Abstract
In addition to tyrosine sites, FAK (focal adhesion kinase) is phosphorylated on multiple serine residues. In the present study, the regulation of two of these sites, Ser-722 (S1) and Ser-911 (S4), was investigated. Phosphorylation of S1 (but not S4) decreased in resuspended cells, and recovered during spreading on fibronectin, indicating adhesion-dependent regulation. GSK3 (glycogen synthase kinase 3) inhibitors decreased S1 phosphorylation, and siRNA (short interfering RNA) silencing indicated further the involvement of GSK3β. Furthermore, GSK3β was found to become activated during cell spreading on fibronectin, and to physically associate with FAK. S1 phosphorylation was observed to decrease in wounded cell monolayers, while GSK3β underwent inactivation and later was observed to increase to the original level within 24 h. Direct phosphorylation of S1, requiring pre-phosphorylation of Ser-726 in the +4 position, was demonstrated using purified GSK3 and a synthetic peptide containing FAK residues 714–730. An inhibitory role for S1 phosphorylation in FAK signalling was indicated by findings that both alanine substitution for S1 and dephosphorylation of S1 by PP1 (serine/threonine protein phosphatase type-1) resulted in an increase in FAK kinase activity; likewise, this role was also shown by cell treatment with the GSK3 inhibitor LiCl. The inhibitory role was confirmed by the finding that cells expressing FAK with alanine substitution for S1 displayed improved cell spreading and faster migration in wound-healing and trans-well assays. Finally, the finding that S1 phosphorylation increased in cells treated with the PP1 inhibitor okadaic acid indicated targeting of this site by PP1. These results indicate an additional mechanism for regulation of FAK activity during cell spreading and migration, involving Ser-722 phosphorylation modulated through the competing actions of GSK3β and PP1.
Keywords: cell adhesion, cell migration, focal adhesion kinase (FAK), glycogen synthase kinase 3 (GSK3), protein phosphatase, protein phosphorylation
Abbreviations: CAS, Crk-associated substrate; CDK, cyclin-dependent kinase; Erk, extracellular-signal-regulated kinase; FAK, focal adhesion kinase; FGF, fibroblast growth factor; Fmoc, fluoren-9-ylmethoxycarbonyl; FRNK, FAK-related non-kinase, corresponding to the C-terminal domain of FAK; GFP, green fluorescent protein; GSK3, glycogen synthase kinase 3; GST, glutathione S-transferase; HEK-293, human epithelial kidney 293; MAPK, mitogen-activated protein kinase; PDGF, platelet-derived growth factor; PKA, protein kinase A; PP(1/2A), (type-1/type-2A) protein serine/threonine phosphatase; siRNA, short interfering RNA
INTRODUCTION
FAK (focal adhesion kinase) is a non-receptor tyrosine kinase found in focal adhesion events (reviewed in [1,2]). FAK is activated by tyrosine phosphorylation upon cell attachment and spreading, and signalling events downstream of FAK are strongly implicated in cell motility and survival [1–4]. Deregulation of FAK signalling may contribute to human cancer progression, as high FAK levels correlate with tumour invasiveness and metastasis [1,5]. Although FAK regulation by tyrosine phosphorylation has been extensively studied, FAK is also phosphorylated on serine residues. Four major serine phosphorylation sites are located in the C-terminal domain of chicken FAK [6]: S1 (Ser-722), S2 (Ser-842), S3 (Ser-845) and S4 (Ser-911), corresponding to serine residues 722, 840, 843 and 910 of human and mouse FAK. Extensive FAK serine phosphorylation occurs in mitosis, when FAK also undergoes tyrosine dephosphorylation and inactivation [6–8]. Mitotic phosphorylation involves S1 and S4 [6], two sites displaying the consensus recognition sequence for CDKs (cyclin-dependent kinases). It has been proposed that, in mitosis, when focal adhesions disassemble, phosphorylation of these residues favours dissociation of FAK from other members of its signalling complex, such as CAS (Crk-associated substrate) and paxillin [6,7]. FAK serine dephosphorylation occurs in early G1 phase, before cell spreading [8]. We reported that PP1 (serine/threonine protein phosphatase type-1) associates with FAK at this time and dephosphorylates it [8]. On the other hand, no PP1 was found to associate with mitotic FAK [8].
Much less is known about FAK serine phosphorylation in non-mitotic cells. FAK serine phosphorylation may be easily lost, possibly due to dephosphorylation by the FAK-associated PP1 [6]. Phosphorylation of FRNK (FAK-related non-kinase, corresponding to the C-terminal domain of FAK) was reported as a late response to integrin binding, potentially performed by PKA (protein kinase A) [9]. Recently, phosphorylation of Ser-910 in response to PDGF (platelet-derived growth factor) and FGF (fibroblast growth factor) was reported [10]. Phosphorylation involved Erk (extracellular-signal-regulated kinase) and a signalling pathway different from the one used by PDGF to induce FAK phosphorylation at Tyr-397 [10].
GSK3 (glycogen synthase kinase 3) is a component of a large number of cellular processes and diseases, and is regulated by insulin and growth factors, as well as during development [11–13]. GSK3 displays two isoforms, α and β, whose specific roles are little known [11,12]. Enzyme regulation is achieved by a combination of serine and tyrosine phosphorylations, subcellular localization and interaction with binding proteins [12]. Specifically, phosphorylation of the inhibitory N-terminal serine (Ser-21 in GSK3α and Ser-9 in GSK3β) is accomplished by different kinases (such as Akt, PKA, protein kinase C or p90Rsk), suggesting regulation of specific GSK3 pools that are differentially distributed within the cell [12]. Most GSK3 substrates need to be pre-phosphorylated at a ‘priming’ serine located at the +4 position with respect to the GSK3 site [11–13]. By this means, the signalling pathways which regulate the various priming kinases may influence GSK3 activity [11]. Little is known of the role of GSK3 in cell adhesion, and most of the studies involved β-catenin phosphorylation by GSK3, an essential event in cadherin-based cell–cell interactions [13].
Several years ago, we detected PP1δ in focal adhesions [14,15], which are multimolecular structures of contact between the cytoskeleton and extracellular matrix [3]. Subsequently, we found that PP1δ associated with FAK in immunoprecipitation and pull-down assays, and dephosphorylated FAK in vitro [8]. The association occurred also with FRNK [8,16], suggesting the C-terminal serine residues of FAK to be potential PP1 dephosphorylation sites.
PP1 is a ubiquitous serine/threonine phosphatase, which localizes to virtually all cell compartments and is involved in diverse cellular pathways [17,18]. The PP1 catalytic subunit displays three isoforms: α, γ1 and δ (also called β; [18,19]). The use of isoform-specific antibodies [20,21] indicated the differential subcellular localization of the isoforms [14,15,20], suggesting their differential functions. The PP1 catalytic subunits interact with a variety of regulatory subunits, which target the enzyme to specific substrates [18,22]. The targeting subunit of the FAK-directed PP1 is not known, and results indicated a direct interaction between the PP1 catalytic subunit and FAK [8,16].
The objective of the present study was to investigate the potential regulation by serine kinases and by PP1 of specific FAK phosphoserine residues. We report the identification of the spreading- and migration-dependent phosphorylation of FAK S1 (Ser-722), its inhibitory role on FAK activity and the involvement of GSK3β and PP1 in targeting this site. Most of the experiments were performed in cells of two different origins, rat fibroblasts and HEK-293 (human embryonic kidney 293) cells, obtaining the same results.
MATERIALS AND METHODS
Antibodies, enzymes and other materials
The antibodies used were as follows: anti-FAK (C-20) and anti-p-(phospho)Tyr-576/577 from Santa Cruz Biotechnology; anti-pSer-722 (pS1), anti-pSer-910 (of human FAK, pS4; the antibody also recognized pSer-911 of chicken and pSer-913 of rat FAK) and anti-pTyr-397 from BioSource; anti-GST (glutathione S-transferase) from Amersham Biosciences; anti-pS21 of GSK3α and anti-pS9 of GSK3β from Cell Signaling; anti-GSK3α and anti-GSK3β [kindly given by Dr J. R. Vandenheede (Katholieke Universiteit Leuven, Belgium)]; and PP1 isoform-specific antibodies, which were raised by us [20,21]. The PP1 catalytic subunit was purified from rabbit muscle [23]. One unit of PP1 releases 1 nmol of H3PO4/min. GSK3 (a mix of the α and β isoforms) was purified from rabbit muscle [24]. One unit of GSK3 incorporates 1 nmol of H3PO4/min, assayed with the specific GSK3 substrate ARRAAVPPSPSLSRHSSPHQS(P)EDEEE [21]. The Erk-1/2 inhibitor UO126, the p38 inhibitor SB203580, the GSK3 inhibitor kenpaullone, the CDK inhibitor roscovitine and okadaic acid (potassium salt) were from Calbiochem. The GSK3 inhibitor SB216763 was from Tocris Cookson Ltd (Bristol, U.K.). FAK−/− cells and FAK−/− cells expressing tetracycline-repressed FAK have been described previously [4].
Plasmid preparation and transfection
Point mutations were performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and PAGE-purified synthetic oligonucleotides (Primm), and were confirmed by DNA sequencing (Primm). GST–FRNK (residues 684–1053 of chicken FAK, subcloned into pGEX-2TK) was a gift from Dr J. T. Parsons (University of Virginia, Charlottesville, VA, U.S.A.). The following PAGE-purified synthetic oligonucleotides (Primm) were used: 5′-CCTGGTTACCCCGCCCCAAGGTCCAGTG-3′ and 5′-CACTGGACCTTGGGGCGGGGTAACCAGG-3′ (antisense) for the Ser-722 (S1)→Ala (i.e. S1A) mutation; 5′-CC-TGATGTGCGGCTCGCCAGAGGCGCCATTGAACGGGAGGAC-3′ and 5′-GTCCTCCCGTTCAATGGCGCCTCTGGCGAGCCGCACATCAGG-3′ (antisense) for the S2A/S3A mutations; and 5′-GCCACAGGAAATCGCCCCTCCTCCTACGG-3′ and 5′-CCGTAGGAGGAGGGGCGATTTCCTGTGGC-3′ (antisense) for the S4A mutation.
Escherichia coli BL-21 protease-minus bacteria were used to produce the GST fusion proteins. Full-length chicken FAK cDNA in pBluescript SK was subcloned into the pcDNA/HisMax TOPO vector. The S1A and S4A mutations were introduced as above. Lipofectamine™ 2000 (Invitrogen) was used to transfect the FAK constructs in FAK−/− fibroblasts [4].
Wild-type and S1A FAK stable transfectants
FAK−/− fibroblasts [4] were co-transfected with both the pcDNA/HisMax TOPO vector expressing either wild-type or S1A FAK (see above) and the biCS2puro/GFP (green fluorescent protein) puromycin-resistance vector, or with the puromycin-resistance vector alone [25]. Cells were exposed to 4 μg/ml puromycin starting from 24 h after transfection, grown for 2–3 weeks and subcloned by dilution in the presence of 40 μg/ml Zeocin™ (the Zeocin™-resistance gene was present in the pcDNA/HisMax TOPO vector). Clones expressing equal levels of wild-type and mutant proteins were used in the experiments.
GSK3α and GSK3β silencing
Rat fibroblasts were co-transfected with the hairpin siRNA (short interfering RNA) expression vectors U6-GSK3αHP1 or U6-GSK3βHP1 [25] and the biCS2puro/GFP puromycin-resistance vector, or with the puromycin-resistance vector alone, using Lipofectamine™ 2000 [25]. Cells were exposed to 10 μg/ml puromycin starting from 24 h after transfection, collected at 72 h and lysed or re-plated on fibronectin.
Bacterial growth, extracts and pull-down assays
Bacteria growth, induction and extraction have been described elsewhere [8]. For pull-down assays, the GST fusion proteins were bound to 25–50 μl of glutathione–Sepharose beads and processed as described in [8].
Cell treatments, extracts and staining
HEK-293 cells, Fisher rat fibroblasts and FAK−/− fibroblasts [4] were grown as described in [8]. For adhesion experiments, tissue-culture dishes were first treated with either 10 μg/ml fibronectin or 20 μg/ml poly(L-lysine) (Sigma–Aldrich) for 30 min at room temperature (≈25 °C). Inhibitor treatments of 90% confluent or resuspended cells were performed as described specifically in the Figure legends. Cells were lysed in 50 mM Tris/HCl, pH 7.5, containing 250 mM NaCl, 5 mM EDTA, 0.1% (v/v) Triton X-100, 1 mM orthovanadate, 7.5 mM 2-mercaptoethanol, protease inhibitors [8] and 1 μM okadaic acid or 50 mM NaF, unless they were for extracts used to assay PP1 activity. For staining, cells were washed in PBS, fixed in 3% (w/v) paraformaldehyde/2% sucrose in PBS, pH 7.5, at room temperature for 10 min, washed in PBS and stained with Giemsa stain.
In vitro cell migration into a wounded area and trans-well assays
For the scratch wound assay, 50 scratches were made on each 60 mm plate of confluent rat fibroblasts using a yellow pipette tip [26]. At the indicated time points, the cells were either lysed or fixed for Giemsa staining (as detailed above). Trans-well assays used Trans-well permeable supports (12 mm diameter, 8 μm pore size; Corning), adding medium with 10% (v/v) fetal-calf serum to the lower chamber and 0.2% BSA in serum-free medium to the upper insert. Following overnight migration, cells were fixed as described above, stained with Crystal Violet, counted under the microscope and lysed in 10% (v/v) acetic acid for measurement of attenuance at 595 nm.
Immunoprecipitation and immunoblotting
Anti-FAK or anti-GSK3α or -β antibodies or anti-PP1δ hyperimmune rabbit serum (10 μl/ml of extract) were used in immunoprecipitation experiments with Protein A–Sepharose [8,20]. Electrophoresis, transblotting, immunodetection and re-probing after stripping were as described previously [8,20].
FAK kinase assay
FAK was immunoprecipitated and incubated in 50 μl of 25 mM imidazole, pH 7.5, containing 50 mM NaCl, 12 mM MgCl2, 4 mM MnCl2, 0.2 mM EGTA, 0.05% Triton X-100, 3% glycerol, 0.1 mM orthovanadate, 5 mg/ml poly(Glu-Tyr) (Sigma–Aldrich), 100 μM [γ-32P]ATP (Amersham Biosciences; 2000–2500 c.p.m./pmol) at 30 °C for 15 min [27]. The reaction was terminated by spotting 15 μl on to Whatman P81 chromatography paper (2 cm×1 cm), followed by washing in 10 mM H3PO4 and counting. The kinase radioactivity [fmol of Pi incorporated·min−1·(mg of cell extract)−1] was calculated after subtracting the c.p.m. of control pre-immune complexes. The Src kinase inhibitor PP2 (Tocris Cookson Ltd) was added to some assays to rule out a contribution from FAK-bound Src.
Phosphatase assay
PP1 activity was assayed on immunocomplexes in the presence of 5 nM okadaic acid [to inhibit any PP2A (protein serine/threonine phosphatase type-2A)] and using 32P-labelled phosphorylase a as substrate [8]. Phosphatase activity {fmol of [32P]H3PO4 released·min−1·(mg of cell extract)−1} was calculated after subtracting the c.p.m. of pre-immune control complexes run in parallel.
Peptide synthesis
The peptide KPSRPGYPSPRSpSEGFY (residues 714–730 of FAK) and its mutated derivative with Ser-722 replaced by alanine were synthesized by automated solid-phase synthesis utilizing Fmoc (fluoren-9-ylmethoxycarbonyl) chemistry with an Applied Biosystems Model 431A Synthesizer on a 4-(hydroxymethyl)-phenoxymethyl-co-polystyrene/1% divinylbenzene resin (PerkinElmer). Fmoc amino acids were activated by adding HBTU [2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate] and 1-hydroxybenzotriazole [28]. The phosphopeptide was obtained by direct incorporation of phosphoserine as the Fmoc-serine[PO(O-benzyl)-OH] derivative (Novabiochem). Peptidyl resins were cleaved and deprotected following the procedure of King et al. [29]. The peptides were purified on a preparative C18 reversed-phase column. The resultant peptides were 95% pure, as judged by analytical HPLC on a 5 μm C18 Symmetry 300 column (4.6 mm×250 mm; Waters Instruments) using a linear gradient of 10–40% acetonitrile in 0.1% trifluoroacetic acid at 1 ml/min. The molecular masses of the peptides were confirmed by mass spectrometry with direct infusion on a Micromass ZMD-4000 Mass Spectrometer (Waters Micromass).
Phosphorylation of S1 of FRNK and of the synthetic phosphopeptide by GSK3
Wild-type and S1A GST–FRNK were pulled-down as described above and divided into five aliquots. One aliquot was incubated with 0.1 mg of HeLa cell extract [8], as a source of the S726 kinase, and three aliquots were incubated with extract, 500 μM ATP and 10 mM MgCl2 (one with additional 50 mM LiCl). The incubation was carried out at 30 °C for 20 min with shaking. After washing with 25 mM imidazole, pH 7.5, three reactions were terminated by adding electrophoresis sample buffer, whereas one tube was incubated further with 62 m-units of purified GSK3 [24] diluted in 25 mM imidazole, pH 7.5/25 mM 2-mercaptoethanol in the presence of 500 μM ATP and 10 mM MgCl2 at 30 °C for 40 min with shaking. The remaining fifth bead aliquot was incubated with GSK3, ATP and MgCl2 as described above. For peptide phosphorylation, the indicated amounts of the peptide KPSRPGYPSPRSpSEGFY (residues 714–730 of FAK) or its S722A mutant were incubated with 10.3 m-units of purified GSK3 [24] in 50 μl of 25 mM imidazole, pH 7.5, 10 mM MgCl2, 50 mM [γ-32P]ATP (1500–2000 c.p.m./pmol) at 30 °C for 10 min [21]. The reaction was terminated and processed as in the case of FAK assay. The kinase activity (pmol of Pi incorporated/min) was calculated after subtracting the c.p.m. of controls prepared without peptide.
GSK3 assay on anti-FAK immunocomplexes
FAK, immunoprecipitated from rat fibroblasts plated on fibronectin, was resuspended in 50 μl of 25 mM imidazole, pH 7.5/25 mM 2-mercaptoethanol containing 200 μM of the synthetic phosphopeptide KPSRPGYPSPRSpSEGFY, 10 mM MgCl2, 50 μM [γ-32P]ATP (1500–2000 c.p.m./pmol) and then incubated at 30 °C for 20 min. The reaction was terminated and processed as in the case of FAK activity. The kinase activity [fmol of Pi incorporated·min−1·(mg of extract used to immunoprecipitate FAK)−1] was calculated after subtracting the c.p.m. of controls, prepared without peptide.
RESULTS
S1 is phosphorylated during cell spreading
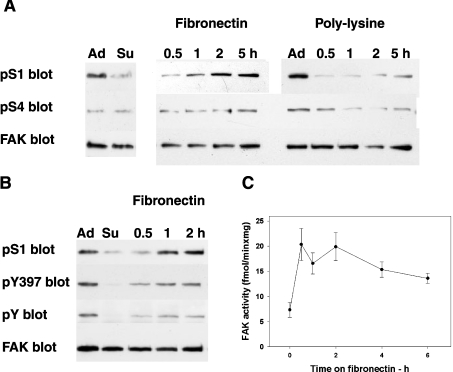
The changes in the phosphorylation of S1 (Ser-722) and S4 (Ser-911 in chicken and Ser-910 in human and mouse FAK) were investigated during cell adhesion and spreading, events that activate FAK tyrosine phosphorylation and signalling [1,2,4]. For this purpose, cells were plated either on fibronectin, which enhances cell adhesion and spreading, or on polylysine, which delays these processes. The experiments were performed in both rat fibroblasts and HEK-293 cells. Cells were grown overnight, collected by trypsin/EDTA treatment, re-plated on dishes coated with either fibronectin or polylysine and grown for up to 5–6 h. The impaired spreading on polylysine was confirmed by microscopic observation (results not shown). Cell extracts were prepared from adherent, resuspended and re-plated cells (collected at the indicated time points; Figure 1A). Phospho-S1 (pS1) decreased in cells in suspension and increased again in cells plated on fibronectin, reaching the level detected in adherent cells in approx. 2 h, whereas it remained low in cells plated on polylysine (Figure 1A). By contrast, phospho-S4 (pS4) was always low (Figure 1A), indicating that S4 phosphorylation was not affected by the adhesion/spreading processes. As expected, FAK underwent activation during spreading on fibronectin, as indicated by the increases in FAK pTyr-397, FAK tyrosine phosphorylation (Figure 1B) and FAK kinase activity [assayed with the exogenous substrate poly(Glu/Tyr); Figure 1C]. Assays performed in the presence of the Src inhibitor PP2 indicated that Src did not contribute to the kinase activity assayed (results not shown). Comparison between the kinetics of FAK activity and pS1 indicated that FAK activation preceded the increase in pS1. Similar results were obtained in rat fibroblasts (results not shown). In addition, S1 phosphorylation did not require FAK activation, since it also occurred in the case of the inactive Y397F FAK mutant (results not shown).
Figure 1. FAK phosphorylation and activation during spreading on fibronectin.
(A) Phosphorylation of FAK at sites S1 (Ser-722) and S4 (Ser-911 in chicken, Ser-910 in human and mouse and Ser-913 in rat FAK) during adhesion/spreading on fibronectin or polylysine. Rat fibroblasts were collected by trypsin/EDTA treatment, re-plated on dishes coated with 10 μg/ml fibronectin or 20 μg/ml polylysine and grown for up to 5 h. Lysates obtained from adherent (Ad) or resuspended (Su) fibroblasts, or from fibroblasts collected at the indicated time points were subjected to electrophoresis and Western blotting and probed separately to detect phospho-S1 (pS1), phospho-S4 (pS4) or FAK. (B) Activation of FAK during spreading on fibronectin. Cell lysates obtained as described in (A) were probed for pS1, pTyr-397 of FAK (pY397), phosphotyrosine of FAK (pY) and FAK. (C) FAK activity during spreading on fibronectin. HEK-293 cells were resuspended (at zero time), re-plated on fibronectin and collected at the indicated time points. FAK was immunoprecipitated and assayed for kinase activity using the substrate poly(Glu-Tyr). Results are shown as the means±S.E.M. for 6 separate experiments.
S1 phosphorylation decreases the kinase activity of FAK
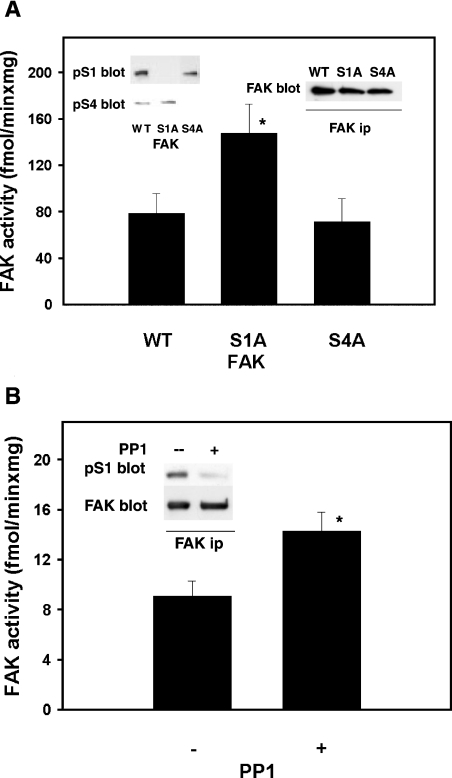
To test the effect of S1 phosphorylation on FAK activity, FAK constructs [either wild-type or mutated to encode the serine-toalanine mutation of S1 (S1A) or S4 (S4A)], were prepared in the pcDNA/HisMax TOPO vector and expressed in FAK−/− fibroblasts (Figure 2A, left inset). Immunoprecipitation of the three FAK types 24 h after transfection (Figure 2A, right inset), followed by kinase assay, indicated that S1A FAK was significantly more active than wild-type or S4A FAK (Figure 2A, bar graph). This result suggests that S1 phosphorylation acts to impair FAK activity. To test this hypothesis further, FAK was immunoprecipitated from fibroblasts or HEK-293 cells, incubated in vitro with purified PP1 catalytic subunit (the serine phosphatase that associates with FAK; [8] and Figure 8), and tested for pS1 level and FAK activity. PP1 treatment decreased pS1 (Figure 2B, inset) and increased FAK activity to some degree (Figure 2B, bar graph). Thus S1 phosphorylation functions to negatively modulate FAK activity, and this can be overcome through PP1-mediated dephosphorylation of this site.
Figure 2. Activation state of wild-type and FAK mutants, and FAK activity following dephosphorylation by PP1.
(A) Activation state of wild-type FAK and the S1A and S4A mutants. FAK−/− cells were transfected with a vector encoding either wild-type chicken FAK (WT) or the FAK point mutants S1A or S4A. After 24 h, cell lysates were prepared and subjected to electrophoresis, Western blotting and probing for pS4 and pS1 in sequence (left inset). FAK was immunoprecipitated (ip) from cell lysates and probed for FAK (right inset) or assayed for the protein kinase activity, as described in Figure 1(C) (bar graph). Results shown are the means±S.E.M. for 5 determinations (6 for S1A); *P≤0.05 (Student's t test) for S1A compared with WT. (B) Kinase activity of FAK treated in vitro with PP1. FAK was immunoprecipitated from rat fibroblasts in duplicate, and the immunocomplexes were incubated further with either 1 unit of PP1 catalytic subunit (purified from muscle; +) or PP1 inhibited by 0.6 μM okadaic acid (−) at 30° for 20 min and assayed for kinase activity (bar graph). Results are shown as means±S.E.M. for 5 determinations; *P≤0.05 (Student's t test). The inset shows dephosphorylation of S1 by PP1. FAK was immunoprecipitated and treated with PP1 as above. This was followed by electrophoresis, Western blotting, probing for pS1, stripping and re-probing for FAK.
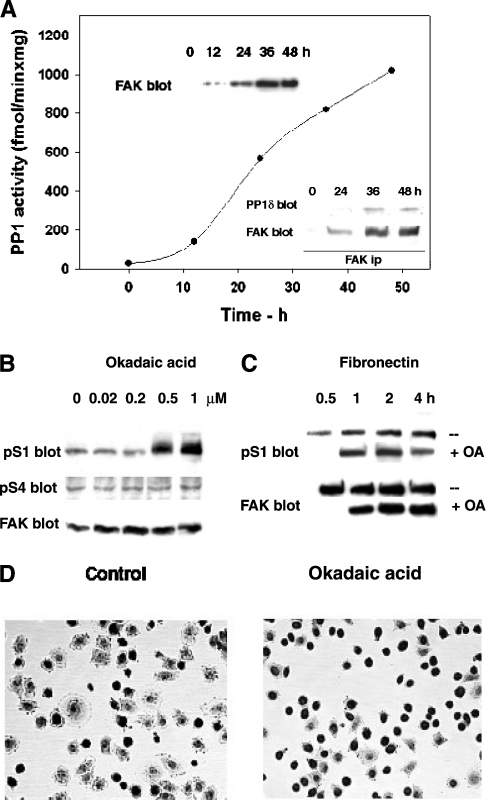
Figure 8. Association of PP1 and FAK, and FAK dephosphorylation by PP1.
(A) PP1 binding to FAK during FAK induction. FAK−/− fibroblasts carrying a tetracycline-repressed FAK construct were grown in the absence of tetracycline for up to 48 h, and collected at the indicated time points. FAK was immunoprecipitated from cell lysates and assayed for the associated PP1 activity (see the Materials and methods section). Top inset: FAK expression. FAK was immunodetected in lysates from cell extracts obtained as above. Lower inset: association of PP1 and FAK. FAK was immunoprecipitated, subjected to electrophoresis and Western blotting and probed for the presence of PP1δ and FAK, after membrane stripping. (B) S1 and S4 phosphorylation in adherent cells treated with okadaic acid. Rat fibroblasts were treated with the indicated amounts of the PP1 inhibitor okadaic acid for 1 h, and the lysates were subjected to electrophoresis and immunoblotting. Two sets of samples were prepared, one to detect pS4 and FAK following membrane stripping, the second to detect pS1. (C) S1 phosphorylation in cells treated with okadaic acid during spreading on fibronectin. Rat fibroblasts were plated on fibronectin and exposed to 0.5 μM okadaic acid (+) or DMSO (−) 30 min after plating. Cells were collected as indicated and the lysates were subjected to electrophoresis and Western blotting to detect pS1 and FAK. (D) Cells at 2 h from plating on fibronectin in the presence of okadaic acid. Rat fibroblasts were plated on fibronectin in the absence (control) or in the presence of okadaic acid (as in C), fixed at 2 h from plating and stained with Giemsa stain.
GSK3 inhibition decreases S1 phosphorylation and activates FAK in spreading cells
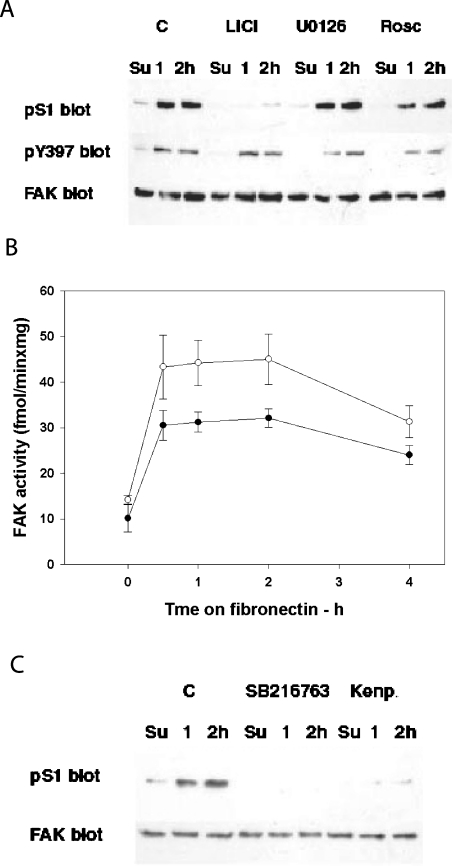
We next sought to determine which cellular kinase acts to phosphorylate S1. The S1 site displays a consensus sequence for proline-directed kinases [30], such as MAPKs (mitogen-activated protein kinases) and CDKs. Additionally, a serine residue at +4 makes it a potential GSK3 substrate [11–13,30]. Cells were treated with several kinase inhibitors [31], and the pS1 level was assayed. Adherent fibroblasts treated with the GSK3 inhibitor LiCl for 0.5–1 h displayed decreased pS1 at 10 mM LiCl and total S1 dephosphorylation at 50 mM LiCl (results not shown). LiCl and other inhibitors were tested during cell spreading on fibronectin. pS1 was greatly reduced in the presence of LiCl (Figure 3A), whereas neither the Erk-1/2 inhibitor UO126 nor the p38 inhibitor SB203580 (results not shown), nor the CDK inhibitor roscovitine, decreased pS1 (Figure 3A). None of these treatments, including LiCl, affected the increase in FAK Tyr-397 phosphorylation, the hallmark of FAK reactivation in cell adhesion (Figure 3A). The targeting of S1 by GSK3 was confirmed by the use of two other GSK3 inhibitors, SB216763 and Kenpaullone (Figure 3C). However, the inhibitors did not indicate which GSK3 isoform was involved, since they inhibited both GSK3-α and -β (results not shown).
Figure 3. FAK phosphorylation in cells treated with the inhibitors of Pro-directed kinases, and effect of LiCl on FAK activity during spreading on fibronectin.
(A) Phosphorylation of S1 and Tyr-397 of FAK in cells treated with the inhibitors of Pro-directed kinases. Rat fibroblasts were plated on fibronectin in the presence of 40 mM LiCl (to inhibit GSK3), 10 μM UO126 [to inhibit MAPK (Erk-1/2)], 30 μM roscovitine (to inhibit CDKs) or DMSO as a control (C). Lysates from cells collected at the indicated time points were used to prepare three sets of immunoblots, which were then probed for pS1, pTyr-397 (pY397) and FAK. (B) Effect of LiCl on FAK activity during spreading on fibronectin. Lysates obtained from cells treated with LiCl as in (A) (○) or from untreated controls (●) were used to immunoprecipitate FAK and assay FAK kinase activity (as described in legend for Figure 1C). Results are shown as means±S.E.M. for 4 determinations. (C) Phosphorylation of S1 in cells treated with the GSK3 inhibitors SB216763 or Kenpaullone (Kenp.). Cells were treated with either 5 μM SB216763 or 10 μM Kenpaullone; other treatments were as for (A). Su, resuspended fibroblasts.
We also investigated whether the dephosphorylation of S1 induced by the GSK3 inhibitors affected the kinase activity of FAK. For this purpose, rat fibroblasts were plated on fibronectin in the presence of LiCl. The kinase activity of immunoprecipitated FAK was 20–35% higher in fibroblasts (Figure 3B) or HEK-293 cells (results not shown) treated with LiCl. This confirmed that pS1 may regulate FAK activity in a negative way.
GSK3β is involved in S1 phosphorylation and associates with FAK during spreading
To test which GSK3 isoform was involved in S1 phosphorylation, we silenced either GSK3α or GSK3β in rat fibroblasts using isoform-specific hairpin siRNAs, synthesized in the cell from transiently transfected DNA-expression vectors [25]. To increase the system efficiency, cells were co-transfected with a puromycin-resistance vector and the non-transfected cells (70–80%) were eliminated by exposing them to puromycin [25]. Figure 4(A) shows the almost complete silencing of the targeted isoform obtained within 72 h from transfection. Silencing of GSK3β was accompanied by loss of S1 phosphorylation, whereas S1 was phosphorylated in GSK3α-silenced and control cells (Figure 4A). As a further test, the GSK3β-silenced cells were allowed to spread on fibronectin. pS1 did not increase in the GSK3β-silenced cells, even under fibronectin stimulation (Figure 4B).
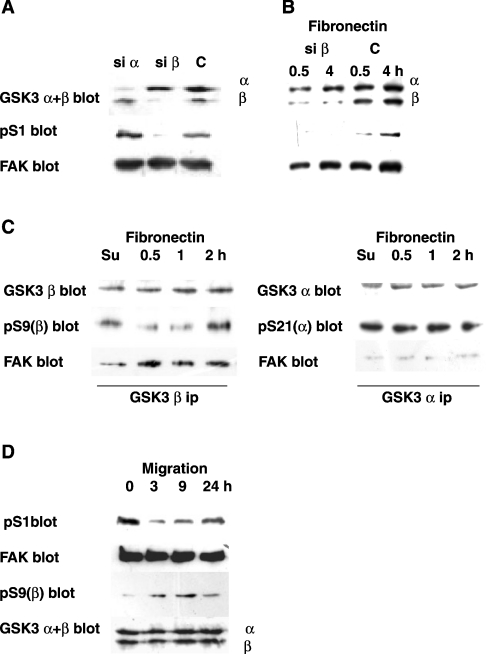
Figure 4. Involvement of GSK3b in FAK phosphorylation during spreading on fibronectin and migration.
(A) Involvement of GSK3β in S1 phosphorylation. Rat fibroblasts were co-transfected with either the GSK3α (si α) or the GSK3β (si β) hairpin siRNA vector, together with a vector that expresses the puromycin-resistance gene. Control cells (C) were transfected with the puromycin-resistance vector only. At 24 h after transfection, the cells were exposed to 10 μg/ml puromycin to kill non-transfected cells, and collected at 72 h. Cell lysates were used to detect the GSK3 isoforms (using a mix of anti-GSK3α and β antibodies; GSK3 α+β blot), pS1 and FAK. (B) Effect of GSK3β silencing on S1 phosphorylation during spreading on fibronectin. Transfected rat fibroblasts (si β and C, as in A) were collected at 72 h and re-plated on fibronectin. Lysates obtained at 0.5 or 4 h were used to detect GSK3 α and β, pS1 and FAK. (C) GSK3β activation and association with FAK during spreading on fibronectin. Lysates from rat fibroblasts collected during spreading on fibronectin were used to immunoprecipitate GSK3β (GSK3β ip) or GSK3α (GSK3α ip). Following electrophoresis and transblotting, the blots were probed to detect GSK3β, pS9 of GSK3β [pS9(β) blot] and FAK, in sequence or GSK3α, pS21 of GSK3α [pS21(α) blot] and FAK, in sequence. (D) S1 phosphorylation in scratch-wound healing. Confluent rat fibroblasts were scratched and induced to migrate, as described in the Materials and methods section. Cells were collected at 3, 9 and 24 h from scratching, as well as from an untreated control (zero time). Lysates were subjected to electrophoresis (loading the same amounts of protein in each lane) and Western blotting. Separate blots were probed to detect pS1, FAK, pS9 of GSK3β and GSK3 α+β.
To confirm further the role of GSK3β, we investigated its changes in activity during spreading on fibronectin through the phosphorylation level of its Ser-9 inhibitory site. GSK3β and GSK3α were immunoprecipitated from cells collected during spreading on fibronectin. pS9 was higher in resuspended cells, indicating GSK3β inactivation, than in spreading cells, indicating GSK3β activation during spreading (Figure 4C). No activity change was detected in GSK3α (based on its Ser-21 inhibitory site; Figure 4C), thus confirming that GSK3α is not affected by spreading. Little FAK co-immunoprecipitated with GSK3β in resuspended cells, but the amount increased during spreading, whereas none did with GSK3α (Figure 4C) or control complexes (results not shown). Detection of the physical association between GSK3β and FAK supported further the role of GSK3β in S1 phosphorylation.
S1 phosphorylation decreases and GSK3β is inactivated in migrating cells
Since FAK activation promotes cell migration [1], we investigated the changes in S1 phosphorylation in an in vitro wound-healing experiment. Confluent fibroblast monolayers were scratched and incubated further for up to 24 h. At 3 h, cells were starting to migrate from the wound edges; at 9 h, more cells were migrating and by 24 h, the wound closure was almost complete (results not shown). pS1 decreased at 3 h, increased at 9 h and was almost back to the control level at 24 h (Figure 4D). The data suggested that pS1 might have a role in regulating FAK also in cell migration. Phosphorylation of GSK3β at Ser-9 increased at 3 and 9 h, and decreased again at 24 h, whereas the total level of GSK3β remained constant. The concomitant decrease in both pS1 and GSK3β activity supported the hypothesis that GSK3β also phosphorylates S1 during cell migration.
Serine-to-alanine mutation of S1 favours spreading
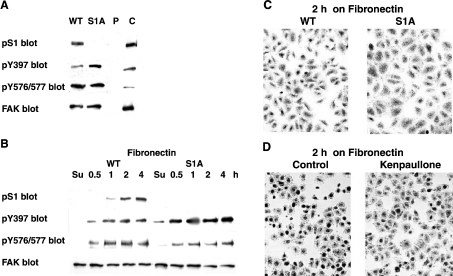
To test further the biological relevance of pS1, we prepared FAK−/− stable transfectants expressing either wild-type FAK or the S1A mutant (described further in the Materials and methods section). The recombinant proteins displayed Mr values slightly higher than FAK from fibroblasts due to the presence of a polyhistidine tag (Figure 5A). The absence of S1 (Figure 5A) was accompanied by an increase in FAK phosphorylation at its Tyr-397 autokinase site. This indicates FAK activation, and is in agreement with the FAK activation observed when loss of pS1 was due to dephosphorylation by PP1 or GSK3 inhibition (Figures 2 and 3). Cells expressing wild-type or S1A FAK were also investigated during spreading. The results showed that also during fibronectin stimulation the S1A mutant was more active than wild-type FAK, based on the increased pTyr-397 (Figure 5B). On the other hand, FAK phosphorylation at Tyr-576/577 (two other regulatory sites, targeted by Src-family kinases) was slightly decreased in the S1A mutant (Figures 5A and 5B). Furthermore, cells expressing S1A FAK were more spread, particularly at 2 h after plating on fibronectin, than cells expressing wild-type FAK (Figure 5C) or cells expressing the puromycin vector alone (results not shown). Improved spreading can be explained by the detected FAK activation. A similar result was obtained when pS1 loss was induced by exposing the cells to the GSK3 inhibitor Kenpaullone (Figure 5D). Taken together, the data strongly support the hypothesis that the absence of S1 phosphorylation favours FAK activation and cell spreading. This confirms the inhibitory role of pS1 towards FAK activity and its occurrence during late spreading (see Figure 10).
Figure 5. Effect of stable expression of the S1A FAK mutant or of GSK3 inhibition on cell spreading on fibronectin.
(A) Stable expression of wild-type and S1A FAK in FAK−/− cells. Stable transfectants of chicken FAK were prepared and subcloned (as described further in the Materials and methods section). Cell lysates prepared from these cells, as well as from cells transfected only with the puromycin-resistance vector (P) or from rat fibroblasts (C), were used to immunodetect pS1, pTyr-397 (Y397), phospho-Y576/Y577 of FAK (pY576/577) and FAK. (B) Phosphorylation of S1, Y397 and Y576/577 during spreading on fibronectin of FAK−/− cells expressing WT or S1A FAK. Cells, as in (A), were re-plated on fibronectin and collected at the indicated time-points (as in Figure 1A). Immunodetection was as in (A). (C) FAK−/− cells expressing WT or S1A FAK at 2 h from plating on fibronectin. Cells, as in (A), were plated on fibronectin, fixed at 2 h and stained with Giemsa stain. (D) Rat fibroblasts at 2 h from plating on fibronectin in the presence of the GSK3 inhibitor Kenpaullone. Fibroblasts were plated on fibronectin in the absence (control) or in the presence of 10 μM Kenpaullone (see Figure 3), fixed and stained as in (C).
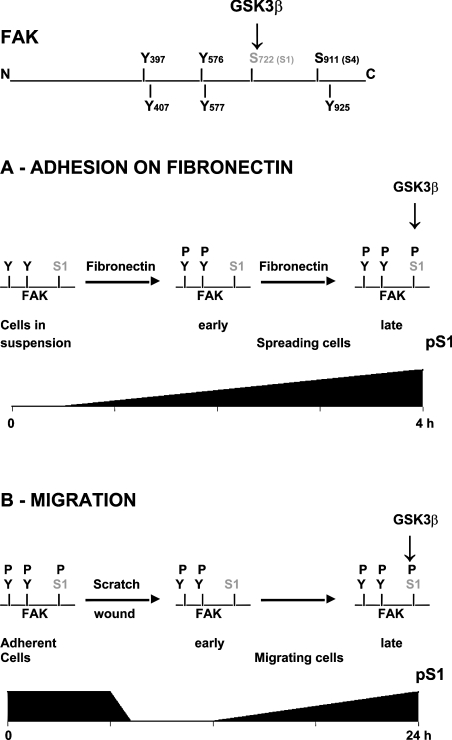
Figure 10. FAK scheme depicting the major tyrosine and serine phosphorylation sites.
S1 phosphorylation by GSK3β and changes in S1 phosphorylation during cell adhesion on fibronectin and cell migration are shown.
Serine-to-alanine mutation of S1 enhances cell migration
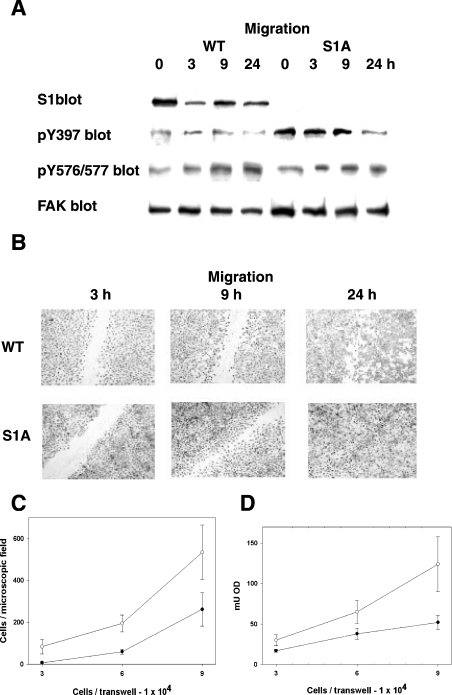
We also tested the relevance of pS1 during cell migration, an event that involves changes in pS1 (Figure 4D). Monolayers of cells expressing either wild-type or S1A FAK were scratched and incubated further for up to 24 h. At all time points considered, S1A FAK displayed higher pTyr-397 than wild-type FAK, suggesting FAK activation, whereas pTyr-576/577 was unchanged (Figure 6A). Indeed, cells expressing S1A FAK migrated faster (Figure 6B, ‘9 h’ panel) and the in vitro wound was completely closed by 24 h, whereas it was still detectable in cells expressing wild-type FAK (Figure 6B, ‘24 h’ panel). The higher motility of the S1A cells was confirmed further by trans-well migration experiments. Different amounts of cells were applied to trans-well inserts, and cell migration was assessed by two different approaches used sequentially. First, the migrated cells were stained and counted under the microscope (Figure 6C); then cells were lysed, and the attenuance of the stain released from the cells was measured (Figure 6D). This approach also indicated the higher motility of the cells expressing the S1A mutant.
Figure 6. Effect of stable expression of the S1A FAK mutant on cell migration.
(A) Phosphorylation of S1, Tyr-397 and Y576/577 in scratch-wound healing of FAK−/− cells expressing WT or S1A FAK. Confluent cells were induced to migrate by scratching (as in Figure 4D). The lysates of cells collected at the indicated time points were used to detect pS1, pTyr-397 (pTyr397), pY576/577 and FAK. (B) Scratch-wound healing of FAK−/− cells expressing WT or S1A FAK. Cells as in (A) were fixed at the indicated time-points and stained with Giemsa stain. (C and D) Trans-well migration assay of FAK−/− cells expressing WT or S1A FAK. The indicated amounts of cells were applied to the upper chamber of trans-well membranes, grown overnight and fixed (as detailed further in the Materials and methods section). The migrated cells were stained with Crystal Violet, counted (C) and lysed for subsequent O.D. (attenuance) determination (D).
GSK3 phosphorylates S1 directly
To test whether the phosphorylation by GSK3 is direct, we investigated S1 phosphorylation of FRNK. Many GSK3 substrates display a consensus sequence for Pro-directed kinases, with an additional serine residue at +4, whose phosphorylation is required to support the phosphorylation of the GSK3 site. We reasoned that this might be the case also with S1. Not knowing the identity of the supporting kinase, we used HeLa cell extracts to pre-phosphorylate FRNK (Figure 7A). Such extracts contained also GSK3, as indicated by the decrease in pS1 in the presence of LiCl (results not shown). However, subsequent incubation with purified GSK3 increased pS1 further. No pS1 was detected without pre-phosphorylation with extract, or in the case of FRNK carrying the S1A mutation (Figure 7A), suggesting that GSK3 phosphorylates S1 provided that the serine position at +4 is also phosphorylated.
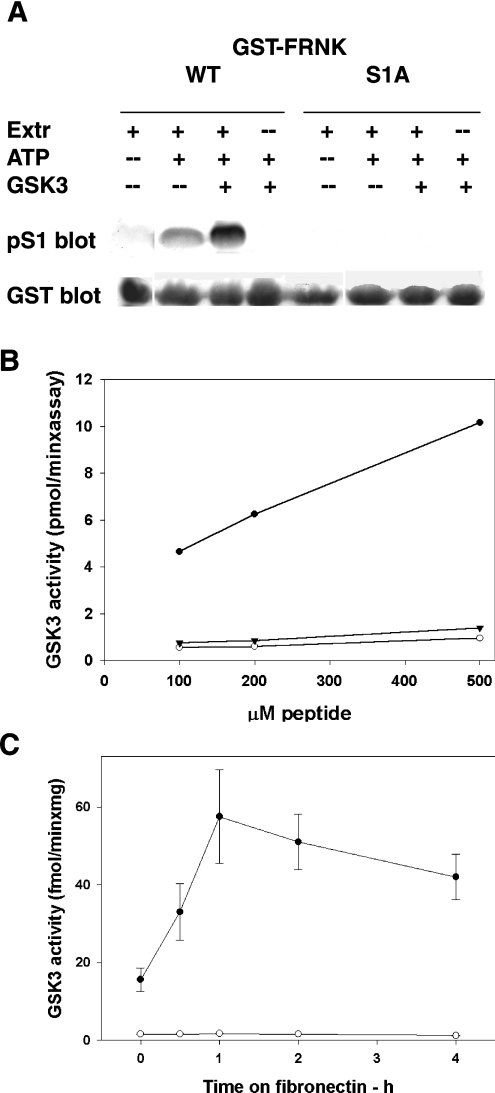
Figure 7. Phosphorylation of FRNK or of a synthetic peptide by GSK3, and association of FAK and GSK3 during spreading on fibronectin.
(A) Phosphorylation of S1 of FRNK by GSK3. GST–FRNK (C-terminus of FAK), either the wild-type or the S1A mutant protein, was bound to glutathione–Sepharose beads, divided into aliquots and incubated as follows: one aliquot with 0.1 mg of HeLa cell extract (Extr) and two aliquots in the presence of extract and ATP/MgCl2. One tube was incubated further with 62 m-units of purified GSK3. GSK3 and ATP/MgCl2 were also added to a remaining fourth aliquot. This was followed by electrophoresis, Western blotting, probing for pS1, stripping and re-probing for GST. (B) Phosphorylation of a peptide mimicking the FAK-S1 site by GSK3. The phosphopeptide KPSRPGYPSPRSpSEGFY (FAK residues 714–730) (●) or its mutated S1A derivative (○) were incubated with 10.3 m-units of purified GSK3, under conditions described further in the Materials and methods section. The wildtype peptide was also incubated in the presence of 50 mM LiCl (▼). Results shown are the mean values for 6 determinations. (C) FAK-associated GSK3 kinase activity during cell spreading on fibronectin, assayed with the peptide mimicking the S1 site. FAK was immunoprecipitated from rat fibroblasts at the indicated time points and assayed for the associated kinase activity with the phosphopeptide KPSRPGYPSPRSpSEGFY (FAK residues 714–730) in the absence (●) or the presence (○) of 50 mM LiCl, under conditions described further in the Materials and methods section. Results are shown as the means±S.E.M. for 4 determinations.
To definitively prove the direct phosphorylation of S1 by GSK3, we prepared the phosphopeptide KPSRPGYPSPRSpSEGFY, which represents residues 714–730 of FAK and is pre-phosphorylated at the +4 serine position. Its mutated S1A derivative was used as a control. Purified GSK3 was able to phosphorylate the phosphopeptide but not its mutated derivative, indicating that GSK3 phosphorylates S1. Moreover, phosphorylation did not occur in the presence of LiCl, confirming further the GSK3 involvement (Figure 7B).
The phosphopeptide was also used to investigate the association of GSK3 with FAK during spreading on fibronectin. FAK was immunoprecipitated from rat fibroblasts plated on fibronectin, and assayed for the associated kinase activity using the peptide KPSRPGYPSPRSpSEGFY as substrate (Figure 7C). The results indicated that the activity increased, attaining a peak after 1 h, which was sustained for up to 4 h. This fits with the changes in pS1 described in Figure 1(A), and supports further the role of GSK3 as the S1 kinase.
S1 is targeted by PP1 in adherent and spreading cells
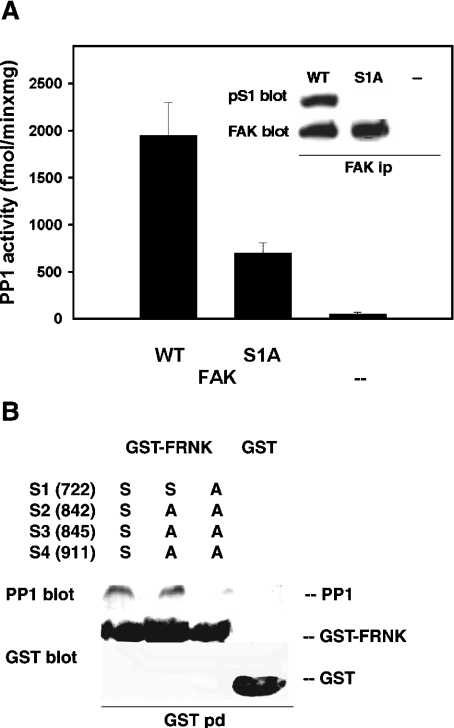
PP1 localizes to focal adhesions [14,15], co-immunoprecipitates with FAK [8] and dephosphorylates S1 in vitro (Figure 2B). The association of PP1 with FAK was confirmed in FAK−/− fibroblasts carrying a tetracycline-repressed FAK construct [4]. Following tetracycline removal, as FAK progressively accumulated (Figure 8A, top inset), progressively more PP1 activity was found to co-immunoprecipitate with FAK (Figure 8A, graph and lower inset). This result suggests constitutive association of PP1 with FAK.
To test the involvement of PP1 in dephosphorylating pS1 in the cell, we treated fibroblasts with the PP1/PP2A inhibitor okadaic acid. Such a treatment increases the phosphorylation of the serine/threonine residues that are targeted by the inhibited phosphatase. Okadaic acid below 0.2 μM inhibited only PP2A, whereas higher concentrations also inhibited PP1 (results not shown, and [32,33]). In adherent cells, only 0.5 and 1 μM okadaic acid increased pS1, suggesting the presence of an active pS1-directed PP1, whereas pS4 was not affected (Figure 8B). To investigate further the S1-directed PP1, we allowed the cells to spread on fibronectin in the presence of 0.5 μM okadaic acid added 30 min after plating. The results indicated maximal activation of the S1-directed PP1 at 1–2 h after plating on fibronectin, and decrease at 4 h (Figure 8C). This suggested S1 targeting by both GSK3 and PP1 at the same time, which may result in a finer control of site phosphorylation. Cell observation indicated that okadaic acid impaired cell spreading (Figure 8D), thus confirming the role of PP1 in the control of this process. Although okadaic acid may inhibit several other PP1 functions, the inhibitory role of pS1 may contribute to the impaired cell spreading.
PP1 binding to FAK decreases upon serine-to-alanine mutation of S1
To test further the targeting of S1 by PP1, wild-type or S1A FAK were expressed in FAK−/− fibroblasts (Figure 9A, inset). FAK immunoprecipitation followed by PP1 assay detected significantly less PP1 associated with the S1A mutant than with wild-type FAK (Figure 9A, bar chart), hence indicating S1 targeting by PP1. However, the presence of PP1 still co-immunoprecipitating with the S1A FAK suggests that PP1 might also target other FAK sites. To test PP1 binding further, we mutated S1 and three other potential PP1 targets in FRNK, i.e. S2 (Ser-842), S3 (Ser-845) and S4 (Ser-911 of chicken FAK). The S2A/S3A/S4A and the S1A/S2A/S3A/S4A mutants, as well as wild-type FRNK, were used to pull-down purified PP1 catalytic subunit (Figure 9B). The results indicated a significant loss of PP1 only upon addition of the S1 mutation, thus confirming the prominent role of S1 in binding PP1.
Figure 9. PP1 binding to FAK and FRNK mutants.
(A) PP1 binding to S1A FAK. FAK−/− cells were transfected with the vector encoding either wild-type (WT) or S1A FAK, as described in the legend to Figure 2. After 24 h the cells were lysed, and FAK was immunoprecipitated and assayed for the associated PP1 activity (as in Figure 8A). Results are shown as the means±S.E.M. for 3 determinations. Inset: FAK was immunoprecipitated from cells expressing the indicated FAK type or the empty vector (−), and then subjected to electrophoresis, Western blotting and probing for pS1 and FAK. (B) PP1 binding to FRNK point mutants in vitro. The indicated serine-to-alanine mutants of S1 (Ser-722), S2 (Ser-842), S3 (Ser-845) and S4 (Ser-911) of FRNK, or GST alone, were bound to glutathione–Sepharose beads and used to pull-down purified PP1 catalytic subunit, which was then detected by immunoblotting (PP1 blot). This was followed by stripping and detection of FRNK mutants or GST (GST blot).
DISCUSSION
In response to integrin-mediated adhesion, FAK undergoes phosphorylation at multiple tyrosine sites to promote both FAK catalytic activity and its interaction with other signalling effectors, including Src [1,2,4]. FAK also is subject to phosphorylation at multiple serine residues [6], but little is known regarding integrin regulation of FAK serine sites, the nature of the protein kinases and phosphatases that target them, and their effects on FAK signalling. In the present study we found that FAK Ser-722 (S1) undergoes integrin-mediated phosphorylation with delayed kinetics relative to the sites of tyrosine phosphorylation, and becomes dephosphorylated during monolayer wound-healing cell migration. We have presented strong evidence that GSK3β is the protein kinase that phosphorylates S1, and that PP1 is the phosphatase responsible for S1 dephosphorylation. We have also shown that S1 phosphorylation has a negative effect on FAK catalytic activity. Taken together, these results indicate that FAK S1 phosphorylation, modulated through the competing actions of GSK3β and PP1, represents an additional mechanism for regulation of FAK activity during cell spreading and migration.
Our results indicate that GSK3 directly phosphorylates S1, provided that the supporting site serine +4 has been phosphorylated previously by another kinase whose identity remains unknown. The involvement of GSK3β is supported by the following evidence: (1) cell treatment with three different GSK3 inhibitors decreased S1 phosphorylation; (2) GSK3β silencing by siRNA decreased S1 phosphorylation; (3) GSK3β associated with FAK during spreading, and was activated at the time when pS1 increased; (4) S1 phosphorylation decreased in wounded cell monolayers while GSK3β underwent inactivation; and (5) direct phosphorylation of S1 by purified GSK3 was demonstrated in a synthetic peptide (residues 714–730 of FAK), provided that Ser-726 was pre-phosphorylated. These results fulfil the key requirements in establishing that a protein is a GSK3 substrate [13], and indicate that cell spreading on fibronectin stimulates GSK3β to phosphorylate the FAK S1 site.
GSK3 is a component of numerous signal transduction pathways [11–13]. In the cell adhesion field, the role of GSK3 has been established in cadherin-based cell adhesion [13]. Much less is known on GSK3 involvement in integrin-stimulated cell spreading and migration, and GSK3 has never been linked directly to FAK. In IEC-18 epithelial cells plated on fibronectin, GSK3 was transiently inhibited by the ILK (integrin-linked kinase) [34] which was, in turn, transiently stimulated upon attachment of cells to fibronectin [35]. GSK3 was then reactivated within 45 min [34]. Our results are in agreement with the reported GSK3 reactivation, which, in our experiments, was detectable at 30 min and lasted for at least 2 h from plating on fibronectin (Figure 4C). Another recent report linked GSK3 to cell migration by showing a cdc42-dependent GSK3β phosphorylation at the inhibitory Ser-9 residue soon after stimulating migration by scratching an astrocyte monolayer and lasting for at least 12 h [36]. This agrees with our results, and suggests that GSK3β regulation might be a general mechanism associated with cell migration, although involving different substrates [36]. Our studies have identified for the first time FAK as a GSK3β substrate. Given the positive role of FAK in cell migration [1,2], the decrease in the inhibitory S1 phosphorylation may contribute to the increase in FAK activity required during migration. This picture is confirmed by the finding that cells expressing the FAK mutant that lacks S1 migrate faster that the wild-type counterpart, strongly supporting the biological relevance of pS1.
Previous studies detected the presence of PP1 at focal adhesions in all cell types examined, and its association with FAK [8], thus suggesting that PP1 is a permanent component of focal adhesions. In the present study, the role of PP1 in focal adhesions was extended by several findings: (1) in FAK−/− fibroblasts expressing exogenous FAK, the amount of PP1 co-immunoprecipitating with FAK increased as FAK accumulated; (2) PP1 targeted pS1 of FAK both in vitro and in the cell; (3) a serine-to-alanine mutation of S1 decreased the amount of PP1 associating with FAK; and (4) increase in pS1 by okadaic acid treatment impairs cell spreading. With regard to other sites, our results excluded targeting of S4 (Ser-911), another major FAK-phosphorylation site [6], during spreading. On the other hand, a recent paper [10] indicated increased S4 phosphorylation in cells treated with PDGF and FGF. We have found that S1 and S4 phosphorylations are differentially regulated also at mitotic exit and in cells treated with phorbol esters (M. Bianchi and E. Villa-Moruzzi, unpublished work). However, targeting of other FAK sites cannot be excluded, since some PP1 still binds to FAK carrying the S1A mutation (Figure 9A). Moreover, other proteins recruited to the activated FAK complex may also be additional PP1 targets.
The role of FAK serine phosphorylation in cell spreading is still little understood. S1 phosphorylation was suggested to modulate FAK binding to the docking protein CAS due to its location close to the major CAS-binding polyproline region of FAK [6]. However, we were unable to provide evidence to support such a role, since S1 mutation did not change CAS binding to FAK (results not shown). The presence of another FAK site involved in CAS binding [6] might explain this discrepancy. On the other hand, our findings suggest that pS1 contributes to FAK regulation by negatively modulating its kinase activity. This conclusion is based on three different approaches: (1) expression of FAK carrying the serine-to-alanine mutation of S1; (2) in vitro FAK dephosphorylation; and (3) cell treatment with the GSK3 inhibitor LiCl. Loss of S1 phosphorylation was accompanied by a modest, although significant, activation of FAK catalytic activity, and by the increase in the phosphorylation of the Tyr-397 autokinase site, the hallmark of FAK activation. FAK regulation occurs through a complex mechanism resulting from the interplay of several kinases and phosphatases, mainly targeting tyrosine residues. Phosphorylation of S1 appears to represent a way not only to modulate FAK, in addition to tyrosine phosphorylation, but also to interfere with the pTyr-397 level.
Based on our results, the physiological role of FAK S1 phosphorylation during cell spreading and migration can be envisaged as follows (Figure 10). In spreading cells, GSK3β becomes activated, although at a later time with respect to FAK activation by tyrosine phosphorylation. FAK activation is already maximal at 30 min, whereas GSK3β and pS1 reach their maximum between 1 and 2 h. The GSK3β-mediated phosphorylation of FAK S1 then acts to tune-down FAK activity after the initial burst following adhesion, and may contribute towards the decrease in FAK activity that is observed at later times of spreading (Figure 1C). When cell migration is stimulated, FAK is quickly activated [1,2], and the decrease in pS1 detected in migrating cells may contribute to achieving a higher degree of FAK activation. This scenario is confirmed by the behaviour of cells expressing the S1A FAK mutant. Relief of cells from the S1 inhibitory site favours cell spreading and migration, as predicted by the model. GSK3 and PP1 may introduce signals originating from pathways distinct from those regulating FAK tyrosine phosphorylation, hence broadening the panoply of signals to which FAK is responsive in relevant biological processes.
Acknowledgments
This work was supported by grants from Telethon-Italy, MIUR (Rome, I, PRIN and FIRB) and the University of Pisa. We thank Dr J. T. Parsons for providing FAK and FRNK cDNA, Dr J. R. Vandenheede for providing anti-GSK3α and GSK3β antibodies, and Dr F. Meggio (Padova, Italy) for fruitful discussions on kinase substrate specificities.
References
- 1.Hanks S. K., Ryzhova L., Shin N., Brabek J. Focal adhesion kinase signalling activities and their implications in the control of cell survival and motility. Front. Biosci. 2003;8:982–986. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 2.Parsons J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 3.Carragher N. O., Frame M. C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik R. K., Parsons J. T. Integrin-mediated signaling in normal and malignant cells: a role of protein tyrosine kinases. Biochim. Biophys. Acta. 1996;1287:73–76. doi: 10.1016/0304-419x(96)00008-x. [DOI] [PubMed] [Google Scholar]
- 6.Ma A., Richardson A., Schaefer E. M., Parsons J. T. Serine phosphorylation of focal adhesion kinase in interphase and mitosis: a possible role in modulating binding to p130CAS. Mol. Biol. Cell. 2001;12:1–12. doi: 10.1091/mbc.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamakita Y., Totsukawa G., Yamashiro S., Fry D., Zhang X., Hanks S. K., Matsumura F. Dissociation of FAK/p130(CAS)/c-Src complexes during mitosis: role of mitosis-specific serine phosphorylation of FAK. J. Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fresu M., Bianchi M., Parsons J. T., Villa-Moruzzi E. Cell-cycle-dependent association of protein phosphatase 1 and focal adhesion kinase. Biochem. J. 2001;350:407–414. doi: 10.1042/0264-6021:3580407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson A., Shannon J. D., Adams R. B., Schaller M. D., Parsons J. T. Identification of integrin-stimulated sites of serine phosphorylation in FRNK, the separately expressed C-terminal domain of focal adhesion kinase: a potential role for protein kinase A. Biochem. J. 1997;324:141–149. doi: 10.1042/bj3240141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunger-Glaser I., Fan R. S., Perez-Salazar E., Rozengurt E. PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. J. Cell Physiol. 2004;200:213–222. doi: 10.1002/jcp.20018. [DOI] [PubMed] [Google Scholar]
- 11.Ali A., Hoeflich K. P., Woodgett J. R. Glycogen synthase kinase-3: properties, functions and regulation. Chem. Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 12.Jope R. S., Johnson G. V. W. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Frame S., Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata K., Hirano K., Villa-Moruzzi E., Hartshorne D. J., Brautigan D. L. Differential localization of myosin and myosin phosphatase subunits in smooth muscle cells and migrating fibroblasts. Mol. Biol. Cell. 1997;8:663–673. doi: 10.1091/mbc.8.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa-Moruzzi E., Tognarini M., Cecchini G., Marchisio P. C. Protein phosphatase 1δ is associated with focal adhesions. Cell Adhesion Commun. 1998;5:297–305. doi: 10.3109/15419069809040299. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi M., De Lucchini S., Vietri M., Villa-Moruzzi E. Reciprocally interacting domains of protein phosphatase 1 and focal adhesion kinase. Mol. Cell. Biochem. 2005;272:85–90. doi: 10.1007/s11010-005-7639-z. [DOI] [PubMed] [Google Scholar]
- 17.Shenolikar S. Protein serine/threonine phosphatases: new avenues for cell regulation. Annu. Rev. Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 18.Ceulemans H., Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki K., Shima H., Kitagawa Y., Irino S., Sugimura T., Nagao M. Identification of members of the protein phosphatase 1 gene family in the rat and enhanced expression of protein phosphatase 1α gene in hepatocellular carcinomas. Jpn. J. Cancer. 1990;81:1272–1280. doi: 10.1111/j.1349-7006.1990.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tognarini M., Villa-Moruzzi E. Analysis of the isoforms of protein phosphatase 1 (PP1) with polyclonal peptide antibodies. Methods Mol. Biol. (Totowa, NJ) 1998;93:169–183. doi: 10.1385/0-89603-468-2:169. [DOI] [PubMed] [Google Scholar]
- 21.Villa-Moruzzi E., Puntoni F., Marin O. Activation of protein phosphatase 1 isoforms and glycogen synthase kinase β in muscle from mdx mice. Int. J. Biochem. Cell Biol. 1996;28:13–22. doi: 10.1016/1357-2725(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 22.Bollen M. Combinatorial control of protein phosphatase-1. Trends Biochem. Sci. 2001;26:426–431. doi: 10.1016/s0968-0004(01)01836-9. [DOI] [PubMed] [Google Scholar]
- 23.Resink T. J., Hemmings B. A., Tung H. Y. L., Cohen P. Characterization of a reconstituted Mg-ATP-dependent protein phosphatase. Eur. J. Biochem. 1983;133:455–461. doi: 10.1111/j.1432-1033.1983.tb07485.x. [DOI] [PubMed] [Google Scholar]
- 24.Hemmings B. A., Yellowlees D., Kernohan J. C., Cohen P. Purification of glycogen synthase kinase 3 from rabbit skeletal muscle. Copurification with the activating factor (FA) of the (Mg-ATP) dependent protein phosphatase. Eur. J. Biochem. 1981;119:443–451. doi: 10.1111/j.1432-1033.1981.tb05628.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu J. Y., Taylor J., DeRuiter S. L., Vojtek A. B., Turner D. L. Simultaneous inhibition of GSK3alpha and GSK3beta using hairpin siRNA expression vectors. Mol. Ther. 2003;7:228–236. doi: 10.1016/s1525-0016(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 26.Lampugnani M. G. Cell migration into wounded areas in vitro. Methods Mol. Biol. (Totowa, NJ) 1999;96:177–182. doi: 10.1385/1-59259-258-9:177. [DOI] [PubMed] [Google Scholar]
- 27.Sieg D. J., Ilic D., Jones K. C., Damsky C. H., Hunter T., Schlaepfer D. D. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 29.King D. S., Fields C. G., Fields G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Protein Res. 1990;36:255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 30.Pinna L. A., Ruzzene M. How do protein kinases recognize their substrates? Biochim. Biophys. Acta. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 31.Bain J., McLauchlan H., Elliott M., Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haysted T. A. J., Sim A. T. R., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. J. Effects of the tumor promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature (London) 1989;237:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- 33.Schoenthal A. H. Analysing gene expression with the use of serine/threonine phosphatase inhibitors. Methods Mol. Biol. (Totowa, NJ) 1998;93:35–40. doi: 10.1385/0-89603-468-2:35. [DOI] [PubMed] [Google Scholar]
- 34.Troussard A. A., Tan C., Yoganathan T. N., Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol. Cell. Biol. 1999;19:7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase-3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature (London) 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]