the cell-free supernatant after treatment with chito-oligomeric–monomeric mixture confirms disintegration of the cell wall. Put together, the results confirmed chito-oligomeric–monomeric mixture to be bactericidal in nature.
Figure 9. SEM of B. cereus and E. coli before (A, C respectively) and after (B, D respectively) treatment with chito-oligomeric–monomeric mixture.
Figure 10. Native PAGE of cell-free supernatants of B. cereus (A) and E. coli (B).
Lanes I and IV, cells subjected to sonication; lanes II and V, control; lanes III and VI, cells treated with chito-oligomeric–monomeric mixture.
Mechanism of bactericidal action by chito-oligomeric–monomeric mixture
Based on the above data, the following mechanism could be proposed for bactericidal action of chito-oligomeric–monomeric mixture.
The mode of action of cationic antibacterial agents is widely believed to be due to interacting with and disrupting the wall/membrane structure [28]. In Gram-positive bacteria (Figure 11), the cell membrane is covered by a cell wall made up of 30–40 layers of peptidoglycans, which contain GlcNAc, N-acetylmuramic acid as well as D- and L-amino acids including isoglutamate and teichoic acid [29], to which the positively charged amino groups of chito-oligomers/GlcN can bind, resulting in cell-wall distortion–disruption, exposure of cell membrane to osmotic shock and exudation of the cytoplasmic contents.
Figure 11. Mechanism of bactericidal action of chito-oligomeric–monomeric mixture towards B. cereus and E. coli: a hypothetical model.
LPS, lipopolysaccharide. The large arrows indicate the sequence of bactericidal actions.
Gram-negative bacteria, on the other hand, contain an outer membrane wherein lipopolysaccharide and proteins are held together by electrostatic interactions with bivalent metal ions, one to two layers of peptidoglycans (cell wall) and a cell membrane (containing lipid bilayer, transmembrane proteins and inner/outer membrane proteins). The negatively charged O-specific antigenic oligosaccharide-repeating units of the E. coli lipopolysaccharide form ionic-type of binding with the amino groups of chito-oligomers (which are cationic in nature below pH 6.2), thus blocking the nutrient flow with concomitant bacterial death due to depletion of the nutrients (Figure 11). Here, the deposition of cationic oligomers on to the cell surface is more prominent than membrane disruption as in the case of Gram-positive bacteria, owing to stronger association of O-chains to the outer membrane structure. The smaller molecular mass of the chito-oligomers/GlcN and more electronegativity on Gram-negative bacteria facilitate effective binding and aggregation of the former, blocking the nutrient flow and ultimately leading to cell lysis.
The papain-derived chito-oligomers with GlcNAc at the non-reducing ends (as determined using N-acetyl-β-glucosaminidase) did show bacterial growth inhibitory activities, indicating a role not played by terminal GlcN/GlcNAc residues. In fact, as shown in Figure 10, oligomeric chain-linked GlcN, through their free –NH3+ groups, could interact with the bacterial cell surface, resulting in bactericidal activity.
In conclusion, the use of papain and Pronase for chitosanolysis as a substitute for expensive unavailable chitosanase could be exploited commercially, for they result in the production of chitooligomers and monomers along with LMMC, which are of considerable biomedical importance. Association of bactericidal activity with the chito-oligomers is of value in their utilization as a natural food additive and food preservative, since they are biocompatible, non-toxic, water-soluble and consumption safe. These results demonstrate value addition to chitosan, which is otherwise a waste material found in the offal of marine food processing industry, and also the use of low-cost and easily available enzymes such as papain and Pronase for better biofunctionalities.
Acknowledgments
A.B.V.K. acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi, for Senior Research Fellowship. We thank Dr A. Rao and Dr S.A. Singh (Protein Chemistry Technology, CFTRI), Mr Anbalagan and Ms Asha (CFTRI) for CD, SEM and IR respectively.
References
- 1.Tharanathan R. N., Kittur F. S. Chitin – the undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 2.Shon D. H. Proceedings of the International Workshop on Bioactive Natural Products, Tokyo, Japan. Tokyo, Japan: The Committee on Science and Technology in Developing Countries (COSTED) and the Science Council of Japan; 2001. Chitosan oligosaccharides for functional foods and microbial enrichment of chitosan oligosaccharides in soy-paste. pp. 56–66. [Google Scholar]
- 3.Suzuki K., Mikami T., Okawa Y., Tokoro A., Suzuki S., Suzuki M. Antitumor effects of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr. Res. 1986;151:403–408. doi: 10.1016/s0008-6215(00)90359-8. [DOI] [PubMed] [Google Scholar]
- 4.Qin C., Du Y., Xiao L., Li Z., Gao X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2003;31:111–117. doi: 10.1016/s0141-8130(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda I., Sugano M., Yashida K., Sasaki E., Iwamoto Y., Hatono K. Dietary fibre and lipid absorption: interference of chitosan and its hydrolyzate with cholesterol and fatty acid absorption and metabolic consequences in rats. In: Kritchevsky D., Bonefield C., editors. Dietary Fibre in Health and Disease. St. Paul, MN: Eagan Press; 1995. pp. 95–106. [Google Scholar]
- 6.Sekiguchi S., Miura Y., Kaneko H., Nishimura S. L., Nishi N., Iwase M., Tokura S. Molecular weight dependency of antimicrobial activity by chitosan oligomers. In: Nishinari K., Doi E., editors. Food Hydrocolloids: Structure, Properties and Functions. New York: Plenum Press; 1994. pp. 71–76. [Google Scholar]
- 7.Jeon Y. J., Park P. J., Kim S. K. Antimicrobial effect of chito-oligosaccharides produced by bioreactor. Carbohydr. Polym. 2001;44:71–76. [Google Scholar]
- 8.Sukwattanasinitt M., Zhu H., Sashiwa H., Aiba S. Utilization of commercial non-chitinase enzymes from fungi for preparation of 2-acetamido-2-deoxy-D-glucose from β-chitin. Carbohydr. Res. 2002;337:133–137. doi: 10.1016/s0008-6215(01)00298-1. [DOI] [PubMed] [Google Scholar]
- 9.Patil R. S., Ghormade V., Deshpande M. V. Chitosanolytic enzymes: an exploration. Enzyme Microbiol. Technol. 2000;36:473–483. doi: 10.1016/s0141-0229(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Pantaleone D., Yalpani M., Scollar M. Unusual susceptibility of chitosan to enzymic hydrolysis. Carbohydr. Res. 1992;237:325–332. [Google Scholar]
- 11.Yalpani M., Pantaleon D. An examination of the unusual susceptibility of aminoglycans to enzymatic hydrolysis. Carbohydr. Res. 1994;265:159–175. [Google Scholar]
- 12.Kittur F. S., Vishu Kumar A. B., Gowda L. R., Tharanathan R. N. Chitosanolysis by a pectinase isozyme of Aspergillus niger – a non-specific activity. Carbohydr. Polym. 2003;53:191–196. [Google Scholar]
- 13.Vishu Kumar A. B., Varadaraj M. C., Lalitha R. G., Tharanathan R. N. Low molecular weight chitosan: preparation with the aid of papain and characterization. Biochim. Biophys. Acta. 2004;1670:137–146. doi: 10.1016/j.bbagen.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Vishu Kumar A. B., Gowda L. R., Tharanathan R. N. Non-specific depolymerization of chitosan by Pronase and characterization of the resultant products. Eur. J. Biochem. 2004;271:713–723. doi: 10.1111/j.1432-1033.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- 15.Kittur F. S., Kumar K. R., Tharanathan R. N. Functional packaging properties of chitosan films. Eur. Food Res. Technol. 1998;206:44–47. [Google Scholar]
- 16.Ottoy M. H., Varum K. M., Smidsord O. Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohydr. Polym. 1996;29:17–24. [Google Scholar]
- 17.Domard A. Determination of N-acetyl content in chitosan sample by c.d. measurement. Int. J. Biol. Macromol. 1987;9:333–336. [Google Scholar]
- 18.Ottoy M. H., Varum K. M., Smidsord O. Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohydr. Polym. 1996;29:17–24. [Google Scholar]
- 19.Domard A., Cartier N. Glucosamine oligomers I. Preparation and characterization. Int. J. Biol. Macromol. 1989;11:297–302. doi: 10.1016/0141-8130(89)90023-8. [DOI] [PubMed] [Google Scholar]
- 20.Imoto T., Yagishita K. A simple activity measurement of lysozyme. Agric. Biol. Chem. 1971;33:1154–1157. [Google Scholar]
- 21.Chen C., Lian W., Isai G. Antimicrobial effects of N-sulfonated and N-sulfobenzyl chitosan and application to oyster preservation. J. Food Prot. 1998;61:1124–1128. doi: 10.4315/0362-028x-61.9.1124. [DOI] [PubMed] [Google Scholar]
- 22.Lynn K. R., Yaguchi M., Roy C. Homology of the N-terminal sequences of asclepains and papain. Biochim. Biophys. Acta. 1980;624:579–580. doi: 10.1016/0005-2795(80)90098-7. [DOI] [PubMed] [Google Scholar]
- 23.Brugnerotto J., Lizardi J., Goycoolea F. M., Argiielles M. W., Desbrieres J., Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569–2580. [Google Scholar]
- 24.Focher B., Beltrame P. L., Naggi A., Tori G. Alkaline N-deacetylation of chitin enhanced by flash treatments: reaction kinetics and structure modifications. Carbohydr. Polym. 1990;12:405–418. [Google Scholar]
- 25.Focher B., Naggi A., Tori G., Cosani A., Terbojevich M. Chitosans from Euphausia superba. 2: characterization of solid-state structure. Carbohydr. Polym. 1992;18:43–49. [Google Scholar]
- 26.Liu X. F., Guan Y. L., Yang D. Z., Li Z., Yao K. D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001;79:1324–1335. [Google Scholar]
- 27.Laemmli U. K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Helander I. M., Latva-Kala K., Lounatmaa K. Permeabilizing action of polyethyleneimine on Salmonella typhimurium involves disruption of the outer membrane and interactions with lipopolysaccharide. Microbiology. 2003;344:385–390. doi: 10.1099/00221287-144-2-385. [DOI] [PubMed] [Google Scholar]
- 29.Voet J., Voet D. 2nd edn. New York: John Wiley & Sons, Inc.; 1995. Biochemistry; pp. 360–362. [Google Scholar]