Abstract
C2GnT-I [core2 β(1,6)-N-acetyglucosaminyltransferase-I] and FucT-VII [α(1,3)-fucosyltransferase-VII] are the key enzymes for the biosynthesis of sialyl-Lewis x determinants on selectin ligands and therefore they represent good drug targets for the treatment of inflammatory disorders and other pathologies involving selectins. In the present study, we examined the importance of N-glycosylation for the ability of C2GnT-I and FucT-VII to generate functional selectin ligands, particularly the PSGL-1 (P-selectin glycoprotein ligand-1). We found that (i) both enzymes have their two N-glycosylation sites occupied, (ii) for C2GnT-I, the N-glycan chain linked to Asn-95 significantly contributes to the synthesis of functional PSGL-1 and is required to localize the enzyme to the cis/medial-Golgi compartment, (iii) all N-glycosylation-deficient proteins of FucT-VII displayr a dramatic impairment of their in vitro enzymatic activities, but retain their ability to fucosylate the core2-modified PSGL-I and to generate P- and L-selectin binding, and (iv) the glycomutants of FucT-VII fail to synthesize sialyl-Lewis x or to generate E-selectin binding unless core2-modified PSGL-1 is present. All combined, our results show a differential functional impact of N-glycosylation on C2GnT-1 and FucT-VII and disclose that a strongly reduced FucT-VII activity retains the ability to fucosylate PSGL-1 on the core2-based binding site(s) for the three selectins.
Keywords: core2 β(1,6)-N-acetyglucosaminyltransferase-I (C2GnT-I); α(1,3)-fucosyltransferase-VII (FucT-VII); N-glycosylation; PNGase F; P-selectin glycoprotein ligand-1 (PSGL-1); selectin
Abbreviations: C2GnT-I, core2 β(1,6)-N-acetyglucosaminyltransferase-I; CHO, Chinese-hamster ovary; sLex, sialyl-Lewis x; CSLEX-1, anti-sLex; EGFP, enhanced green fluorescent protein; FucT-VII, α(1,3)-fucosyltransferase-VII; GlcNAc, N-acetylglucosamine; mAb, monoclonal antibody; α-Man-II, α-mannosidase-II; PNGase F, peptide N-glycosidase F; PSGL-1, P-selectin glycoprotein ligand-1; PL1, anti-PSGL-1; TM, tunicamycin; wt, wild-type
INTRODUCTION
During inflammation, the attachment of leucocytes to endothelium is initiated by the selectin family E- (CD62E), P-(CD62P) and L-selectin (CD62L). E- and P-selectin expression is induced on endothelial cells by inflammatory cytokines (P-selectin is also expressed on platelets), while L-selectin is constitutively expressed on leucocytes to mediate leucocyte–leucocyte and leucocyte–endothelium interactions [1]. These interactions cause a slow downstream movement of leucocytes along the endothelium via transient, reversible, adhesive interactions called leucocyte rolling [2]. All three selectins bind, in a calcium-dependent manner, the tetrasaccharide sLex [sialyl-Lewis x; AcNeu α2-3Gal β1-4 (Fucα1-3)GlcNAc, where AcNeu stands for N-acetylneuraminic acid (sialic acid), Gal stands for galactose and GlcNAc stands for N-acetylglucosamine] carried by a variety of molecules including N- and O-glycoproteins as well as glycolipids [3]. In the immune system, the sLex moiety that is recognized by the three selectins is carried by mucins (O-glycoproteins) [4] including the PSGL-1 (P-selectin glycoprotien ligand-1), the major counter-receptor for P-selectin, which can also serve as a ligand for L- and E-selectin [5]. Three other mucins have been identified as L-selectin counter-receptors on high endothelial venules of lymph nodes: the GlyCAM-1 (glycosylation-dependent cell adhesion molecule-1), the CD34 and the MAdCAM-1 (mucosal addressin cell adhesion molecule-1) [6].
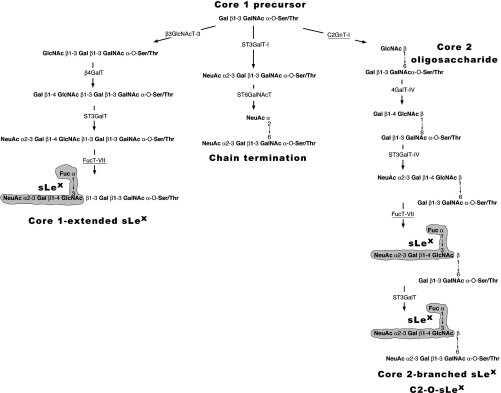
Stuctural studies of the glycans involved in selectin-mediated adhesion and data from mice with targeted deletions of glycosyltransferase and sulphotransferase genes disclose that a high-affinity selectin–ligand interaction requires core2-based O-glycans synthesized by the C2GnT-I [core2 β(1,6)-N-acetyglucosaminyltransferase; EC 2.4.1.102] and a collaborative action of other glycosyltransferases including the FucT-VII [α(1,3)-fucosyltransferase-VII; EC 2.4.1.152)] as well as sulpho-transferases [7–10]. As illustrated in Figure 1, the synthesis of sLex on core2-branched O-glycans involves the C2GnT-I that adds a β(1,6)-linked GlcNAc to the GalNAc of the core1 precursor, thereby initiating the synthesis of a sialyllactosamine backbone that is further α(1,3)-fucosylated on the GlcNAc residue by FucT-VII [11]. Two other enzymes ST3Gal-I and the β3GlcNAcT-3, compete with C2GnT-I for the same core1 substrate, to undergo either chain termination and core1-extension respectively (Figure 1). Interestingly, it has been recently shown that, in the absence of C2GnT-I activity, core1-extended sLex can be formed on recombinant PSGL-1 to support a significant degree of shear-dependent tethering and rolling of neutrophils and lymphocytes, although to a lesser extent than with core2-modified PSGL-1 [11].
Figure 1. Involvement of C2GnT-I and FucT-VII in the biosynthesis of sLex structures.
The accepted pathway for the synthesis of sLex structures on O-linked glycans starts with a common core1 precursor and follows three different directions [11]: the formation of core2-branched sLex is initiated by C2GnT-I (underlined) followed by β4GalT-IV, ST3GalT-IV and FucT-VII (underlined). The core 1 precursor can also be modified by β3GlcNAcT-3, forming an extended-core1 oligosaccharide, which is further galactosylated by β4GalT (probably the β4GalT-I) and fucosylated by FucT-VII resulting in the expression of core1-extended sLex structures. No sLex antigen is synthesized (chain termination) if the core1 precursor undergoes sialylation catalysed by ST3GalT-I and ST6GalNAcT-I.
The leucocyte PSGL-1 is a homodimer of disulphide-linked subunits with an apparent molecular mass of 120 kDa each and extensively decorated by O-glycans; some are modified by sLex [12,13]. Furthermore, studies to map the segments of PSGL-1 that interact with P-selectin revealed that the reacting sLex moiety is located within an anionic polypeptide segment containing three tyrosine residues, of which at least one is sulphated [14–16]. PSGL-1 can support the binding of the three selectins, although with specific requirements for glycosylation and sulphation. For example, both P- and L-selectin interact with PSGL-1 through the same core2-branched sugar chain on Thr-57 but, while P-selectin requires the three adjacent sulphated tyrosines Tyr-46, Tyr-48 and Tyr-51 [16], only the sulphated Tyr-51 seems to be important for L-selectin–PSGL-1 interaction [17]. In contrast, neither a core2-branched O-glycan nor tyrosine sulphation was found to be necessary for E-selectin–PSGL-1 interaction [3], but the presence of C2GnT-I was found to significantly enhance the affinity of PSGL-1 for E-selectin [18].
We and others have shown that C2GnT-I and FucT-VII are N-glycosylated proteins [19–21]. Given the essential contribution of these glycosyltransferases to the synthesis of selectin ligands, and since N-glycosylation has been reported to influence folding, stability, sorting and transport of proteins [22,23], we herein investigated the impact of this post-translational modification on C2GnT-I and FucT-VII functions in vitro and in vivo. We therefore generated N-glycosylation mutants of these enzymes, expressed them in CHO (Chinese-hamster ovary) cells in the presence of PSGL-1 and analysed E, P- and L-selectin binding. We found that N-glycans of C2GnT-I are crucial for its in vitro and P-selectin binding activities. In FucT-VII however, all mutants were able to construct functional PSGL-1 molecules and to trigger P- and L-selectin binding sites. However, none of them was active in vitro or able to synthesize sLex structure or to generate E-selectin binding, unless core2-modified PSGL-1 is present.
EXPERIMENTAL
Construction of FucT-VII and C2GnT-I glycomutants
The constructs FucT-VII–EGFP (where EGFP stands for enhanced green fluorescent protein) and C2GnT-I–EGFP in pcDNA3.1(+) vector (Invitrogen) have been described previously [19]. The FucT-VII single mutations N81Q (Asn81→Gln) and N291Q (Asn-81 and Asn-291 substituted with glutamine residues) and the double-mutant N81Q/N291Q were generated by PCR as described in [24] using FucT-VII–EGFP as template and the sense and antisense primers listed in Table 1. Reactions were performed using Pfu polymerase (Stratagene, Saint Quentin en Yvelines, France) and PCR products were treated with DpnI and used directly to transform TOP10 F′ ultracompetent Escherichia coli (Invitrogen). The C2GnT-I mutants were generated as above except that the threonine residue in the sequence Asn-Xaa-Thr was converted into alanine as described by Toki et al. [20]. The three C2GnT-I mutants were named T60A, T97A and T60A/T97A. The nucleotide sequence of each construct was confirmed by sequencing.
Table 1. Mutants and primers.
The sense and antisense primers for T60A correspond respectively to nt 151–192 and 127–168 of the C2GnT-I coding sequence and those for T97A correspond to nt 261–303 and 238–279 respectively. The sense and antisense primers for N81Q correspond respectively to nt 220–255 and 235–267 of the FucT-VII coding sequence and those for N291Q correspond to nt 850–891 and 860–903 respectively. In the sense primers of T60A and T97A, the codon ACC (coding for Thr) was replaced by GCC (coding for Ala). In the N81Q mutant of FucT-VII, the codon AAC (Asn) was replaced by CAG (Gln), and in N291Q the codon AAT (Asn) was replaced by CAA (Gln). The mutated nucleotides are italicized and marked by ‘*’. The overlapped sequences are underlined.
| Species | Mutations | Primers |
|---|---|---|
| C2GnT-I | T60A | Sense: 5′-GAGAATCCTAGTAGTGATATTAATTGC*GCCAAAGTTTTACAG-3′ |
| Antisense: 5′-ATCACTACTAGGATTCTCCCCAGCAAGCTCCAAGTGTCTGAC-3′ | ||
| T97A | Sense: 5′-TGGACACCTGACGACTATATAAACATG*GCCAGTGACTGTTCT-3′ | |
| Antisense: 5′-ATAGTCGTCAGGTGTCCACCGAGGGCGCTTTTTAAATTTCAC-3′ | ||
| FucT-VII | N81Q | Sense: 5′-GCCCGCTGCCACCTGAGTGCC*CA*GCGAAGCCTG-3′ |
| Antisense: 5′-GTCGGCGCTGGCCAGCAGGCTTCG*CT*GGGCACT-3′ | ||
| N291Q | Sense: 5′-GCGGCTTTCCTCACTGGCATG*CA*AGAGAGCCGATACCAACGC-3′ | |
| Antisense: 5′-CCAGGCAAAGAAGCGTTGGTATCGGCTCTC*TT*GCATGCCAGT-3′ |
Cell culture and transfections
CHO-K1 and HEK-293T (human embryonic kidney 293T) cells were cultured in Ham-F12 medium and Dulbecco's modified Eagle's medium respectively. All media (Invitrogen) contained 10% (v/v) fetal calf serum and antibiotics. Transfections were performed using Lipofectamine™ reagent (Invitrogen) according to the manufacturer's instructions. The production of CHO-K1 cells stably expressing FucT-VII (CHO/F7) and those expressing C2GnT-I–EGFP (CHO/C2) have been described previously [25,26]. To study P- and L-selectin binding, we developed a complementation system in which PSGL-1 was co-expressed with either C2GnT-I (CHO/C2P1) or FucT-VII (CHO/F7P1), so that cells do not exhibit P- or L-selectin binding unless the enzyme of interest is present. Accordingly, the CHO/F7P1 cells were used to study C2GnT-I and its mutants, while CHO/C2P1 cells were used to study FucT-VII and its mutants. CHO/F7P1 cells were made by stably transfecting CHO/F7 cells with 1 μg of pZeoSV/PSGL-1 [11]. The bulk stable transfectants were collected and sorted by an FACS using the PL1 (anti-PSGL-1) mAb (monoclonal antibody) [27] followed by an CSLEX-1 (anti-sLex) mAb (prepared as described in [28]). To generate CHO/C2P1 cells, we transfected CHO/C2 cells with 1 μg of pZeoSV/PSGL-1 and the zeocin-resistant clones were first checked for C2GnT-I expression with the anti-C2GnT-I antibody 1719.39 as described in [29] and then sorted by FACS using PL1 immunoreactivity as above. In all cases, the impact of N-glycosylation on glycosyltransferase function in vivo was assessed by the binding of selectin-IgM chimaeras to CHO/F7P1 or CHO/C2P1 cells after transient or stable transfections with C2GnT-I or FucT-VII constructs or their corresponding mutants. To determine the in vitro enzyme activities, cell monolayers were harvested using the non-enzymatic dissociation solution (Sigma), and pellets were solubilized with the appropriate buffers for 30 min at 4 °C. To evaluate the effect of TM (tunicamycin) on C2GnT-I, CHO/C2 cells were cultured in the absence or in the presence of 1 μg/ml of the drug for 72 h, with a daily medium change, and prepared for either in vitro enzyme activity or SDS/PAGE and immunoblotting, as described below. Recombinant selectin-IgM chimaeras were produced by transiently transfecting HEK-293T cells with selectin–IgM plasmids as described in [28].
In vitro enzyme assays
For C2GnT-I activity, cell pellets were solubilized in water containing 0.4% Triton X-100, 150 mM NaCl and EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN, U.S.A.) and used as described previously [30]. The oligosaccharide Galβ1,3GalNAc-O-p-nitrophenyl (Toronto Research Chemicals, North York, Ontario, Canada) was used as an acceptor and UDP-[6-3H]GlcNAc (400000 c.p.m./assay) as a donor. After 1 h of incubation at 37 °C, mixtures were diluted to 5 ml with water and applied to Sep-Pak C18 cartridges (Waters, Milford, MA, U.S.A.). Columns were then rinsed with 20 ml of water and bound labelled acceptors were eluted with methanol and counted. For FucT-VII activity, cell pellets were solubilized in solubilization buffer (50 mM Hepes, pH 7.5, containing 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 20%, v/v, glycerol and EDTA-free protease inhibitor cocktail) and centrifuged at 10000 g for 15 min. Supernatants were then used to assay for FucT-VII activity as described in [31], using GDP-[14C]fucose (100000 c.p.m.) as a donor and either fetuin (2.5 mg/ml final concentration) or the synthetic oligosaccharide AcNeu α(2,3)Gal β(1,4)GlcNAc-octyl (1 mM final concentration) as an acceptor. Fetuin was separated from the reaction mixture by WGA (wheat germ agglutinin)-agarose chromatography [32] and counted. Asialofetuin was taken as a reference. When oligosaccharides were used as acceptors, the reaction mixtures were diluted to 5 ml with 250 mM ammonium formate buffer (pH 4) and applied to Sep-Pak C18 cartridges. Columns were then rinsed with 20 ml of ammonium formate buffer and bound labelled acceptors were eluted with 70% (v/v) methanol/water and counted. On the basis of equimolarity between glycosyltransferases and EGFP in cell extracts (i.e. one molecule of glycosyltransferase conjugated to one molecule of EGFP), the enzyme concentration was calculated using the amount of EGFP/assay using the value of molar absorption coefficient ϵ=55000 M−1·cm−1 for EGFP as described previously [19].
In some instances, crude membrane fractions were prepared from cells by sonication and assayed for α(1,3)-fucosyltransferase activity as described above. Briefly, cells were resuspended in the solubilization buffer without Triton X-100 and sonicated for 20 s at maximum intensity. This step was repeated five times with 5 min incubation on ice between each sonication.
Fluorescence microscopy and flow cytometry
Confocal microscopy was used to compare the intracellular distribution of C2GnT-I and its mutants with that of the medial-Golgi marker α-Man-II (α-mannosidase-II), essentially as described in [26]. Methods for selectin binding on cell monolayers and CSLEX-1 immunoreactivity have been described previously [28]. Alternatively, selectin binding was quantified by FACS as follows: cells were harvested as above and resuspended in OptiMEM/BSA at 106 cells/ml, incubated with selectin-IgM chimaeras for 30 min, rinsed and incubated with phycoerythrin-conjugated anti-human IgM antibodies (Jackson Immunoresearch Laboratories, Baltimore, PA, U.S.A.). Fluorescence was then analysed by flow cytometry as described in [28]. In some selectin-binding experiments, cells were pretreated with increasing concentrations of PL1 mAb before selectin-binding and FACS analyses.
PNGase F (peptide N-glycosidase F) treatment, SDS/PAGE and Western immunoblotting
PNGase F treatment was performed essentially as described in [19]. In brief, cell pellets were prepared as above and lysed in 100 mM Tris/HCl buffer (pH 8.6) containing 1% Triton X-100, 1% 2-mercaptoethanol and EDTA-free protease inhibitor cocktail and clarified by centrifugation at 14000 g for 15 min at 4 °C. Unless otherwise specified, supernatants were then treated with 20 units/ml PNGase F at 37 °C for 120 min and proteins were separated by SDS/PAGE after the addition of 5-fold concentrated SDS sample buffer. To analyse EGFP-fused proteins or PSGL-1, cell pellets were dissolved in SDS sample buffer under reducing conditions and separated on SDS/PAGE (7.5% polyacrylamide). Proteins were then detected on blots with the anti-EGFP JL-8 mAb (Invitrogen) or the anti-PSGL-1 PL1 mAb (Immuno-tech, Marseille, France) as described in [19,28].
RESULTS
On the basis of their amino acid sequences, both C2GnT-I and FucT-VII have two potential N-glycosylation sites: Asn-58 and Asn-95 for C2GnT-I and Asn-81 and Asn-291 for FucT-VII. The mutants of FucT-VII were generated by substituting the aspar-agine residues in the sequence Asn-Xaa-Ser/Thr by glutamine, and those of C2GnT-I mutants were made by substituting the threonine residues into alanine. Accordingly, the C2GnT-I single mutations were named T60A, T97A and T60A/T97A for the double mutant while those of FucT-VII were named N81Q, N291Q and N81Q/N291Q respectively. Each N-glycosylation-deficient mutant will be referred to as glycomutant throughout the paper.
N-glycosylation patterns of C2GnT-I and FucT-VII
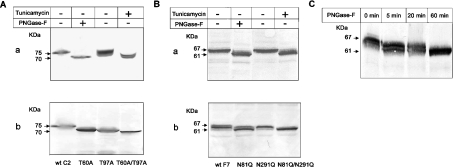
The elimination of one or two N-glycosylation sites from C2GnT-I or FucT-VII was estimated by Western blotting using the anti-EGFP mAb and compared with wt (wild-type) proteins after PNGase F or TM treatments. As shown in Figure 2(A, a), the C2GnT-I–EGFP fusion protein migrates as a 75 kDa glycoprotein, which is converted by PNGase F into a 70 kDa species. After treating the cells with TM, two bands appear, the lower being similar to the PNGase F-treated molecule and the upper one having a slightly higher size, suggesting that a residual N-glycosylation may still occur in the presence of the drug. Obliterating either the first (T60A) or the second (T97A) N-glycosylation site of C2GnT-I results in a significant increase in electrophoretic mobilities of the proteins, while the double mutant T60A/T97A migrates as the PNGase F-treated form or the lower band derived from TM-treated cells (Figure 2A, b). These data indicate that the two N-glycosylation sequons of C2GnT-I are occupied and both sites carry virtually equivalent amounts of N-glycans.
Figure 2. Effects of treatment by TM or PNGase F or removal of N-glycosylation sites on the molecular masses of C2GnT-I and FucT-VII.
Cells expressing the wt C2GnT-I (A) or wt FucT-VII (wt F7) (B) were cultured in the presence of 1 μg/ml TM for 72 h, extracted in SDS sample buffer and separated by SDS/PAGE (8% polyacrylamide). After blotting, proteins were probed with an anti-EGFP mAb and developed by the alkaline phosphatase-based method. For treatment with PNGase F, cells expressing C2GnT-I or FucT-VII were extracted in the PNGase F buffer, as described in the ‘Experimental’ section, treated with 20 units/ml PNGase F at 37 °C for 2 h and analysed by SDS/PAGE and immunoblotting as above (see A, a, and B, a). Cells expressing C2GnT-I or its glycomutants (A, b) or FucT-VII or its mutants (B, b) were extracted in SDS sample buffer and analysed as above. (C) Time course of PNGase F treatment (20 units/ml) of the wt FucT-VII. Otherwise conditions were the same as in (A) and (B).
Regarding FucT-VII, we found that the EGFP-conjugated protein migrates as a 67 kDa complex that could be converted into a 61 kDa species either after PNGase F or TM treatment (Figure 2B, a). The N81Q glycomutant displays a significantly higher shift compared with its N291Q counterpart (Figure 2B, b), indicating that the largest amount of N-glycans is attached to Asn-81. This result suggests that either the N-glycan moiety carried by Asn-291 is quantitatively negligible or even that this site may not be occupied. To address the latter possibility, we performed a time-course de-N-glycosylation by PNGase F, which hydrolyses N-glycan chains sequentially and with different kinetics [33]. As shown in Figure 2(C), the 67 kDa complex of FucT-VII–EGFP was rapidly (5 min) converted into a lower form that still harbours N-glycans, since it could be further hydrolysed after a longer incubation time (60 min) to generate the fully de-N-glycosylated 61 kDa protein. Collectively, these results indicate that all N-glycosylation sites of FucT-VII and C2GnT-I are occupied.
The lack of N-glycosylation differentially affects properties of C2GnT-I
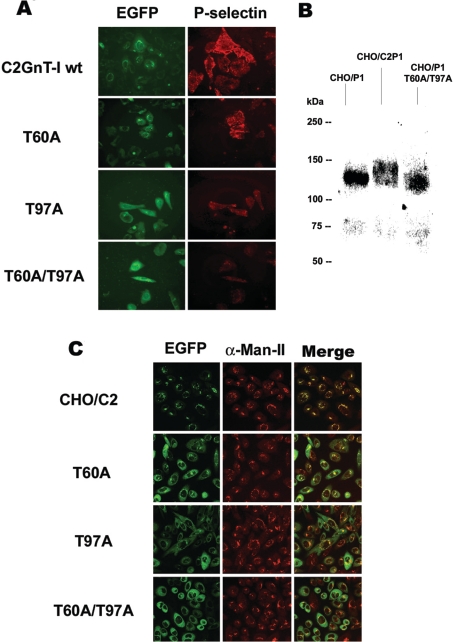
The biological activities of C2GnT-I and its variants were measured by a complementation P-selectin binding assay using CHO/F7P1 cells. This complementation system is based on the fact that P-selectin does not bind to cells unless they express PSGL-1 molecules carrying C2GnT-I-branched O-glycans (referred to as core2-modified PSGL-1) that have been fucosylated by FucT-VII [3,13,16–18]. As illustrated in Figure 3(A), transfection of CHO/F7P1 cells with the T60A mutant results in a cell-surface binding of P-selectin-IgM chimaera similar to that observed for the wt enzyme. The T97A mutant was also able to generate P-selectin binding, albeit to a significantly lower extent, while the unglycosylated form T60A/T97A was totally unable to do so. This result points to an additive effect of the two mutations and demonstrates the crucial role of C2GnT-I glycosylation in vivo, particularly the N-glycan chain linked to Asn-95.
Figure 3. Comparison of P-selectin-binding and subcellular distribution between C2GnT-I and its N-glycosylation-deficient variants.
(A) CHO/F7P1 cells were transiently transfected by DNAs coding for the wt C2GnT-I or its glycomutants (EGFP) and incubated with P-selectin–IgM chimaeras. The binding was then revealed by fluorescence microscopy using an RITC (rhodamine isothiocyanate)-conjugated anti-human IgM secondary antibody. Gain and exposure time were kept constant between images. (B) Western-blot analysis of PSGL-1 synthesized in CHO cells in the absence of C2GnT-I (CHO/P1) or in the presence of the enzyme (CHO/C2P1) or the double mutant T60A/T97A (CHO/P1 T60A/T97A). Proteins were separated by SDS/PAGE followed by immunoblotting using the anti-PSGL-1 mAb PL1. (C) Confocal microscopy images of EGFP-conjugated proteins and α-Man-II immunostaining of cells expressing the wt (CHO/C2) or the mutants T60A, T97A and T60A/T97A. The images shown are the Z sections at 5 μm from the top of the cells. The results are representative of two independent experiments.
Thus the T97A and T60A/T97A glycomutants of the C2GnT-I would not be able to synthesize the branched O-glycans that mediate P-selectin–PSGL-1 interaction. To address this issue, we used immunoblotting to evaluate the structural alterations of PSGL-1 synthesized in the presence of the wt C2GnT-I or its double mutant T60A/T97A. Figure 3(B) shows that in CHO cells, PSGL-1 subunits are synthesized as 115–120 kDa species (CHO/P1), which are converted by C2GnT-I into polydispersive higher molecular mass glycoforms of 130–140 kDa (CHO/C2P1), consistent with previously reported results [11,13]. In contrast, PSGL-1 synthesized in the presence of the double-mutant T60A/T97A, exhibits a lower molecular mass of approx. 115 kDa (CHO/P1 T60A/T97A) indicating that this mutant has lost the ability of branching PSGL-1 O-glycans. Combined with data from Figure 3(A), this result demonstrates that the lack of P-selectin binding to cells expressing C2GnT-I mutants is related to a defect in core2-based O-glycans on PSGL-1.
While performing these experiments, we have observed that together with the alteration of P-selectin binding, there was an obvious change in the intracellular distribution pattern of C2GnT-I glycomutants. It is likely that a defect in N-glycosylation could prevent the enzyme from reaching the cis/medial-Golgi compartments where its activity is optimally expressed [26,29], thus providing a possible explanation for the decreased in vivo activities of T97A and T60A/T97A mutants. To this end, we used confocal fluorescence microscopy to compare the localization of the wt C2GnT-I and the mutants with that of the well-known medial-Golgi marker α-Man-II [34]. As shown in Figure 3(C) and as expected, the wt EGFP-conjugated C2GnT-I shows a typical Golgi staining which perfectly co-localizes with α-Man-II, consistent with the results reported by us [26,29] and others [35]. However, the C2GnT-I glycomutants exhibit a broad distribution that is clearly different from that of the wt enzyme or α-Man-II, suggestive of an endoplasmic reticulum staining, albeit some co-localization could still be seen for the T60A mutant. Thus there is a good correlation between the alteration in P-selectin binding activity and the degree of mis-localization of C2GnT-I mutants.
The in vitro catalytic activities of C2GnT-I glycomutants
Carbohydrate addition probably precedes polypeptide folding into functional conformation operated by the endoplasmic reticulum quality control system [23]. It was therefore reasoned that, if the alteration of the intracellular distribution of C2GnT-I mutants is due to a glycosylation-dependent unfolding, then their in vitro catalytic activities would be altered as well. As shown in Table 2, compared with the intact enzyme, the activity of the T60A mutant is slightly decreased [1.22 versus 1.92 nmol·(nmol EGFP)−1·h−1 for the control], whereas the activities of T97A and T60A/T97A were dramatically reduced up to 0.45 and 0.12 nmol·(nmol EGFP)−1·h−1 respectively (Table 2). This result can be paralleled with data from P-selectin binding assay and indicates that the folding of T97A and the T60A/T97A double mutant is much more affected than that of the T60A species, confirming the crucial role of the Asn-95 N-glycan chain for the biological activity of C2GnT-I.
Table 2. In vitro β(1,6)-N-acetylglucosaminyltransferase activity of C2GnT-I and its glycomutants.
Detergent cell extracts were assayed for β(1,6)-N-acetylglucosaminyltransferase activity as described in [30] using the synthetic oligosaccharide Galβ1,3GalNAc-O-p-nitrophenyl as an acceptor. Unless otherwise specified, the activity was determined as nmol of GlcNAc transferred·(nmol of EGFP)−1·h−1 (ϵ=55000 M−1·cm−1).
| Catalytic activity [nmol·(nmol of EGFP)−1·h−1] | Inhibition (%) | |
|---|---|---|
| Wild-type | 1.92±0.20 | |
| T60A | 1.22±0.15 | 36.4 |
| T97A | 0.45±0.07 | 76.6 |
| T60A/T97A | 0.12±0.02 | 93.7 |
| Control | 116.65* | |
| TM-treated | 58.41* | 50.1 |
*The activity was calculated as nmol of GlcNAc transferred·(mg of protein)−1·h−1.
To ensure that the decreased catalytic activity of C2GnT-I mutants is due to the absence of N-glycans rather than to a sequence change (i.e. substitution of Thr-60 and Thr-97 by Ala in the Asn-Xaa-Thr sequence), we measured the C2GnT-I activity after treatment with TM, a widely used N-glycosylation inhibitor [36]. As shown in Table 2, TM treatment results in approx. 50% inhibition of C2GnT-I activity, consistent with data from a previous study [20], which rules out the possibility of an amino acid substitution effect on the activities of C2GnT-I glycomutants.
A defect in N-glycosylation of FucT-VII does not alter its P-selectin binding activity
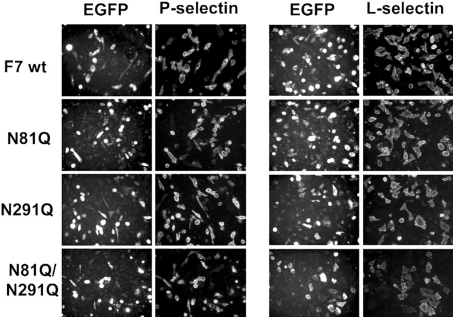
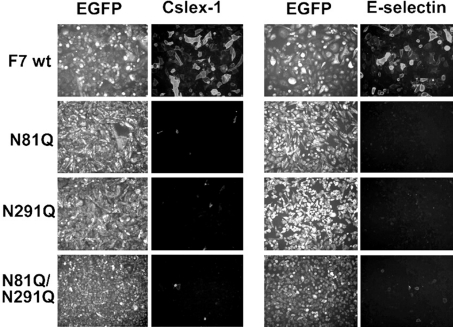
Collaborating with other glycosyltransferases, including C2GnT-I, FucT-VII is the other enzyme that makes an essential contribution in the synthesis of selectin ligands [3,37]. P-selectin binding was analysed on CHO/C2P1 after transient transfection with genes for FucT-VII or its glycomutants. Preliminary experiments showed that without FucT-VII or its mutants, CHO/C2P1 cells do not bind P-selectin (results not shown). As shown in Figure 4, neither the localization of the enzyme nor the binding of P-selectin–IgM chimaera were affected by the lack of N-glycans on Asn-81 (N81Q), Asn-291 (N291Q) or on both sites (N81Q/N291Q). Since CHO/C2P1 do not bind P-selectin unless FucT-VII or its mutants are expressed, this result indicates that despite N-glycosylation deficiency, FucT-VII mutants are able to create P-selectin binding sites on PSGL-1. Thus FucT-VII differs from C2GnT-I with respect to the requirement of N-glycans for their activities in vivo.
Figure 4. P- and L-selectin-binding activities of cells expressing the wt FucT-VII or its glycomutants.
CHO/C2P1 cells were transiently transfected by DNAs coding for the wt FucT-VII (F7 wt) or its glycomutants (N81Q, N291 and N81Q/N291Q) and incubated with the human IgM-conjugated P-selectin (left panel) or L-selectin (right panel). The binding was then revealed by fluorescence microscopy as in Figure 3(A). Gain and exposure time were kept constant between images.
As for C2GnT-I, we investigated whether the elimination of N-glycans from FucT-VII affects its in vitro catalytic activity. Surprisingly, none of the N-glycosylation-deficient variants of FucT-VII was found to be active in vitro (Table 3). Compared with an activity of 5.28 nmol·(nmol EGFP)−1·h−1 found for the wt, the activities of N81Q (0.22), N291Q (0.65) and the double mutant N81Q/N291Q (0.19) correspond to 95.8, 87.7 and 96.4% inhibition respectively. What then could compensate in vivo, but not in vitro, for the unfolding effect caused by the lack of N-glycans on FucT-VII? To rule out the possibility of a membrane-bound effect, which could explain the maintenance of FucT-VII glycomutant activities in vivo, we sonicated the cells in order to destroy their architecture while keeping the membranal state of the enzymes. On the other hand, to look for a possible proteolytic degradation of FucT-VII mutants in vitro, detergent cell extracts were incubated for 2 h at 37 °C (i.e. the conditions used for the in vitro enzyme assays) and analysed by SDS/PAGE and immunoblotting as above. As shown in Supplementary Figure 1 (see at http://www.BiochemJ.org/bj/391/bj3910491add.htm), there is no significant difference between sonicated and detergent-extracted enzyme activities and no significant change could be noted between the wt FucT-VII and its glycomutants with respect to SDS/PAGE protein patterns.
Table 3. In vitro α(1,3)-fucosyltransferase activity of FucT-VII and its glycomutants.
Detergent cell extracts were assayed for α(1,3)-fucosyltransferase activity using fetuin as an acceptor. The activities were determined as pmol of fucose transferred·(nmol EGFP)·h−1 (ϵ=55000 M−1·cm−1) (see also Table 2).
| Catalytic activity [pmol·(nmol EGFP)−1·h−1] | Inhibition (%) | |
|---|---|---|
| Wild-type | 5.28±1.12 | – |
| N81Q | 0.22±0.10 | 95.8 |
| N291Q | 0.65±0.20 | 87.7 |
| N81Q/N291Q | 0.19±0.14 | 96.4 |
The lack of N-glycosylation of FucT-VII moderately affects L-selectin binding
PSGL-1 is one of the physiological ligands for L-selectin [17,38,39]. It was therefore of relevance to analyse the effect of FucT-VII N-glycosylation on L-selectin–PSGL-1 interaction. As for P-selectin, the binding of L-selectin–IgM chimaera was performed on CHO/C2P1 cells transiently transfected with FucT-VII or its N-glycosylation-deficient variants. Preliminary experiments showed that L-selectin binding to these cells is achieved only when FucT-VII is present (results not shown). After transfection, we found that while the double mutant N81Q/N291Q displays a slight decrease, none of the single mutations N81Q or N291Q results in a visible alteration of L-selectin binding (Figure 4, right panel). Together with P-selectin data, these results suggest that FucT-VII glycomutants are able to construct P- and L-selectin binding sites on PSGL-1.
FucT-VII glycomutants fail to synthesize sLex antigens or to generate E-selectin binding, unless PSGL-1 and C2GnT-I are present
In contrast with the two others, E-selectin does not require core2-branched O-glycans or tyrosine sulphation to bind to cells, but the presence of both PSGL-1 and C2GnT-I has been shown to significantly enhance the binding [18]. It was therefore interesting to evaluate the impact of FucT-VII N-glycosylation on E-selectin binding in the absence or the presence of C2GnT-I and PSGL-1. In a first set of experiments, we made stable CHO transfectants expressing only FucT-VII or its glycomutants and tested CSLEX-1 immunoreactivity and E-selectin binding. The results are presented in Figure 5. Surprisingly, compared with cells expressing the intact enzyme (F7 wt), the CSLEX-1 immunostaining (left panel) and E-selectin binding (right panel) were strongly inhibited on all cells having the monoglycosylated species (N81Q and N291Q) or the unglycosylated form N81Q/N291Q, suggestive of the inability of FucT-VII glycomutants to fucosylate the sLex precursors in these cells. Similar results were obtained using KM-93, another mAb specific for sLex antigens (results not shown).
Figure 5. Altered sLex and E-selectin binding to cells expressing the N-glycosylation-deficient variants of FucT-VII.
Cells expressing either the F7 wt or the mutated FucT-VII (N81Q, N291Q and N81Q/N291Q) were assayed for anti-sLex immunostaining using CSLEX-1 mAb (left panel) or E-selectin binding (right panel). The binding of CSLEX-1 mAb was revealed by an RITC (rhodamine isothiocyanate)-conjugated anti-mouse IgM and the binding of E-selectin–IgM chimaeras was revealed by an RITC-conjugated anti-human IgM. Immunostaining was then analysed by fluorescence microscopy. Gain and exposure time were kept constant between images.
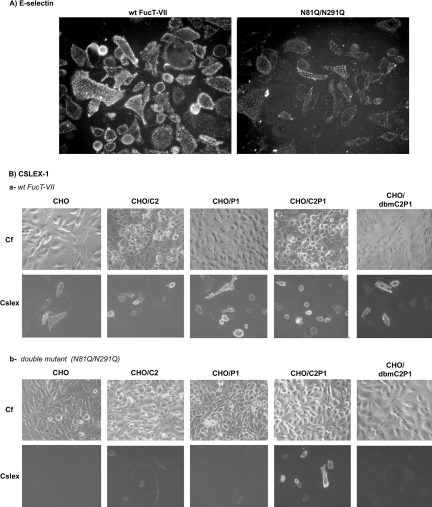
We next questioned whether the same is true when FucT-VII mutants are co-expressed with C2GnT-I and PSGL-1. To this end, CHO/C2P1 cells were transiently transfected with FucT-VII or its double mutant N81Q/N291Q and the result is illustrated in Figure 6(A). Surprisingly and in contrast with the previous result (see Figure 5), the N81Q/N291Q mutant is now able to trigger E-selectin binding, although to a lesser extent compared with the wt. Thus the unglycosylated form of FucT-VII is able to fucosylate E-selectin binding sites and by inference to fucosylate sLex precursors, but only when core2-modified oligosaccharides are present in PSGL-1. To further confirm these results, we analysed CSLEX-1 immunoreactivity on a series of cell lines with different combinations of PSGL-1 and C2GnT-I. The parental CHO cells and those expressing the inactive double mutant of C2GnT-I (T60A/T97A) were chosen as controls. As shown in Figure 6(B) and as expected, the sLex glycotope was expressed on all cells transfected with the wt FucT-VII (Figure 6B, a). However, after transfection with the N81Q/N291Q construct only cells co-expressing PSGL-1 and C2GnT-I were stained with CSLEX-1 mAb (Figure 6B, b). It is noteworthy that the N81Q/N291Q-catalysed sLex synthesis is prevented when PSGL-1 is expressed alone (CHO/P1) or with an unglycosylated variant of C2GnT-I (see CHO/dbmC2P1), consistent with the results from Figure 3(A). Together, these results demonstrate that the N-glycosylation-deficient FucT-VII variants are unable to synthesize sLex antigen or to generate E-selectin binding, unless PSGL-1 and C2GnT-I are present.
Figure 6. Core2-modified PSGL-1 restores sLex expression and E-selectin binding activities of the mutated FucT-VII.
(A) CHO/C2P1 cells were transiently transfected with the wt FucT-VII or its double mutant N81Q/N291Q and analysed for E-selectin binding as in Figure 5. (B) Cells expressing C2GnT-I alone (CHO/C2), PSGL-1 alone (CHO/P1), or both molecules (CHO/C2P1), as well as those expressing PSGL-1 with the double mutant of C2GnT-I (CHO/dbmC2P1) were transiently transfected with the wt FucT-VII or its double mutant N81Q/N291Q and analysed for sLex expression as in Figure 5. The parental CHO cells (CHO) expressing none of these molecules were chosen as control. Gain and exposure time were kept constant between images. Cf, phase contrast.
A defect in FucT-VII N-glycosylation differentially affects PSGL-1 interaction with P- and L-selectin
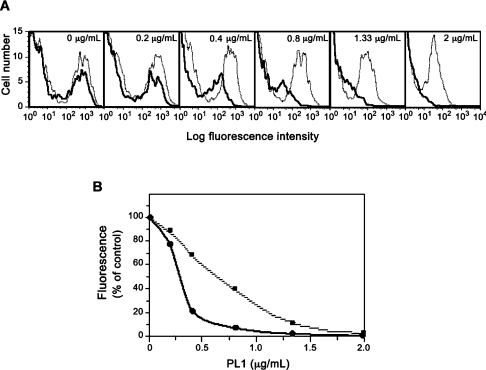
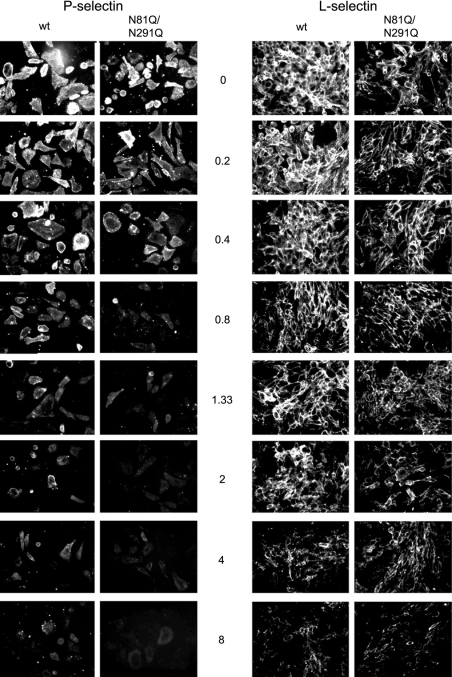
The above results suggest that when the α(1,3)-fucosyltransferase activity is weak, it is restricted to a few oligosaccharide acceptors, including the core2-modified O-glycans in PSGL-1. Yet, we postulated that such a weak activity may not be sufficient to fucosylate all core2-modified molecules and if so, cells expressing the N81Q/N291Q mutant may exhibit reduced avidity towards P-selectin. This parameter can be explored by a binding/competition assay using the anti-PSGL-1 mAb PL1, a function-blocking antibody known to inhibit P-selectin in a dose-dependent manner [28,40]. Cells were pretreated with increasing concentrations of PL1 before P-selectin binding and fluorescence was analysed by flow cytometry. As shown in Figure 7, in the absence of PL1, cells expressing the wt bind roughly the same quantity of P-selectin–IgM chimaeras compared with N81Q/N291Q-expressing cells, consistent with results from Figure 4. PL1 blocks P-selectin binding in a dose-dependent manner for cells expressing either FucT-VII (dotted grey lines) or N81Q/N291Q (bold solid lines) (Figure 7A). The deduced dose–response curve, shown in Figure 7(B), indicates that the concentration of PL1 necessary for 50% inhibition of P-selectin binding (IC50) is 2-fold lower for N81Q/N291Q (0.33 μg/ml) than for FucT-VII (0.70 μg/ml). In the former case, a marked inhibition of approx. 80% is observed at 0.4 μg/ml of PL1, while at this concentration, only 30% of P-selectin binding is displaced from FucT-VII-transfected cells. This result clearly shows that P-selectin binds to cells expressing the unglycosylated FucT-VII with a reduced avidity, suggesting that fewer PSGL-1 molecules are fucosylated by the N81Q/N291Q variant. We next examined whether the same difference, in terms of avidity, could be seen for L-selectin. To this end, cells were pretreated with increasing concentrations of PL1, and L-selectin binding was analysed by fluorescence microscopy and compared with that of P-selectin. As shown in Figure 8, P-selectin binding to N81Q/N291Q-transfected cells is displaced by low concentrations of PL1 compared with wt FucT-VII-expressing cells, consistent with the data from FACS analyses (see Figure 7A). However and interestingly, a much higher concentration of PL1 (i.e. between 4 and 8 μg/ml) is needed to significantly decrease, but not to abolish, L-selectin binding on cells expressing either the wt FucT-VII or the N81Q/N291Q mutant. This result confirms that (i) the glycans that bind to L-selectin remain largely fucosylated in the presence of a weak α(1,3)-fucosyltransferase activity and (ii) L-selectin interacts, only partially, with the P-selectin binding site defined by PL1 mAb.
Figure 7. Flow cytometric analyses of P-selectin binding to cells transfected with the wt or the doubly mutated FucT-VII.
CHO/C2P1 cells were transfected with constructs coding for the wt FucT-VII or its N81Q/N291Q double mutant, pretreated with increasing concentrations of the anti-PSGL-1 mAb PL1 and assayed for P-selectin binding as described in the Experimental section. (A) Dose-dependent inhibition by PL1 of P-selectin binding to cells expressing the wt FucT-VII (dotted grey lines) and to those expressing the N81Q/N291Q mutant (bold solid lines). (B) The dose–response curves deduced from the fluorescence data in (A) expressed as the percentage of the binding obtained in the absence of PL1. ■, The dose–response inhibition of P-selectin binding to cells expressing FucT-VII; ●, values from the double mutant.
Figure 8. PL1-mediated dose-dependent inhibition of P- and L-selectin binding.
Cells expressing the wild-type FucT-VII (wt) or the double mutant N81Q/N291Q were incubated with the human IgM-conjugated P-selectin (left panel) or L-selectin (right panel) in the absence (0 μg/ml) or in the presence of increasing concentrations of PL1 (from 0.2 to 8 μg/ml). The binding of the selectins was then examined by fluorescence microscopy as in Figure 4. Gain and exposure time were kept constant between images.
DISCUSSION
In the present study, we evaluated the structural and functional impact of N-glycosylation on C2GnT-I and FucT-VII, two enzymes that play crucial roles in the synthesis of selectin ligands [3,7–9]. Each of these enzymes carries two potential N-glycosylation sites, the obliteration of which produced lower molecular mass species, as did enzymatic deglycosylation by PNGase F or blockade of N-glycosylation by TM, indicating that all sites carry N-linked sugars (see Figure 2).
Among the two sites Asn-58 and Asn-95 of C2GnT-I, the latter was found to be essential for the Golgi targeting and the ability of the enzyme to initiate the formation of a functional PSGL-1. Simultaneous obliteration of both sites caused a complete inactivation of the enzyme in vivo and in vitro. In general and for a given mutation, a correlation was found between the effect on activity in vitro and features such as Golgi localization and P-selectin binding. For example, the T60A, which has the lowest effect in vitro, was found to cause the least effect on the intracellular distribution of the enzyme and to have virtually no effect on P-selectin binding (compare the results from Table 2 and Figure 3). The same kind of reasoning is valuable for the other glycomutants, suggesting that the functional folding of C2GnT-I is a prerequisite for its targeting to the right Golgi compartment, where it can optimally act on its cognate acceptors including PSGL-1.
Our results are consistent with those from Toki et al. [20], who found that the activity of the recombinant C2GnT-I secreted from SF9 cells was impaired after TM treatment or after the obliteration of the two N-glycosylation sites (T60A/T97A). However our results differ regarding the in vitro activities of the mutants T60A and T97A (see Table 1 in [20]). Since we both used the same mutagenesis strategy, this discrepancy could not be attributed to a sequence alteration but rather to the expression system (Sf9 cells versus CHO cells) and/or to the form of the protein used (soluble versus transmembrane protein).
Regarding FucT-VII, we found that although all glycomutants exhibited dramatically decreased activities in vitro (see Table 3), N-glycosylation was not found to be absolutely required for the ability of FucT-VII to construct P- and L-selectin binding sites on cells expressing core2-modified PSGL-1 (Figure 4). In the absence of the latter, FucT-VII mutants fail to synthesize the sLex glycotope or to generate E-selectin binding. However, when core2-modified PSGL-1 is present, the unglycosylated FucT-VII variant recovers its ability to trigger detectable sLex expression and E-selectin binding (compare data from Figures 5 and 6). Thus a low α(1,3)-fucosyltransferase activity seems to be sufficient to allow P- and L-selectin binding to core2-modified PSGL-1, while a higher activity is needed for E-selectin binding. These results are consistent with data from Knibbs et al. [41], who found that the level of FucT-VII transcripts in lymphoblastic T-cells differentially regulates the synthesis of P- and E-selectin ligands, so that the level of FucT-VII mRNA is high when both E- and P-selectin ligands are expressed and it is low in cells expressing P-selectin ligands alone [41]. Our results are also in line with studies by Borges et al. [42], who found that a low α(1,3)-fucosyltransferase activity is sufficient to ensure P-selectin binding to CD8+ T-cells, whereas a much higher activity is needed for E-selectin binding [42]. Our results confirm these findings and further demonstrate that L-selectin binding is also preserved in the presence of a weak FucT-VII activity.
Why then has a weak α(1,3)-fucosylation such a higher impact on E-selectin than on P-selectin or L-selectin binding sites? One likely explanation could be provided by the fact that the interaction of P- and L-selectin with PSGL-1 requires tyrosine sulphation in addition to core2-based O-glycans, the so-called C2-O-sLex determinant [15–17], while E-selectin does not require such adjacent sulphated tyrosines [3,18]. Yet, the fact that the N81Q/N291Q mutant is able to trigger sLex epitope and E-selectin binding only when core2-modified PSGL-1 is present suggests and confirms that (i) core2-based oligosaccharides are the preferred acceptor for FucT-VII (M. Prorok-Hamon and A. El-Battari, unpublished work), and (ii) the proximity of the sulphated tyrosines may enhance the affinity of FucT-VII glycomutants for the C2-O-sLex precursor carried on Thr-57 of PSGL-1. However, although still active in vivo, these mutants probably do not fucosylate all P-selectin binding sites, since cells expressing the unglycosylated FucT-VII variant exhibit a weaker avidity towards P-selectin (see Figures 7 and 8). A more specific and structurally relevant competing molecule [i.e. the PSGL-1-derived GSP-6 (glycosulphopeptide 6)] [43,44] is needed to address this issue in the future.
Other α(1,3)/α(1,4)-fucosyltransferases including FucT-III, FucT-IV and FucT-VI can also synthesize sialyl-Lewis antigens and generate selectin counter-receptors and the role of N-glycosylation for their function has been addressed previously [45–47]. Surprisingly, none of these studies had used selectins as a functional assay to evaluate the impact of N-glycosylation on the biological properties of these enzymes. The present study is the first in which a structure–function relationship of an α(1,3)-fucosyltransferase is explored with respect to the synthesis of functional PSGL-1, one of the major activities of this enzyme in vivo.
Online data
Acknowledgments
This work was supported by Institut National de la Santé et de Recherche Scientifique et Médicale (INSERM) and Ministère de la Recherche et de la Technologie (MRT) fellowship to M.P.-H. We thank C. Prévôt and M. Barrad for their technical assistance with flow cytometry and confocal microscopy and Dr About I (Laboratoire IMEB ERT 30, Faculté d'Odontologie, Université de la Méditerranée, Marseille Cedex 05, France) for the critical reading of the paper.
References
- 1.Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell (Cambridge, Mass.) 1994;76:301–331. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Maksimowicz-McKinnon K., Bhatt D. L., Calabrese L. H. Recent advances in vascular inflammation: C-reactive protein and other inflammatory biomarkers. Curr. Opin. Rheumatol. 2004;16:18–24. doi: 10.1097/00002281-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Lowe J. B. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Selectin ligands: will the real ones please stand up? J. Clin. Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEver R. P., Cummings R. D. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 1997;100:485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen S. D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 7.Ellies L. G., Tsuboi S., Petryniak B., Lowe J. B., Fukuda M., Marth J. D. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 8.Homeister J. W., Thall A. D., Petryniak B., Maly P., Rogers C. E., Smith P. L., Kelly R. J., Gersten K. M., Askari S. W., Cheng G., et al. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 9.Sperandio M., Thatte A., Foy D., Ellies L. G., Marth J. D., Ley K. Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood. 2001;97:3812–3819. doi: 10.1182/blood.v97.12.3812. [DOI] [PubMed] [Google Scholar]
- 10.Van Zante A., Gauguet J. M., Bistrup A., Tsay D., von Andrian U. H., Rosen S. D. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. J. Exp. Med. 2003;198:1289–1300. doi: 10.1084/jem.20030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitoma J., Petryniak B., Hiraoka N., Yeh J. C., Lowe J. B., Fukuda M. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis X-type L-selectin ligand activity. J. Biol. Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- 12.Norgard K. E., Moore K. L., Diaz S., Stults N. L., Ushiyama S., McEver R. P., Cummings R. D., Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J. Biol. Chem. 1993;268:12764–12774. [PubMed] [Google Scholar]
- 13.Moore K. L., Eaton S. F., Lyons D. E., Lichenstein H. S., Cummings R. D., McEver R. P. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J. Biol. Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- 14.Sako D., Comess K. M., Barone K. M., Camphausen R. T., Cumming D. A., Shaw G. D. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell (Cambridge, Mass.) 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 15.Pouyani T., Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell (Cambridge, Mass.) 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Ramachandran V., Kang J., Kishimoto T. K., Cummings R. D., McEver R. P. Identification of N-terminal residues on P-selectin glycoprotein ligand-1 required for binding to P-selectin. J. Biol. Chem. 1998;273:7078–7087. doi: 10.1074/jbc.273.12.7078. [DOI] [PubMed] [Google Scholar]
- 17.Bernimoulin M. P., Zeng X. L., Abbal C., Giraud S., Martinez M., Michielin O., Schapira M., Spertini O. Molecular basis of leukocyte rolling on PSGL-1. Predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. J. Biol. Chem. 2003;278:37–47. doi: 10.1074/jbc.M204360200. [DOI] [PubMed] [Google Scholar]
- 18.Li F., Wilkins P. P., Crawley S., Weinstein J., Cummings R. D., McEver R. P. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J. Biol. Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 19.El-Battari A., Prorok M., Angata K., Mathieu S., Zerfaoui M., Ong E., Suzuki M., Lombardo D., Fukuda M. Different glycosyltransferases are differentially processed for secretion, dimerization, and autoglycosylation. Glycobiology. 2003;13:941–953. doi: 10.1093/glycob/cwg117. [DOI] [PubMed] [Google Scholar]
- 20.Toki D., Sarkar M., Yip B., Reck F., Joziasse D., Fukuda M., Schachter H., Brockhausen I. Expression of stable human O-glycan core 2 β-1,6-N-acetylglucosaminyltransferase in Sf9 insect cells. Biochem. J. 1997;325:63–69. doi: 10.1042/bj3250063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vries T., Yen T. Y., Joshi R. K., Storm J., van Den Eijnden D. H., Knegtel R. M., Bunschoten H., Joziasse D. H., Macher B. A. Neighboring cysteine residues in human fucosyltransferase VII are engaged in disulfide bridges, forming small loop structures. Glycobiology. 2001;11:423–432. doi: 10.1093/glycob/11.5.423. [DOI] [PubMed] [Google Scholar]
- 22.Helenius A., Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 23.Roth J. Protein N-glycosylation along the secretory pathway: relationship to organelle topography and function, protein quality control, and cell interactions. Chem. Rev. 2002;102:285–303. doi: 10.1021/cr000423j. [DOI] [PubMed] [Google Scholar]
- 24.Ansaldi M., Lepelletier M., Mejean V. Site-specific mutagenesis by using an accurate recombinant polymerase chain reaction method. Anal. Biochem. 1996;234:110–111. doi: 10.1006/abio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 25.Zerfaoui M., Fukuda M., Sbarra V., Lombardo D., El-Battari A. α(1,2)-Fucosylation prevents sialyl Lewis x expression and E-selectin-mediated adhesion of fucosyltransferase VII-transfected cells. Eur. J. Biochem. 2000;267:53–61. doi: 10.1046/j.1432-1327.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 26.Zerfaoui M., Fukuda M., Langlet C., Mathieu S., Suzuki M., Lombardo D., El-Battari A. The cytosolic and transmembrane domains of the β1,6 N-acetylglucosaminyltransferase (C2GnT) function as a cis to medial/Golgi-targeting determinant. Glycobiology. 2002;12:15–24. doi: 10.1093/glycob/12.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Moore K. L., Patel K. D., Bruehl R. E., Li F., Johnson D. A., Lichenstein H. S., Cummings R. D., Bainton D. F., McEver R. P. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathieu S., Prorok M., Benoliel A. M., Uch R., Langlet C., Bongrand P., Gerolami R., El-Battari A. Transgene expression of α(1,2)-fucosyltransferase-I (FUT1) in tumor cells selectively inhibits sialyl-Lewis x expression and binding to E-selectin without affecting synthesis of sialyl-Lewis a or binding to P-selectin. Am. J. Pathol. 2004;164:371–383. doi: 10.1016/s0002-9440(10)63127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skrincosky D., Kain R., El-Battari A., Exner M., Kerjaschki D., Fukuda M. Altered Golgi localization of core 2 β-1,6-N-acetylglucosaminyl-transferase leads to decreased synthesis of branched O-glycans. J. Biol. Chem. 1997;272:22695–22702. doi: 10.1074/jbc.272.36.22695. [DOI] [PubMed] [Google Scholar]
- 30.Yousefi S., Higgins E., Daoling Z., Pollex-Kruger A., Hindsgaul O., Dennis J. W. Increased UDP-GlcNAc:Gal β1-3GaLNAc-R (GlcNAc to GaLNAc) β-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J. Biol. Chem. 1991;266:1772–1782. [PubMed] [Google Scholar]
- 31.Britten C. J., van den Eijnden D. H., McDowell W., Kelly V. A., Witham S. J., Edbrooke M. R., Bird M. I., de Vries T., Smithers N. Acceptor specificity of the human leukocyte α3 fucosyltransferase: role of FucT-VII in the generation of selectin ligands. Glycobiology. 1998;8:321–327. doi: 10.1093/glycob/8.4.321. [DOI] [PubMed] [Google Scholar]
- 32.Cartellieri S., Hamer O., Helmholz H., Niemeyer B. One-step affinity purification of fetuin from fetal bovine serum. Biotechnol. Appl. Biochem. 2002;35:83–89. doi: 10.1042/ba20010067. [DOI] [PubMed] [Google Scholar]
- 33.Kuster B., Hunter A. P., Wheeler S. F., Dwek R. A., Harvey D. J. Structural determination of N-linked carbohydrates by matrix-assisted laser desorption/ionization-mass spectrometry following enzymatic release within sodium dodecyl sulphate-polyacrylamide electrophoresis gels: application to species-specific glycosylation of α1-acid glycoprotein. Electrophoresis. 1998;19:1950–1959. doi: 10.1002/elps.1150191113. [DOI] [PubMed] [Google Scholar]
- 34.Velasco A., Hendricks L., Moreman K. W., Tulsiani D. R. P., Touster O., Farquhar M. G. Cell-type dependent variations in the subcellular distribution of a-mannosidase I and II. J. Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalziel M., Whitehouse C., McFarlane I., Brockhausen I., Gschmeissner S., Schwientek T., Clausen H., Burchell J. M., Taylor-Papadimitriou J. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 2001;276:11007–11015. doi: 10.1074/jbc.M006523200. [DOI] [PubMed] [Google Scholar]
- 36.Lelhe L., Tanner W. The specific site of tunicamycin inhibition in the formation of the dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976;71:167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- 37.Vestweber D., Blanks J. E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 38.Walcheck B., Moore K. L., McEver R. P., Kishimoto T. K. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1: a mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J. Clin. Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperandio M., Smith M. L., Forlow S. B., Olson T. S., Xia L., McEver R. P., Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J. Exp. Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herron M. J., Nelson C. M., Larson J., Snapp K. R., Kansas G. S., Goodman J. L. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science. 2000;288:1653–1666. doi: 10.1126/science.288.5471.1653. [DOI] [PubMed] [Google Scholar]
- 41.Knibbs R. N., Craig R. A., Maly P., Smith P. L., Wolber F. M., Faulkner N. E., Lowe J. B., Stoolman L. M. α(1,3)-Fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J. Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- 42.Borges E., Pendl G., Eytner R., Steegmaier M., Zollner O., Vestweber D. The binding of T cell-expressed P-selectin glycoprotein ligand-1 to E- and P-selectin is differentially regulated. J. Biol. Chem. 1997;272:28786–28792. doi: 10.1074/jbc.272.45.28786. [DOI] [PubMed] [Google Scholar]
- 43.Leppänen A., White S. P., Helin J., McEver R. P., Cummings R. D. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J. Biol. Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 44.Leppänen A., Yago T., Otto V. I., McEver R. P., Cummings R. D. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J. Biol. Chem. 2003;278:26391–26400. doi: 10.1074/jbc.M303551200. [DOI] [PubMed] [Google Scholar]
- 45.Christensen L. L., Jensen U. B., Bross P., Orntoft T. F. The C-terminal N-glycosylation sites of the human α1,3/4-fucosyltransferase III, -V, and -VI (hFucTIII, -V, and -VI) are necessary for the expression of full enzyme activity. Glycobiology. 2000;10:931–939. doi: 10.1093/glycob/10.9.931. [DOI] [PubMed] [Google Scholar]
- 46.Christensen L. L., Bross P., Orntoft T. F. Glycosylation of the N-terminal potential N-glycosylation sites in the human α1,3-fucosyltransferase V and -VI (hFucTV and -VI) Glycoconj. J. 2000;17:859–865. doi: 10.1023/a:1010917229243. [DOI] [PubMed] [Google Scholar]
- 47.Morais V. A., Costa M. T., Costa J. N-glycosylation of recombinant human fucosyltransferase III is required for its in vivo folding in mammalian and insect cells. Biochim. Biophys. Acta. 2003;1619:133–138. doi: 10.1016/s0304-4165(02)00448-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.