Abstract
ACAT1 (acyl-CoA:cholesterol acyltransferase 1) is thought to have two distinct sterol-binding sites: a substrate-binding site and an allosteric-activator site. In the present work, we investigated the structural features of various sterols as substrates and/or activators in vitro. The results show that without cholesterol, the plant sterol sitosterol is a poor substrate for ACAT. In the presence of cholesterol, ACAT1-mediated esterification of sitosterol is highly activated while ACAT2-mediated esterification of sitosterol is only moderately activated. For ACAT1, we show that the stereochemistry of the 3-hydroxy group at steroid ring A is a critical structural feature for a sterol to serve as a substrate, but less critical for activation. Additionally, enantiomeric cholesterol, which has the same biophysical properties as cholesterol in membranes, fails to activate ACAT1. Thus ACAT1 activation by cholesterol is the result of stereo-specific interactions between cholesterol and ACAT1, and is not related to the biophysical properties of phospholipid membranes. To demonstrate the relevance of the ACAT1 allosteric model in intact cells, we showed that sitosterol esterification in human macrophages is activated upon cholesterol loading. We further show that the activation is not due to an increase in ACAT1 protein content, but is partly due to an increase in the cholesterol content in the endoplasmic reticulum where ACAT1 resides. Together, our results support the existence of a distinct sterol-activator site in addition to the sterol-substrate site of ACAT1 and demonstrate the applicability of the ACAT1 allosteric model in intact cells.
Keywords: acyl-CoA:cholesterol acyltransferase (ACAT), allosteric model, enantiomeric cholesterol, sitosterol, sterol esterification
Abbreviations: ACAT, acyl-CoA:cholesterol acyltransferase 1; AcLDL, acetylated low-density lipoprotein; DHE, dehydroergosterol; DMEM, Dulbecco's modified Eagle's medium; ent-cholesterol, enantiomeric cholesterol; ER, endoplasmic reticulum; FBS, fetal bovine serum; PC, phosphatidylcholine; SREBP, sterol-regulatory-element-binding protein
INTRODUCTION
ACAT (acyl-CoA:cholesterol acyltransferase) is a membrane-bound enzyme residing in the ER (endoplasmic reticulum) that plays important roles in cellular cholesterol metabolism. The primary role of ACAT is to convert cholesterol into cholesteryl esters using long chain fatty acyl-CoA as the fatty acyl donor. In mammals, there are two isoforms of ACAT: ACAT1 and ACAT2. These are two homologous enzymes encoded by two different genes [1–4], reviewed in [5–7]. In adult humans, ACAT1 is present in a variety of tissues, including hepatocytes and Kupffer cells [8–10], adrenal glands, and macrophages [9]. The expression of ACAT2 is restricted: in adult humans, ACAT2 is expressed in the intestinal mucosa [10,11] and plays an important role in contributing the cholesteryl esters present in chylomicrons [6,7]. Under various pathological conditions, macrophages can also express ACAT2 [11]. More recently, ACAT2 has been shown to be present at various levels in hepatocytes of patients suffering from gallstones [12].
Both ACAT1 and ACAT2 are integral membrane proteins with multiple transmembrane domains [13,14]. Recombinant ACAT1 expressed in mammalian cells and insect cells have been purified to homogeneity [15]. In intact cells, ACAT uses the cholesterol associated with ER membranes as its primary substrate. In conventional ACAT enzyme assays, microsomal membranes are used as the enzyme source, and externally provided cholesterol or other sterols in lipid vesicles are used as the substrate. It is difficult to determine the true sterol specificity of ACAT by using this type of assay because sterol transfer between the ER membrane and the donor vesicle occurs slowly and the transfer rates among different sterols can vary up to 100-fold [16]. Thus the differential transfer rates of various sterols can mask the intrinsic sterol specificity of the enzyme. To circumvent this problem, we have shown that solubilization of both ACAT1 and ACAT2 in CHAPS detergent preserves enzymatic activity [15]. Solubilized ACAT can then be dispersed into taurocholate-PC (where PC stands for phosphatidylcholine)-based mixed micelles or be reconstituted into unilamellar vesicles [17]. Both procedures place a defined amount of phospholipid, sterol and enzyme in the same phase, thus avoiding the problem of sterol transfer between membranes. When assayed in either mixed micelles or reconstituted vesicles, the cholesterol substrate saturation curve is sigmoidal for both ACAT1 and ACAT2 [10]. In contrast, the substrate saturation curve for long chain fatty acyl-CoA is hyperbolic for both enzymes [15]. These results suggest that both ACAT enzymes are allosterically regulated by cholesterol, with cholesterol serving as an activator as well as a substrate. ACAT1 can also esterify various oxysterols, such as 7-ketocholesterol and 7β-hydroxycholesterol, though at much lower rates than cholesterol [17]. Kinetic experiments with ACAT1 in mixed micelles or in unilamellar vesicles showed that when cholesterol is present, ACAT is able to more efficiently esterify other sterols such as 7-ketocholesterol. However, the result of a converse experiment showed that the presence of 7-ketocholesterol did not lead to an increase in ACAT1 esterification of cholesterol in vitro [17]. These data led us to propose that in addition to a substrate-binding site, ACAT1 contains a putative sterol-activator site, which is preferentially activated by cholesterol over other oxysterols. Upon activation by cholesterol, the substrate site can accommodate cholesterol and oxysterols in a less discriminatory manner, and the enzyme becomes more efficient in esterifying various sterols [17].
It is well known that cholesterol condenses the fluid phospholipid membranes [18]. This condensing effect is thought to be the result of the hydrophobic properties of cholesterol [19]. Cholesterol also changes the thermodynamic properties of lipid phase transitions [20]. Oxysterols (e.g. 7-ketocholesterol) and cholesterol both affect the biophysical properties of phospholipid membranes, but they do so by different mechanisms [21]. Thus the results obtained from our previous study based on oxysterols cannot eliminate the possibility that cholesterol activation of ACAT1 is due to its ability to affect the biophysical properties of phospholipids in membranes, as opposed to a direct interaction between cholesterol and the putative activator site in ACAT1. ent-cholesterol (enantiomeric cholesterol) [22] is the mirror image of cholesterol, and has the same effect on the biophysical properties of phospholipids in monolayer or bilayer as cholesterol (reviewed in [23]). In the present study, we use ent-cholesterol to demonstrate that the activation effect of cholesterol on ACAT1 is due to the specific structural requirement of the putative activator site rather than the biophysical effects on the membrane.
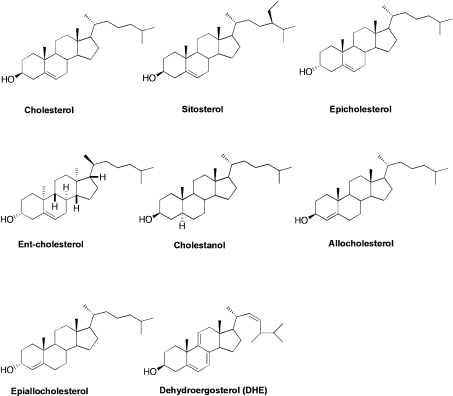
Other sterols with slight variations in structure from cholesterol (Figure 1) are also included in our study to probe the specificity of the ACAT1 substrate site and the putative activator site. For the activation assay, we use ACAT1 or ACAT2 as the enzyme source, and sitosterol as the ACAT substrate. Sitosterol is the major sterol found in most plants. Its structure differs from cholesterol by having an additional ethyl group located at the C-24 position on the iso-octyl side chain (Figure 1). We have previously shown that, in the absence of cholesterol, sitosterol is a poor substrate for human ACAT1 [17]. The potential physiological relevance of the ACAT allosteric model has not been tested in any mammalian cell type. In the present study, we use sitosterol as the probe to test its potential activation in esterification by cholesterol in macrophage cells.
Figure 1. Structures of various sterols used in the present study.
MATERIALS AND METHODS
Materials
[3H]Sitosterol (50 Ci/mmol), [3H]cholesterol (40 Ci/mmol) and [14C]cholesteryl oleate (40 Ci/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO, U.S.A.). [3H]Oleoyl-CoA was synthesized as described previously [24]. Egg PC, taurocholate, CHAPS, sitosterol, cholesterol, 5α,6α-epoxycholesterol, FBS (fetal bovine serum), fatty acid-free BSA, cholesteryl oleate, PMA, Nile Red, oleic acid, acetic anhydride and oleoyl-CoA were obtained from Sigma. Epicholesterol, cholestanol, allocholesterol, epiallocholesterol and DHE (dehydroergosterol) were obtained from Steraloids (Wilton, NH, U.S.A.). ent-cholesterol was synthesized chemically as described previously [22]. All other reagent-grade chemicals and organic solvents were obtained from Fisher Scientific (Pittsburgh, PA, U.S.A.). Reverse phase TLC plates were obtained from J. T. Baker (Phillipsburg, NJ, U.S.A.) or Whatman (Florham Park, NJ, U.S.A.). Tissue culture media RPMI 1640 and DMEM (Dulbecco's modified Eagle's medium) were from Cellgro (Mediatech, Herndon, VA, U.S.A.). Penicillin/streptomycin stock solution was obtained from Invitrogen and Hepes was from Roche (Indianapolis, IN, U.S.A.). Tissue culture flasks or dishes were from Nalge Nunc International (Rochester, NY, U.S.A.) or Bellco (Wineland, NJ, U.S.A.). Ficoll-paq density solution was from Amersham Biosciences. THP-1 cells were from American Type Culture Collection (A.T.C.C., Manassas, VA, U.S.A.).
Methods
Enzyme purification
The source of ACAT1 used in this work is recombinant human ACAT1 tagged with His6 at the N-terminus (His–ACAT1), expressed in insect Hi5 cells and purified to homogeneity by procedures described previously [15]. The source of ACAT2 used was recombinant human ACAT2 tagged with His6 at the N-terminus (His–ACAT2), stably expressed in Chinese-hamster ovary cells as described previously [10]. The His–ACAT2 enzyme was partially purified following the method for recombinant His–ACAT1 [15] except for the nickel column chromatography step used. Based on specific activity analysis, ACAT2 was purified to an average of 190-fold from crude cell extracts, with approx. 6% recovery of total ACAT activity. After purification, the ACAT1 and ACAT2 preparations were stored in 0.5% CHAPS at −80 °C. Stored under this condition, the ACAT activity remains stable for at least 1 year. Specific activity of 1 unit of ACAT is defined as 1 (pmol of cholesteryl oleate formed)·min−1·(μg of protein)−1 assayed under standard mixed micelle conditions [15].
In vitro ACAT enzyme activity assays
Assayed in sterol/PC/taurocholate mixed micelles, using [3H]sitosterol as substrate: [3H]sitosterol (0.5 μCi/0.1 nmol) along with various concentrations of non-radioactive sterol as indicated. The mixed micelles were prepared as described previously [15,17], with 11.2 mM PC/18.6 mM taurocholate. The enzyme in 0.5% CHAPS was added to the mixed micelles at 4 °C. To start the enzyme reaction, 10 nmol non-radioactive oleoyl-CoA/BSA was added and the reaction was continued at 37 °C for 30 min. Control experiments showed that the ACAT1 or ACAT2 activity was linear for at least 30 min (results not shown). The reaction was terminated by adding 2:1 chloroform/methanol and the lipids were extracted as described previously [15]. Lipids were separated on silica TLC plates in 90:10:1 [light petroleum (boiling range 39–54 °C)/ether/acetic acid]. In this system, cholesteryl ester and sitosteryl ester migrate at the same retention factor (Rf) value of 0.89. Sterol ester products were visualized, scraped and counted in a scintillation counter. Sitosteryl oleate served as an internal standard and it was synthesized as described previously [17].
Assayed in sterol/PC/taurocholate mixed micelles, using [3H]oleoyl-CoA as labelled substrate, and various concentrations of non-radioactive sitosterol and/or cholesterol as indicated in Figure 3(A): [3H]oleoyl-CoA/BSA at 3.0×104 d.p.m./nmol was used to start the reaction at 37 °C for 30 min. After lipid extraction by 2:1 chloroform/methanol and water, cholesteryl oleate and sitosteryl oleate were separated as described in the Supplementary Figure 1 (http://www.BiochemJ.org/bj/391/bj3910389add.htm). The Rf value in this system was 0.30 for sitosteryl oleate and 0.34 for cholesteryl oleate [25]. Experiments conducted to determine the degree of crossover between sitosteryl oleate and cholesteryl oleate bands are described in the Supplementary Table 1 (http://www.BiochemJ.org/bj/391/bj3910389add.htm).
Figure 3. Sitosterol substrate saturation curves of ACAT1 in the presence or absence of cholesterol.
The ACAT assays in mixed micelles were performed as described in the Materials and methods section, using [3H]oleoyl-CoA or [3H]sitosterol as the labelled substrate. The final concentrations of sitosterol and cholesterol used in mixed micelles are as indicated. (A) ACAT1 as the enzyme; left panel shows the sitosteryl oleate formed and the right panel shows cholesteryl oleate. (B) [3H]Sitosterol was used as the substrate with ACAT1 as the enzyme source. ACAT activity is depicted as [3H]sitosteryl oleate formed in the presence or absence of cholesterol. Data points and error bars represent the mean and variation between duplicate trials. All results presented are representative of two separate experiments.
Assayed in sterol/PC unilamellar vesicles using either [3H]sitosterol or [3H]oleoyl-CoA as a substrate: the mixed micelles were prepared as described above. The bile salt (taurocholate) was rapidly and efficiently removed by treating the micelles with the cationic resin cholestyramine (30 mg/500 μl of micelles), resulting in unilamellar vesicles. The details regarding the formation and characteristics of the unilamellar vesicles were described previously [17]. Vesicles consisting of two different sterols were made by dissolving the two sterols in the same mixed micelles. Activity assays were conducted as described in the first paragraph of this subsection. Sterol products were scraped at their corresponding Rf values. Rf values for cholestanyl oleate, cholesteryl oleate, sitosteryl oleate, allocholesteryl oleate, epicholesteryl oleate, ent-cholesteryl oleate, dehydroergosteryl oleate and epiallocholesteryl oleate are 0.89, and 0.5 for 5α,6α epoxycholesteryl oleate [17].
Assayed in whole (unfractionated) cell homogenates: monolayers of THP-1 macrophage cells or human monocyte-derived macrophages were washed with PBS, hypo-osmotic shocked for 30 s with a solution of 1 mM EDTA/1 mM Tris (pH 7.4) and harvested by scraping into buffer A (50 mM Tris and 1 mM EDTA, pH 7.4) at room temperature (22 °C), according to the method described previously [26]. Then, 1 μCi of [3H]sitosterol at 50 Ci/mmol sitosterol in 1 μl DMSO stock solution was added directly to 25 μl of cell homogenate and incubated on ice for 30 min. The assay was initiated by adding non-radioactive oleoyl-CoA/BSA. Alternatively, when the formation of cholesteryl oleate was examined, [3H]oleoyl-CoA/BSA (not 3H-labelled sitosterol) was added to initiate the reaction. The reaction was carried out at 37 °C for 5 min. The reaction products were analysed as described in the first paragraph of this subsection.
Cell culture
Undifferentiated THP-1 cells were maintained in RPMI 1640 medium with 10% (v/v) FBS. Cells were differentiated by the addition of 100 nM PMA to the medium. Cells were fully differentiated after 72 h in this medium. With PMA in the medium, the cells could be maintained in a differentiated state, for 2 weeks without loss of viability.
Isolation and maintenance of human monocyte-derived macrophages
Monocytes were collected from the blood of healthy volunteers according to the procedure described in [27]. Briefly, mononuclear leucocytes were separated from whole blood by the Ficoll-paq centrifugation method, and were resuspended in DMEM+10% pooled human serum. Cells were washed every 24 h in culture media for 3 days to remove non-adherent lymphocytes and red blood cells. Adherent monocytes were allowed to differentiate into macrophages for 13 days, with fresh medium added periodically.
Intact cell labelling with [3H]sterol in DMSO or in LDL (low-density lipoprotein)
Differentiated THP-1 macrophages or human monocyte-derived macrophages were grown in medium with or without 50 μg/ml AcLDL (acetylated LDL) for 12 h. AcLDL was prepared as described previously [9]. [3H]Sitosterol (0.5 μCi/100 mm dish; specific radioactivity 50 Ci/mmol) or 0.5 μCi/100 mm dish of [3H]cholesterol (40 Ci/mmol) in either DMSO or LDL was then added to the growth medium for 24 h. [3H]Sterol incorporated into LDL was prepared by directly adding 20 μl of [3H]sterol (20 μCi) in DMSO to 1 ml of 2.5 mg/ml LDL and incubating at 40 °C for 2 h. The [3H]sterol-LDL solution was then dialysed in EDTA-saline for 24 h at 4 °C. The cell culture medium was removed and the cells were washed three times with PBS. The cells were then harvested by incubation in 0.2 M NaOH for 45 min at room temperature. After separating into aliquots for protein concentration determination, lipids were extracted by 2:1 chloroform/methanol and water and then separated by TLC with a mobile phase of 90:10:1 (light petroleum/ether/acetic acid). The cholesteryl ester/sitosteryl ester band was scraped and counted by a scintillation counter (Beckman LS 3801).
Statistical analysis
Statistical comparisons for all activation and substrate assays were made using a two-tailed, unpaired Student's t test generated by GraphPad Prism 4 for Windows & Macintosh (GraphPAD Software, San Diego, CA, U.S.A.). For activation assays, significance was determined by comparing various sterols added to the non-sterol control. For substrate assays, various substrate activities were compared with the background. The difference between two sets of values was considered significant when the P value was the <0.05.
RESULTS
Effect of cholesterol on sitosterol as a substrate for ACAT1 or ACAT2
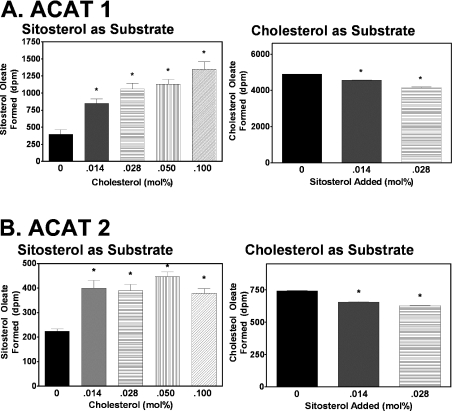
To examine sitosterol as an ACAT substrate, 0.5 μCi of 3H-labelled sitosterol was assayed at a fixed concentration (1.6 mM; at 0.15 mol%) in PC/taurocholate mixed micelles that contained either ACAT1 or ACAT2. The mixed micelle solution contained either no cholesterol or an increasing mol% of cholesterol as indicated in Figure 2. The results showed that, for ACAT1, the inclusion of cholesterol significantly increased the sitosterol esterification in a dose-dependent manner. When the cholesterol mol% reached 0.1, sitosterol esterification increased 4.5-fold as compared with the control value (Figure 2A, left panel). In a separate assay, the converse experiment was performed, testing the effect of sitosterol on ACAT1 cholesterol esterification using 0.5 μCi of [3H]cholesterol as substrate. The result showed that the cholesterol esterification by ACAT1 was slightly inhibited when sitosterol was included in the mixed micelles (Figure 2A, right panel). Parallel experiments, using ACAT2 as the enzyme source, yielded similar results (Figure 2B). However, the fold increase in sitosterol esterification in the presence of cholesterol was less: 0.1 mol% of cholesterol resulted in only a 2-fold increase (Figure 2B, left panel). A separate experiment showed that the addition of sitosterol in the mixed micelle assay using 0.5 μCi of [3H]cholesterol as the substrate also slightly, but statistically significantly, inhibited the cholesterol esterification by ACAT2 (Figure 2B, right panel).
Figure 2. Effects of cholesterol on sitosterol esterification, and effects of sitosterol on cholesterol esterification by ACAT1 or by ACAT2.
(A) Left panel: the enzyme assay consisted of [3H]sitosterol (1.6 mM; 0.5 μCi/assay) and increasing amounts of unlabelled cholesterol in PC/taurocholate mixed micelles; 0.38 unit of ACAT1 was used per assay. Right panel: the enzyme assay consisted of [3H]cholesterol (1.6 mM; 0.5 μCi/assay) and increasing amounts of cold sitosterol in PC/taurocholate mixed micelles; 0.38 unit of ACAT1 was used per assay. (B) Left and right panels: the same conditions as described for (A) were used, except that 0.076 unit of ACAT2 was used per assay in the left panel and 0.019 unit of ACAT2 was used per assay in the right panel. Data points and error bars represent the mean and variation between duplicate trials. Results are representative of two separate experiments. *P<0.05 for activation/inhibition versus control.
Effect of cholesterol on sitosterol saturation curves
The results shown in Figure 2 were performed at a fixed concentration of radiolabelled sitosterol. To extend these results, one needs to perform more detailed kinetic studies, using both cholesterol and sitosterol as a substrate for ACAT. With ACAT2, the maximal activation by cholesterol on sitosterol esterification is only 2-fold. Therefore we focused our efforts on using ACAT1 to study the mechanism. An alternate assay system was developed using [3H]oleoyl-CoA as the labelled substrate, and unlabelled cholesterol and sitosterol as the sterol substrates/activators. The sitosteryl and cholesteryl oleate products were separated by TLC (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/391/bj3910389add.htm) and a cross-contamination assay was performed to verify the counts found in each band (see Supplementary Table 1 at http://www.BiochemJ.org/bj/391/bj3910389add.htm). This assay allowed us to monitor both the cholesteryl and sitosteryl oleate produced from the same reaction. Substrate saturation experiments were then performed in the presence or absence of cholesterol, using this assay system. The results show that the addition of cholesterol increased the sitosterol esterification in a dose-dependent manner for ACAT1 (Figure 3A, left panel). At a higher cholesterol concentration (0.028 mol%), the increase in the maximal velocity (Vmax) value was almost 3-fold (Figure 3A, left panel). In addition, the sterol saturation curve is hyperbolic in the presence of 0.028 mol% but not in the presence of 0.014 mol% cholesterol. These effects parallel the activation by cholesterol when 7-ketocholesterol is used as the ACAT1 substrate [17]. The effect of sitosterol on cholesteryl oleate formation by ACAT1 was also determined in these experiments. The results show that increasing concentrations of sitosterol slightly inhibited cholesteryl oleate formation by ACAT1 (Figure 3A, right panel). In Figure 3(B), a similar sitosterol substrate saturation curve experiment using ACAT1 and [3H]sitosterol (instead of [3H]oleoyl-CoA) as the labelled substrate was performed. This assay method does not require the separation of the sitosteryl and cholesteryl oleate products. The results fully corroborate the data described in Figure 3(A) – at a higher mol% of cholesterol, ACAT1 esterification of sitosterol is activated almost 3-fold, and the shape of the substrate saturation curve is hyperbolic.
Examination of the structural requirements for a sterol to be a substrate for ACAT1
The ability of eight different sterols to serve as a substrate for ACAT1 is shown in Figure 4. The concentrations of various sterols were fixed at a saturating concentration of 1.6 mM [17] in the form of mixed micelles; [3H]oleoyl-CoA was used as the radiolabelled substrate. The results show that compared with cholesterol, the enantiomeric form of cholesterol is a poor substrate at 0.55% (±0.09%) of the capacity of cholesterol. And, at 0.03% (±0.01%) capacity of cholesterol, epicholesterol is a worse substrate. Although ent-cholesterol and epicholesterol both possess a 3α-hydroxy group as opposed to the 3β-hydroxy group present in cholesterol, the 3α-hydroxy group in ent-cholesterol has the same equatorial configuration as it does in cholesterol. Hence, these two steroids are more different than what the nomenclature suggests. Sitosterol and DHE are also worse substrates than cholesterol (at 6.8 and 2% of cholesterol respectively), but are better substrates than ent-cholesterol and epicholesterol. Interestingly, cholestanol, which lacks the C5–C6 double bond of cholesterol, but possesses the 3β-hydroxy group, is a better substrate than cholesterol (at 110% capacity of cholesterol). Allocholesterol, which possesses a double bond at C4–C5, is a better substrate (at 23% of cholesterol) than sitosterol. Interestingly, epiallocholesterol, which only differs from allocholesterol by having a 3α-hydroxy group, is not a substrate for ACAT. These data together show that, to serve as an ACAT1 substrate, the stereochemistry of the 3-hydroxy moiety at steroid ring A is a critical feature. The placement (or even presence) of the double bond in the steroid B ring is not a required feature of an ACAT1 substrate. Side-chain and steroid ring structure modifications, such as those found in sitosterol, DHE and various oxysterols (examined in our previous work [17]) all diminish the enzyme activity, but can all be tolerated as a substrate.
Figure 4. Various sterols as substrates of pure His–ACAT1 in vitro.
The ACAT assays in mixed micelles were performed as described in the Materials and methods section, using [3H]oleoyl CoA as the labelled substrate. The concentration of all the sterols was 1.6 mM. The results show the steryl oleate formed from each sterol. Rf values are listed in the Materials and methods section. Data points and error bars represent the mean and variation between duplicate trials. The results are representative of three separate experiments. All values are significantly (P<0.05) higher than background values except for epiallocholesterol.
Examination of the structural requirements of sterols for activation of ACAT1
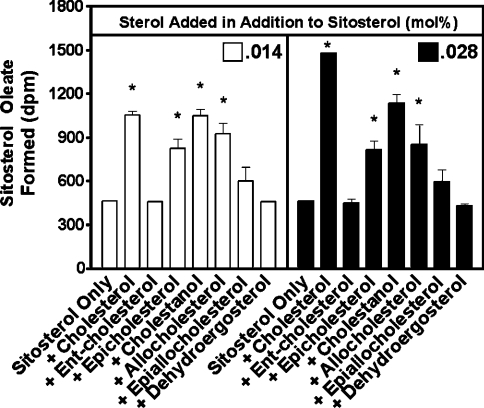
Here, we used ACAT1 as the enzyme source and 1.6 mM [3H]sitosterol in mixed micelles as a substrate, and tested the effect of various unlabelled sterols as potential activators for ACAT1 at two different concentrations (0.014 and 0.028 mol%). Under these conditions, sitosterol is present at a saturating level. The sterol being tested as an activator is present at a 3.5–7-fold lower concentration. Thus the sterol being tested is expected to only occupy the putative activator site. The results show that ent-cholesterol does not activate ACAT1 esterification of sitosterol (Figure 5). The control experiment showed that cholesterol activates ACAT esterification of sitosterol 3-fold. The inability of ent-cholesterol to activate ACAT1 implies that certain specific structural elements are required for the sterol to be recognized by the putative activator site of ACAT1. Interestingly, epicholesterol (with a 3α-hydroxy group at ring A) is a poor substrate for ACAT1, yet is able to activate ACAT1 esterification of sitosterol up to 1.5-fold. However, epiallocholesterol does not significantly activate ACAT1 esterification of sitosterol. Thus, in contrast with the substrate site, the putative activation site of ACAT1 apparently can accommodate both the -α and -β orientations of the 3-hydroxy group. Cholestanol and allocholesterol are also activators of ACAT1. At 0.014 mol%, these sterols are almost as effective as cholesterol (2.1-fold activation) in activating ACAT1 (2-fold and 1.8-fold activation respectively). However, at higher concentrations (0.028 mol%), cholesterol-induced activation increases up to 3-fold but cholestanol-induced activation increases only 2.4-fold. Thus cholesterol is clearly the more effective activator of ACAT1. It was also determined that DHE is not an activator of ACAT1 esterification of sitosterol. DHE is a fluorescent yeast cholesterol analogue that has been found to behave biophysically like cholesterol in membranes [28]. The finding that DHE fails to activate ACAT1 esterification of sitosterol further supports the argument for a putative ACAT1 activator site that interacts directly with cholesterol and has a certain stereospecificity.
Figure 5. Various sterols as activators of pure His–ACAT1 in vitro.
The ACAT activity assays were performed as described in the Materials and methods section with [3H]sitosterol as substrate. Briefly, mixed micelles of [3H]sitosterol (1.6 mM sitosterol/11.2 mM PC/18.6 mM taurocholate) were made with either 0.014 or 0.028 mol% of unlabelled sterols. [3H]Sitosteryl oleate products generated by pure His–ACAT1 were separated by TLC and quantified by scintillation counting. Data points and error bars represent the mean and variation between duplicate trials. The results are representative of four separate experiments. *P<0.05 for activation versus control.
Structural specificity of sterols as substrate or as activator in the reconstituted vesicle system
To allow for direct contact of the sterols and enzymes in solution, a mixed micelle system was used. This assay system also provides a milieu for homogeneous distribution of sterols that precludes the formation of sterol microdomain(s). However, the presence of high concentrations of bile salts in the mixed micelles system could mask the hydrophobic effects of the various sterols. In addition, ACAT does not exist in a mixed micelles environment in intact cells. To test the validity of results obtained in mixed micelles, experiments from Figures 4 and 5 were repeated with reconstituted unilamellar vesicles preloaded with various sterols. The results (Figure 6A) show that ACAT1 prefers cholestanol or cholesterol as a substrate over all other sterols tested. As in mixed micelle experiments, cholestanol proves to be a better substrate than cholesterol. DHE and ent-cholesterol were very poor substrates and when epicholesterol was used as a substrate, we were not able to detect significant ACAT1 activity above background. In Figure 6(B), we show that cholesterol, cholestanol and epicholesterol (P<0.025) are able to activate ACAT esterification of sitosterol, whereas ent-cholesterol and DHE do not activate ACAT. Thus the data obtained in vesicles corroborate the results obtained in mixed micelles. A summary of the sterol structural features required to serve as substrate or as activator of ACAT1 is provided in Table 1.
Figure 6. Various sterols as substrates or activators of pure His–ACAT1 in reconstituted vesicles.
Reconstituted vesicles were generated as described in the Materials and methods section. (A) Various sterols were placed in vesicles (1.6 mM sterol/11.2 mM PC) and tested as substrates for pure His–ACAT1 with [3H]oleoyl-CoA as the radiolabelled substrate. The ACAT activity assays were performed as previously described in the Materials and methods section. Data points and error bars represent the mean and variation between duplicate trials. The results are representative of three separate experiments. (B) Various sterols were tested for their ability to activate His–ACAT1 esterification of [3H]sitosterol in vesicles (1.6 mM sitosterol/11.2 mM PC). Various unlabelled sterols were included in the [3H]sitosterol vesicles at either 0.014 or 0.028 mol%. [3H]Sitosteryl oleate products were separated by TLC and quantified by scintillation counting. Data points and error bars represent the mean and variation between duplicate trials. The results are representative of four separate experiments. *P value <0.05 versus background in (A) and versus control in (B).
Table 1. Structural features required for sterols to serve as a substrate or as an activator for ACAT1.
| Structural feature | Substrate | Activator |
|---|---|---|
| Hydroxy orientation at C-3 | 3β-required | 3β-preferred, 3α-tolerated |
| Double bond at C-5, C-6 or at C-4, C-5 | Not important | Not important |
| Variation at iso-octyl side chain | Reduces the enzyme activity but tolerated as substrate | Not tolerated |
Sitosterol esterification in human macrophage cells
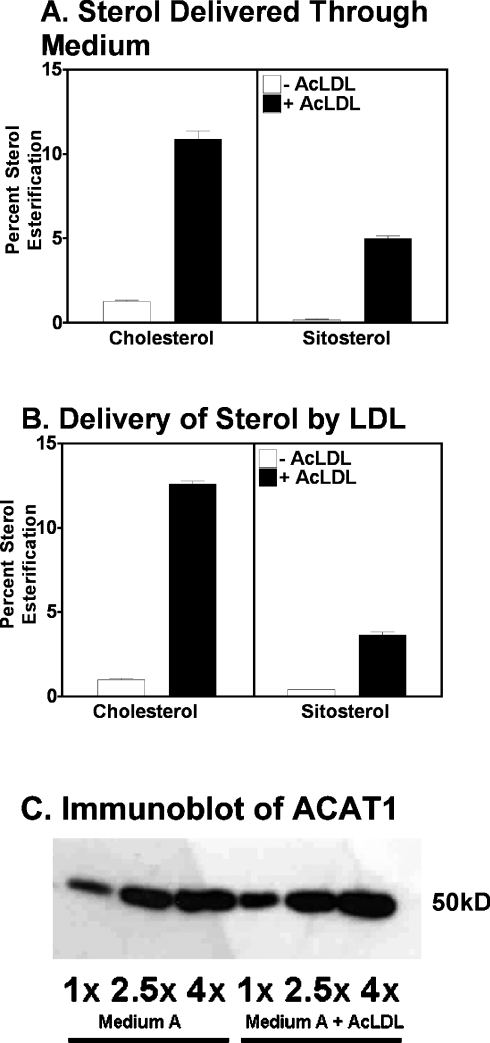
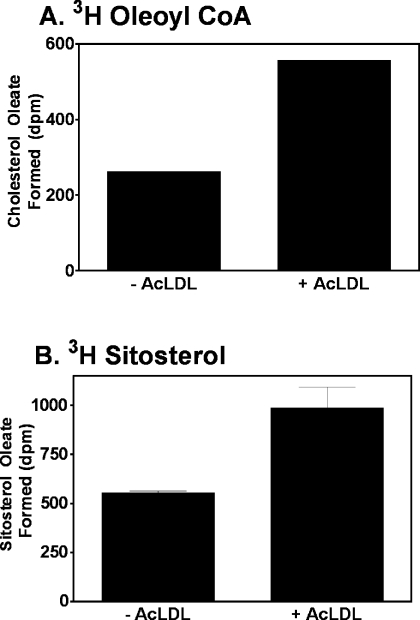
The in vitro data described above support the model that cholesterol is recognized stereospecifically by ACAT1 and serves as an allosteric activator for esterification of various sterols, including sitosterol. This model was then tested under physiological conditions. ACAT1 is the major isoform in human macrophages [9,29]. Phorbol ester (PMA)-activated THP-1 cells were the cell model. THP-1 cells are derived from acute monocytic leukaemia. Upon activation with PMA, THP-1 cells differentiate into human macrophage-like cells, and express scavenger receptors that are responsible for the uptake of various modified LDLs, including AcLDL. PMA-treated THP-1 cells were grown in cell culture medium with or without 50 μg/ml AcLDL for 12 h. AcLDL is taken up by the scavenger receptors in the THP-1 cells, causing the cells to become cholesterol loaded [30]. Radiolabelled sitosterol or radiolabelled cholesterol was then delivered directly to the cells by adding the sterols to the tissue culture medium in DMSO stock solutions. After 24 h, the percentage esterification of the radiolabelled sterols was measured. The results show that in cells grown in a medium without AcLDL, only 1.2% of the labelled cholesterol was esterified. However, when the cells were preincubated with AcLDL, the percentage esterification increased to above 10%: an 8-fold increase (Figure 7A, left panel). For labelled sitosterol without AcLDL, there was a very low percentage (0.2%) of sitosterol esterification. However, in cells loaded with AcLDL, 5% of the sitosterol was esterified (Figure 7A, right panel). The sterol delivery method was then altered by delivering radiolabelled sitosterol or radiolabelled cholesterol to cells as free (unesterified) sterol present in LDL. Once again, there was a significant increase in percentage of sterol esterification in both cholesterol (10-fold increase) and sitosterol (7.5-fold increase) when cells were preloaded with cholesterol via AcLDL (Figure 7B). These results suggest that loading cells with cholesterol renders sitosterol a more active ACAT substrate. A control experiment showed that incubation with AcLDL does not cause an increase in the protein levels of ACAT1 in these THP-1 cells (Figure 7C). This is consistent with earlier findings in human macrophages [11]. In a Western-blot analysis using anti-ACAT2 antibodies, ACAT2 protein was not detected in THP-1 cells whether they were grown with or without AcLDL (results not shown). The same sets of experiments were carried out in human monocyte-derived macrophages, using LDL as the method for delivering radiolabelled sterols. The results again show a significant increase in percentage of esterification of both radiolabelled cholesterol and radiolabelled sitosterol when macrophages were preincubated with AcLDL (results not shown). The results of control experiments showed that acetyl-LDL increases the cholesterol content in the ER (Figure 8A). When [3H]sitosterol was monitored as a substrate, the ability of ACAT1 to esterify sitosterol in acetyl-LDL-treated cells also increased 2-fold. This result shows that an increase in ER cholesterol correlates with an increase in the capacity of ACAT1 to esterify sitosterol (Figure 8B).
Figure 7. Esterification of sitosterol and cholesterol in intact THP-1 macrophages.
The experiments were conducted as described in the Material and methods section. THP-1 macrophage cells were grown in medium A (DMEM, 10% FBS and 10 nM PMA); treated without or with 50 μg/ml AcLDL for 12 h. (A) [3H]Sitosterol or [3H]cholesterol was added to the growth medium for 24 h. (B) [3H]Sitosterol or [3H]cholesterol in LDL was added to the growth medium for 24 h. Cells were harvested and lipids were extracted and separated. Sterol and sterol ester products were counted by scintillation counter. The assays were run in duplicate wells. Error bars represent mean and variation between duplicates. Results shown represent one of the two experiments with similar results. (C) Immunoblot of ACAT1 enzyme in THP-1 macrophages grown in the presence or absence of 50 μg/ml AcLDL for 36 h. Cellular proteins from extracts of cells grown under each growth condition were loaded at doses of 20, 50 or 80 μg (1×, 2.5× or 4×) as indicated. The immunoblot was performed according to procedures described in [11], using the polyclonal anti-ACAT1 antibodies as the primary antibody. Data shown are representative of two separate experiments.
Figure 8. Increased cholesterol content in the ER correlates with the increased sitosterol esterification in THP-1 macrophage cells.
THP-1 macrophages were grown in medium A with or without 50 μg/ml AcLDL for 12 h. Cell homogenates were prepared according to the procedure described in the Materials and methods section. (A) [3H]Oleoyl-CoA/BSA was added to the cell homogenates. (B) [3H]Sitosterol and non-radioactive oleoyl-CoA/BSA were added to cell homogenates. ACAT activity assays were conducted at 37 °C for 5 min. Data points and error bars represent the mean and variation between duplicate trials. Results shown are representative of two separate experiments.
DISCUSSION
In a previous report, we proposed that ACAT1 is an allosteric enzyme, with two types of sterol-binding sites, a substrate site and an activator site. The substrate site is able to accommodate a wide variety of sterols while the putative activator site may only recognize cholesterol [17]. In our present work, we employed sterols with various structural variations to probe the structural requirements for the substrate site and the putative activator site. The results show that the substrate site of ACAT1 preferentially recognizes cholestanol and cholesterol over all other sterols tested. The double bond on steroid ring B is not a critical feature; the side-chain modification of sitosterol at C-24 greatly diminishes, but does not completely inactivate, ACAT1's ability to esterify sterols. The stereochemistry of the 3-hydroxy group in ring A is a critical feature in determining whether a sterol can serve as an ACAT1 substrate. Epicholesterol is an extremely poor substrate for ACAT1, suggesting that the axial orientation of the 3-OH moiety in this steroid precludes its binding to the ACAT1 substrate site. In a different but related cholesterol-sensing system, Adams et al. [31] show that the stereochemistry at the 3-OH on cholesterol is also an important feature that determines the ability of the sterol to mediate the processing of SREBP (sterol-regulatory-element-binding protein) in mammalian cells. Furthermore, Radhakrishnan et al. [32] have shown that the SREBP cleavage activating protein (SCAP) binds to cholesterol with high affinity but fails to bind epicholesterol (3α-OH).
ent-cholesterol is the mirror image of cholesterol, and although this molecule contains a 3α-OH group, this OH group has the same equatorial configuration as it has in cholesterol. In fact, the relative configurations of all stereocentres (a total of eight centres) in the two molecules are the same. Thus the physical properties of cholesterol and ent-cholesterol are identical. The finding that cholesterol and not ent-cholesterol is a strong substrate for ACAT-1 indicates that the substrate-binding site recognizes the overall shape of the cholesterol molecule, and not just the axial or equatorial orientation of the 3-OH group.
To address the putative ACAT1 activator site, our current results show that unlike cholesterol, the enantiomeric form of cholesterol fails to activate ACAT1 esterification of sitosterol. It has been shown that monolayers of egg sphingomyelin containing either cholesterol or ent-cholesterol compressed identically [23]. Additionally, Mannock et al. [33] reported that bilayers containing cholesterol or ent-cholesterol share the same buoyant density and X-ray diffraction patterns, and the enantiomers had the same effects on the gel/liquid–crystalline phase transitions of the bilayers as well. Thus cholesterol and ent-cholesterol affect the biophysical properties of phospholipids in lipid monolayer and bilayer studies in the same manner [23]. However, certain enzymes such as cholesterol oxidase are known to prefer cholesterol over ent-cholesterol [23,34]. Our finding that ACAT1 is activated by cholesterol but not by ent-cholesterol strongly argues that structural considerations take precedence over biophysical considerations in determining a sterol's ability to activate ACAT1. An alternative interpretation of our data could be that ent-cholesterol does act as an activator. However, its effect is masked by competitive inhibition at the substrate site. This interpretation is unlikely, as we have shown that ent-cholesterol is a poor substrate. DHE, another sterol that displays very similar biophysical properties in membranes as cholesterol, also fails to activate ACAT1. Together, these results strongly support the existence of a putative activator site present within the ACAT1 protein that interacts with cholesterol in a stereo-specific manner. Interestingly, we found that the putative activator site allows for variation in the orientation of the 3-hydroxy group: epicholesterol is able to activate ACAT1, although not as effectively as its 3β isomer (cholesterol). Thus, within ACAT1, the putative activator site may have a distinct structural arrangement from that of the substrate site. The existence of the ACAT1 substrate and allosteric-activator site is deduced based on enzyme kinetic analysis. In the future, when purified ACAT1 becomes available in more abundant quantities, binding analysis and other protein biochemical methods will be employed to identify the two sterol-binding sites.
Our results herein show that sitosterol esterification is highly activated by cholesterol in ACAT1, but only moderately activated by cholesterol in ACAT2. Previously, Temel et al. [35] showed that ACAT2 was less likely than ACAT1 to use sitosterol as a substrate (as opposed to cholesterol, which both isoenzymes prefer). Our results provide a rationale to explain these findings. ACAT2 is more selective in its choice of substrates than ACAT1, because it is less responsive to cholesterol-dependent activation. To address the physiological relevance, our model predicts that in animals and in humans, under high cholesterol loading conditions, the capacity of ACAT1 (and that of ACAT2 to a lesser extent) to esterify various sterols, including sitosterol and various oxysterols, will be significantly augmented. Sitosterol is a major plant sterol and is normally present in mammals at very low levels. In the sitosterolaemia disease, the presence of sitosterol and other plant sterols are highly elevated [36]. Salen et al. [37] have shown that not only does free sitosterol accumulates in the plasma and tissues, but significant amounts of sitosteryl esters are also found in certain tissues. Results of the present study provide a plausible explanation for the observation that esterified sitosterol accumulates in the atherosclerotic thoracic aortic tissue of a human patient with sitosterolaemia [37]. Accumulation of these fatty acid esters in plaque macrophages may result in cell death and a decrease in plaque stability leading to plaque rupture and atherothrombosis.
Online data
Acknowledgments
This work was supported by an NIH grant (HL60306 to T.-Y.C.) and the grant GM 47969 (to D.F.C.). J.L. has been supported by an NIH predoctoral training grant (T32 GM008704) awarded to Dartmouth and E.J.W. by NIH Cardiovascular Research Training grant (55935). We thank Dr N. Sakashita for advice on preparation of human macrophages and for kindly providing THP-1 cells and AcLDL, H. Josephson (Department of Biochemistry, Dartmouth Medical School) and N. Ballew (GlycoFi, Lebanon, NH, U.S.A.) for careful reading and preparation of this paper, B. Costine (Department of Physiology, Dartmouth Medical School) for advice on statistical analysis, and all the members of the TYC Laboratory (Department of Biochemistry, Dartmouth Medical School) for helpful discussion and for sharing of reagents.
References
- 1.Chang C. C. Y., Huh H. Y., Cadigan K. M., Chang T. Y. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 1993;268:20747–20755. [PubMed] [Google Scholar]
- 2.Anderson R. A., Joyce C., Davis M., Reagan J. W., Clark M., Shelness G. S., Rudel L. L. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 3.Cases S., Novak S., Zheng Y. W., Myers H., Lear S. R., Sande E., Welch C. B., Lusis A. J., Spencer T. A., Krause B. R., et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 4.Oelkers P., Behari A., Cromley D., Billheimer J. T., Sturley S. L. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 1998;273:26765–26771. doi: 10.1074/jbc.273.41.26765. [DOI] [PubMed] [Google Scholar]
- 5.Buhman K. F., Accada M., Farese R. V., Jr Mammalian acyl-CoA:cholesterol acyltransferases. Biochim. Biophys. Acta. 2000;1529:142–154. doi: 10.1016/s1388-1981(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 6.Rudel L., Lee R., Cockman T. Structure, function, and regulation of ACAT. Curr. Opin. Lipidol. 2001;12:121–127. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Chang T. Y., Chang C. C. Y., Lin S., Yu C., Li B. L., Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr. Opin. Lipidol. 2001;12:289–296. doi: 10.1097/00041433-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Smith J. L., Rangaraj K., Simpson R., Maclean D. J., Nathanson L. K., Stuart K. A., Scott S. P., Ramm G. A., de Jersey J. Quantitative analysis of the expression of ACAT genes in human tissues by real-time PCR. J. Lipid Res. 2004;45:686–696. doi: 10.1194/jlr.M300365-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki A., Sakashita N., Lee O., Takahashi K., Horiuchi S., Hakamata H., Morganelli P. M., Chang C. C., Chang T. Y. Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes-macrophages. Arterioscler. Thromb. Vasc. Biol. 1998;18:1568–1574. doi: 10.1161/01.atv.18.10.1568. [DOI] [PubMed] [Google Scholar]
- 10.Chang C. C. Y., Sakashita N., Ornvold K., Lee O., Chang E. T., Dong R., Lin S., Lee C. Y., Strom S. C., Kashyap R., et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 2000;275:28083–28092. doi: 10.1074/jbc.M003927200. [DOI] [PubMed] [Google Scholar]
- 11.Sakashita N., Miyazaki A., Chang C. C. Y., Morganelli P., Chang T. Y., Nakamura O., Kiyota E., Hakamata H., Satoh M., Tamagawa H., et al. The presence of ACAT2 in human and mouse macrophages: in vivo and in vitro studies. Lab. Invest. 2003;83:1–13. doi: 10.1097/01.lab.0000095687.17383.39. [DOI] [PubMed] [Google Scholar]
- 12.Parini P., Davis M., Lada A. T., Erickson S. K., Wright T. L., Gustafsson U., Sahlin S., Einarsson C., Eriksson M., Angelin B., et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 2004;110:2017–2023. doi: 10.1161/01.CIR.0000143163.76212.0B. [DOI] [PubMed] [Google Scholar]
- 13.Lin S., Cheng D., Liu M. S., Chen J., Chang T. Y. Human acylCoA:cholesterol acyltransferase-1 in the endoplasmic reticulum contains seven transmembrane domains. J. Biol. Chem. 1999;274:23276–23285. doi: 10.1074/jbc.274.33.23276. [DOI] [PubMed] [Google Scholar]
- 14.Lin S., Lu X., Chang C. C. Y., Chang T. Y. Human acyl-coenzyme A:cholesterol acyltransferase 2 (hACAT2) expressed in Chinese hamster ovary cells: membrane topology and active site location. Mol. Biol. Cell. 2003;14:2447–2460. doi: 10.1091/mbc.E02-11-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang C. C. Y., Lee C. Y. G., Chang E. T., Cruz J. C., Levesque M. C., Chang T. Y. Recombinant human acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) purified to essential homogeneity utilizes cholesterol in mixed micelles or vesicles in a highly cooperative manner. J. Biol. Chem. 1998;273:35132–35141. doi: 10.1074/jbc.273.52.35132. [DOI] [PubMed] [Google Scholar]
- 16.Theunissen J. J., Jackson R. L., Kempen H. J. M., Demel R. A. Membrane properties of oxysterols. Interfacial orientation, influence on membrane permeability and redistribution between membranes. Biochim. Biophys. Acta. 1986;860:66–74. doi: 10.1016/0005-2736(86)90499-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Yu C., Liu J., Spencer T. A., Jr, Chang C. C. Y., Chang T. Y. Cholesterol is superior to 7-ketocholesterol or 7alpha-hydroxycholesterol as an allosteric activator for acyl-coenzyme A:cholesterol acyltransferase 1. J. Biol. Chem. 2003;278:11642–11647. doi: 10.1074/jbc.M211559200. [DOI] [PubMed] [Google Scholar]
- 18.Leathes J. B. Role of fats in vital phenomena. Lancet. 1925;208:853–856. [Google Scholar]
- 19.Cao H., Tokutake N., Regen S. L. Unraveling the mystery surrounding cholesterol's condensing effect. J. Am. Chem. Soc. 2003;125:16182–16183. doi: 10.1021/ja039172x. [DOI] [PubMed] [Google Scholar]
- 20.Cheetham J. J., Wachtel E., Bach D., Epand R. M. Role of the stereochemistry of the hydroxyl group of cholesterol and the formation of nonbilayer structures in the phosphatidylethanolamines. Biochemistry. 1989;28:8928–8934. doi: 10.1021/bi00448a036. [DOI] [PubMed] [Google Scholar]
- 21.Verhagen J. C., ter Braake P., Teunissen J., van Ginkel G., Sevanian A. Physical effects of biologically formed cholesterol oxidation products on lipid membranes investigated with fluorescence depolarization spectroscopy and electron spin resonance. J. Lipid Res. 1996;37:1488–1502. [PubMed] [Google Scholar]
- 22.Westover E. J., Covey D. F. First synthesis of ent-desmosterol and its conversion to ent-deuterocholesterol. Steroids. 2003;68:159–166. doi: 10.1016/s0039-128x(02)00174-5. [DOI] [PubMed] [Google Scholar]
- 23.Westover E. J., Covey D. F. The enantiomer of cholesterol. J. Membr. Biol. 2004;202:61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- 24.Bishop J. E., Hajra A. K. A method for the chemical synthesis of 14C-labeled fatty acyl coenzyme A's of high specific activity. Anal. Biochem. 1980;106:344–350. doi: 10.1016/0003-2697(80)90531-x. [DOI] [PubMed] [Google Scholar]
- 25.Billheimer J. T., Avart S., Milani B. Separation of steryl esters by reversed-phase liquid chromatography. J. Lipid Res. 1983;24:1646–1651. [PubMed] [Google Scholar]
- 26.Chang T. Y., Limanek J. S., Chang C. C. Y. A simple and efficient procedure for the rapid homogenization of cultured animal cells grown in monolayer. Anal. Biochem. 1981;161:298–302. doi: 10.1016/0003-2697(81)90360-2. [DOI] [PubMed] [Google Scholar]
- 27.Sakai M., Miyazaki A., Hakamata H., Sato Y., Matsumura T., Kobori S., Chichiri M., Horiouchi S. Lysophosphatidylcholine potentiates the mitogenic activity of modified LDL for human monocyte-derived macrophages. Arterioscler. Thromb. Vasc. Biol. 1996;16:600–605. doi: 10.1161/01.atv.16.4.600. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder F., Jefferson J. R., Kier A. B., Knittel J., Scallen T., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc. Soc. Exp. Biol. Med. 1991;196:235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- 29.Sakashita N., Miyazaki A., Takeya M., Horiuchi S., Chang C. C. Y., Chang T. Y., Takahashi K. Localization of human acyl-coenzyme A:cholesterol acyltransferase-1 in macrophages and in various tissues. Am. J. Pathol. 2000;156:227–236. doi: 10.1016/S0002-9440(10)64723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kritharides L., Christian A., Stoudt G., Morel D., Rothblat G. H. Cholesterol metabolism and efflux in human THP-1 macrophages. Arterioscler. Thromb. Vasc. Biol. 1998;18:1589–1599. doi: 10.1161/01.atv.18.10.1589. [DOI] [PubMed] [Google Scholar]
- 31.Adams C. M., Reitz J., De Brabander J. K., Feramisco J. D., Li L., Brown M. S., Goldstein J. L. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- 32.Radhakrishnan A., Sun L. P., Kwon H. J., Brown M. S., Goldstein J. L. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol. Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Mannock D. A., Mcintosh T. J., Jiang X., Covey D. F., McElhaney R. N. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys. J. 2003;84:1038–1046. doi: 10.1016/S0006-3495(03)74920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luker G. D., Pica C. M., Kumar A. S., Covey D. F., Piwnica-Worms D. Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry. 2000;39:7651–7661. doi: 10.1021/bi9928593. [DOI] [PubMed] [Google Scholar]
- 35.Temel R. E., Gebre A. K., Parks J. S., Rudel L. L. Compared with acyl-CoA:cholesterol O-acyltransferase (ACAT) 1 and lecithin:cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J. Biol. Chem. 2003;278:47594–47601. doi: 10.1074/jbc.M308235200. [DOI] [PubMed] [Google Scholar]
- 36.Miettenen T. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 37.Salen G., Shefer S., Nguyen L., Ness G., Tint G. S., Shore V. Sitosterolemia. J. Lipid Res. 1992;33:945–955. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.