Abstract
Although the physiological roles of the individual bile acid synthetic enzymes have been extensively examined, relatively little is known regarding the function of intracellular bile acid-binding proteins. Male L-FABP (liver fatty-acid-binding protein) gene-ablated mice were used to determine a role for L-FABP, the major liver bile acid-binding protein, in bile acid and biliary cholesterol metabolism. First, in control-fed mice L-FABP gene ablation alone increased the total bile acid pool size by 1.5-fold, especially in gall-bladder and liver, but without altering the proportions of bile acid, cholesterol and phospholipid. Loss of liver L-FABP was more than compensated by up-regulation of: other liver cytosolic bile acid-binding proteins [GST (glutathione S-transferase), 3α-HSD (3α-hydroxysteroid dehydrogenase)], key hepatic bile acid synthetic enzymes [CYP7A1 (cholesterol 7α-hydroxylase) and CYP27A1 (sterol 27α-hydroxylase)], membrane bile acid translocases [canalicular BSEP (bile salt export pump), canalicular MRP2 (multidrug resistance associated protein 2), and basolateral/serosal OATP-1 (organic anion transporting polypeptide 1)], and positive alterations in nuclear receptors [more LXRα (liver X receptor α) and less SHP (short heterodimer partner)]. Secondly, L-FABP gene ablation reversed the cholesterol-responsiveness of bile acid metabolic parameters such that total bile acid pool size, especially in gall-bladder and liver, was reduced 4-fold, while the mass of biliary cholesterol increased 1.9-fold. The dramatically reduced bile acid levels in cholesterol-fed male L-FABP (−/−) mice were associated with reduced expression of: (i) liver cytosolic bile acid-binding proteins (L-FABP, GST and 3α-HSD), (ii) hepatic bile acid synthetic enzymes [CYP7A1, CYP27A1 and SCP-x (sterol carrier protein-x/3-ketoacyl-CoA thiolase)] concomitant with decreased positive nuclear receptor alterations (i.e. less LXRα and more SHP), and (iii) membrane bile acid transporters (BSEP, MRP2 and OATP-1). These are the first results suggesting a physiological role for the major cytosolic bile acid-binding protein (L-FABP) in influencing liver bile metabolic phenotype and gall-bladder bile lipids of male mice, especially in response to dietary cholesterol.
Keywords: bile acid, cholesterol, cholesteryl ester, fatty-acid-binding protein, gene ablation, liver
Abbreviations: 3α-HSD, 3α-hydroxysteroid dehydrogenase; ACAT, acyl-CoA:cholesterol acyltransferase; ACBP, acyl-CoA-binding protein; apo A1, apolipoprotein A1; BSEP, bile salt export pump; CYP27A1, sterol 27α-hydroxylase; CYP7A1, cholesterol 7α-hydroxylase; DEXA, dual-energy X-ray absorptiometry; FTM, fat tissue mass; FXR, farnesoid X receptor; GST, glutathione S-transferase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LDL, low-density lipoprotein; L-FABP, liver fatty-acid-binding protein; LTM, lean tissue mass; LXRα, liver X receptor α; MRP2, multidrug resistance associated protein 2; OATP-1, organic anion transporting polypeptide 1; PPARα, peroxisome-proliferator-activated receptor α; SCP-2, sterol carrier protein-2; SCP-x, sterol carrier protein-x/3-ketoacyl-CoA thiolase; SHP, short heterodimer partner; SRB-1, scavenger receptor class B type I; SREBP-1, sterol-regulatory-element-binding protein 1
INTRODUCTION
Bile acids emulsify lipids and facilitate absorption of dietary fat and fat-soluble vitamins (reviewed in [1,2]). The majority (95%) of secreted bile acid is reabsorbed from the intestine back to the liver (reviewed in [1,2]). Faecal loss of the remaining bile acid (∼5%) represents the major route of excess cholesterol removal (reviewed in [1,2]). Bile acid synthetic enzymes as well as their genetics and regulation have been examined, many in gene-ablated mice (reviewed in [2]). Likewise, membrane bile acid transporters at the hepatocyte sinusoid and canaliculus are well studied (reviewed in [2–4]). In contrast, mechanism(s) for the rapid (seconds to minutes) vectorial transport of bile acids within hepatocytes are only poorly understood and suggest a role for soluble bile acid-binding proteins rather than vesicular bile acid transport (reviewed in [3,5]). Liver cytosol contains three potential bile acid-binding proteins: L-FABP (liver fatty-acid-binding protein), GST (glutathione S-transferase) and 3α-HSD (3α-hydroxysteroid dehydrogenase) (reviewed in [3,5]). Although physiological roles have not been demonstrated for GST and HSD in bile acid transport within the hepatocyte (reviewed in [5,6]), L-FABP is the major cytoplasmic protein labelled by photoreactive bile acid probes in intact hepatoctyes and liver slices [6]. L-FABP is the most abundant liver bile acid-binding protein, representing 3–5% of cytosolic protein or 200–400 μM (reviewed in [7]), and since radioligand-binding assays yield a dissociation constant Kd of 1–6 μM [8], L-FABP may contribute significantly to bile acid binding in hepatocyte cytosol. Finally, there is indirect evidence suggesting a physiological role of L-FABP in regulating bile acid metabolism and determining bile acid pool size. L-FABP expression is up-regulated 5-fold in control-fed male SCP-x/SCP-2 (−/−) mice (where SCP-x stands for sterol carrier protein-x/3-ketoacyl-CoA thiolase and SCP-2 for sterol carrier protein-2), along with 4-fold increased CYP7A1 (cholesterol 7α-hydroxylase), decreased bile acid pool size and altered serum and liver lipids [9–11]. Since SCP-x/SCP-2 (−/−) mice are deficient in SCP-x and SCP-2, both of which also participate in bile acid metabolism [12–15], it is difficult to attribute which specific alteration in bile acid metabolism is due to L-FABP up-regulation, absence of SCP-x, and/or loss of SCP-2.
To begin to resolve these issues, advantage was taken of L-FABP gene-ablated mice [16–18]. Male mice were chosen because: (i) livers of male mice express much more SCP-x, the peroxisomal enzyme essential for the terminal step in cholesterol branched side chain oxidation during bile acid formation [12], and (ii) male mice are more resistant to deleterious effects of high levels of branched-chain lipids such as phytol [12,16] and cholesterol [19]. To determine directly the effect of L-FABP gene ablation and cholesterol diet on bile acid pool size, male mice were fed on either a control chow or cholesterol-rich diet, and levels of hepatic bile acid synthetic enzymes, hepatic bile acid nuclear regulatory proteins, bile acid composition and lipid phenotype were determined. The results were consistent with L-FABP having a key physiological role in regulating bile acid metabolism and cholesterol-responsiveness in male mouse liver and gall-bladder.
EXPERIMENTAL
Proteins and antibodies
Protease inhibitor cocktail for mammalian tissue was from Sigma–Aldrich (St. Louis, MO, U.S.A.). Proteins were quantified utilizing the Protein Assay Dye Reagent (Bio-Rad Laboratories, Richmond, VA, U.S.A.). Rabbit polyclonal antisera to recombinant rat L-FABP, mouse ACBP (acyl-CoA-binding protein), mouse SCP-2 and mouse SCP-x were as described in [20]. Rabbit polyclonal anti-human ACAT-1 (acyl-CoA:cholesterol acyltransferase 1), anti-mouse FATP-1 (fatty-acid transport protein 1), anti-human PPARα (peroxisome-proliferator-activated receptor α), anti-human SREBP-1 (sterol-regulatory-element-binding protein 1) and goat polyclonal anti-mouse LDL (low-density lipoprotein) receptor were from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Rabbit polyclonal anti-human ACAT-2 was from Cayman Chemical (Ann Arbor, MI, U.S.A.). Goat polyclonal anti-human CYP7A1, goat polyclonal anti-human CYP27A1 (sterol 27α-hydroxylase), rabbit polyclonal anti-human FXR (farnesoid X receptor), goat polyclonal anti-human LXRα (liver X receptor α) and goat polyclonal anti-mouse SHP (short heterodimer partner protein) were from Santa Cruz Biotechnology. Rabbit polyclonal anti-mouse GST and rabbit polyclonal anti-Pseudomonas 3α-HSD were from USB (Swampscott, MA, U.S.A.). Goat polyclonal anti-mouse BSEP (bile salt export pump protein) and goat polyclonal anti-human MRP2 (multidrug resistance associated protein 2) were from Santa Cruz Biotechnology; rabbit polyclonal anti-rat OATP-1 (organic anion transporting polypeptide 1) was from Alpha Diagnostic International (San Antonio, TX, U.S.A.). Rabbit polyclonal anti-mouse SRB-1 (scavenger receptor class B type I) was from Novus Biologicals (Littleton, CO, U.S.A.), while rabbit polyclonal anti-human HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase was from Upstate Cell Signaling Solutions (Lake Placid, NY, U.S.A.). Rabbit polyclonal anti-mouse caveolin-1 was from Affinity Bioreagents (Golden, CO, U.S.A.), while alkaline phosphatase-conjugated goat anti-rabbit IgG and alkaline phosphatase-conjugated rabbit anti-goat IgG were from Sigma–Aldrich. Protein was quantified by the Bradford protein assay (Bio-Rad Laboratories) [21].
Animals
Chimaeric L-FABP null (−/−) C57Bl/6 mice were obtained as described earlier [22], crossed with C57/Bl6 wild-type mice to produce heterozygous offspring, and interbred to obtain the N2 generation L-FABP null (−/−) and wild-type (+/+) littermate control mice [16]. Mice had free access to water and standard low fat (5% of calories from fat) rodent chow, Teklad Rodent Diet (W8604) from Harlan Teklad (Madison, WI, U.S.A.). Mice were housed in a temperature-controlled (25 °C) facility on a 12-h light/dark cycle. Male mice were chosen for the studies presented herein because of: (i) the known sexual dimorphism of CYP7A1 [19], CYP27A1 [19], SCP-x [12], and other bile acid synthetic enzymes (e.g. oxysterol 7α-hydroxylases) [23] and (ii) concomitant with their much higher level of SCP-x, male mice are normally much more resistant to the actions of high dietary levels of branched-chain lipids such as phytanic acid [12] and cholesterol [19,23]. Before the dietary study, male mice aged 7 weeks were housed individually, allowed free access to water, and acclimated for 1 week on a defined control diet AIN-76A (#D11243, Research Diets, New Brunswick, NJ, U.S.A.) which is a phytol-free and phytoestrogen-free diet, but has 5% of calories from fat as described earlier [18]. Then, 16 mice [eight L-FABP (−/−) and eight L-FABP (+/+)] were continued for 5 weeks on the control diet (#D11243, Research Diets), while 16 mice [eight L-FABP (−/−) and eight L-FABP (+/+)] were placed on an isocaloric cholesterol-rich (1.25%) diet based on the control diet (5% of calories from fat, #D01091702, Research Diets).
At the end of the study, each mouse was fasted for 12 h, weighed, anaesthetized (ketamine, 100 mg/kg body weight; zylaxine, 10 mg/kg body weight), and blood collected by cardiac puncture was immediately processed to serum and stored at −80 °C. After killing by cervical dislocation, liver and gall-bladder were removed. A small piece of the liver was used immediately for histological analysis, while the rest was divided into small portions, flash-frozen with solid CO2, and stored at −80 °C. Bile collected from gall-bladder was flash-frozen with solid CO2, and stored at −80 °C. All experimental methods for the use of laboratory animals were approved by the University Laboratory Animal Care Committee and met AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) guidelines.
Whole body phenotype analysis
Mice and food remaining in the cage were weighed every other day; mice were returned to their respective cages and fed with preweighed amounts of respective diet. Neither cholesterol-rich diet nor L-FABP gene ablation altered food consumption, indicating the absence of dietary preferences. At the beginning and conclusion of the dietary study the mice were fasted for 12 h, weighed, anaesthetized (ketamine, 100 mg/kg body weight; zylaxine, 10 mg/kg body weight), and examined by DEXA (dual-energy X-ray absorptiometry) using a Lunar PIXImus densitometer (Lunar Corp., Madison, WI, U.S.A.) as described earlier [12]. The proportion of bone mass, FTM (fat tissue mass) to LTM (lean tissue mass) was determined from these sequential images using PIXImus densitometer software version 1.46 as described earlier [12].
Bile acid and biliary lipid quantification
Liver (0.1 g, flash-frozen, stored at −80 °C) was finely minced with a razor blade, suspended in 0.5 ml of ice-cold PBS (pH 7.4) with protease inhibitor cocktail (Sigma–Aldrich), homogenized (20 strokes, Potter–Elvehjem homogenizer), centrifuged at 600 g and 4 °C for 10 min (to remove cell debris), and the PNS (post-nuclear supernatant) was centrifuged at 105000 g and 4 °C for 90 min to obtain a supernatant (hereinafter referred to as 105K supernatant) containing soluble proteins. Bile acids, measured with Bile Acids-L3K Assay kit (Diagnostic Chemicals, Oxford, CT, U.S.A.) and the most common murine bile acid tauro-β-muricholate (Steraloids, Newport, RI, U.S.A.) as standard, were expressed as nmol/mg of protein (liver 105K supernatant and serum) or total mass (from bile acid and protein in each fraction/tissue). Biliary cholesterol was determined with kit no. 274-47109 (Wako Diagnostics, Richmond, VA, U.S.A.) using cholesterol as a standard. Since phosphatidylcholine is the major biliary phospholipid and 2-linoleoyl-1-palmitoyl-sn-glycero-3-phosphocholine is the major phosphatidylcholine species [24], biliary phospholipids were quantified with kit no. 990-54009 (Wako Diagnostics) using 2-linoleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (Sigma–Aldrich) as a standard. Biliary bile acid, cholesterol and phospholipid content were expressed in mM or g of lipid/dl of bile, or as total mass determined from the biliary lipid concentration and volume. The ratio phospholipid/(bile acid+phospholipid) was calculated from molar concentrations of the respective species. mol% cholesterol=[mol of cholesterol/(mol of cholesterol+mol of bile acid+mol of phospholipid)]×100. The biliary cholesterol saturation index was calculated from the critical tables for determining cholesterol saturation of bile as described previously [25].
Histological analysis of liver
Liver slices, excised near the porta hepatis, were fixed in 10% (v/v) neutral buffered formalin for 24 h, transferred to 70% (v/v) ethanol, processed and embedded in paraffin, and sections (5 μm thick) were stained with haematoxylin and eosin (H&E). After examination under a light microscope, hepatocytes were scored for severity of fatty vacuolation on a 0–4 scale: 0, normal; 1, minimal fatty change; 2+, mild fatty change; 3+, moderate fatty change and 4+, severe fatty change.
Liver Western-blot analysis
Liver (0.1 g, flash-frozen, stored at −80 °C) was finely minced using a razor blade, suspended in 0.5 ml of ice-cold PBS (pH 7.4) with protease inhibitor cocktail (Sigma–Aldrich), homogenized (20 strokes, Potter–Elvehjem), and centrifuged (600 g, 4 °C, 10 min) to remove cell debris [26]. Aliquots of homogenates (10–30 μg) were run on Tricine SDS/polyacrylamide gels, proteins were transferred on to 0.45 μm nitrocellulose paper (Sigma, St. Louis, MO, U.S.A.) by electroblotting in a continuous buffer system utilizing a Miniprotean II transblot apparatus (Bio-Rad Laboratories) at 40 V/gel for 2 h at 4 °C. After transfer, the blots were rinsed, blocked and treated using primary affinity-purified antisera (see the Proteins and antibodies subsection above), blots were then incubated with the appropriate secondary antibody, the colour was developed, blots were digitally imaged, and proteins were quantified using a single-chip CCD (charge-coupled-device) video camera, FluorChemimager and FluorChem image analysis software (version 2.0) from Alpha Innotech (San Leandro, CA, U.S.A.) as described earlier [20].
Lipid analysis
Liver lipids were analysed as described in [17,22]. Serum total cholesterol (Wako # 276-64909), free cholesterol (Wako # 274-47109), non-esterified fatty acid (Wako # 994-75409), triacylglycerol (Wako # 998-40391/# 994-40491) and phospholipid (Wako # 990-54009) were determined with the above kits (Wako Diagnostics). Phospholipids were quantified using 2-linoleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (Sigma–Aldrich) as standard since this phosphatidylcholine is the most common murine bile phospholipid (see above). Serum cholesteryl ester was quantified as (total cholesterol–free cholesterol).
Statistics
SigmaPlot 2000 for Windows version 6.10 (SPSS, Chicago, IL, U.S.A.) was used for graphics. Values are means±S.E.M. (n=7 or 8). ANOVA with Newman–Keuls post-test utilized the GraphPad Prism data analysis package version 3.02 for Windows (GraphPad Software, San Diego, CA, U.S.A.).
RESULTS
Effect of L-FABP gene ablation on bile acid mass distribution in liver, serum and gall-bladder of male mice
In control-fed wild-type L-FABP (+/+) male mice the quantity of bile acid was highest in gall-bladder ≫ liver ≫ serum in the relative proportion 11000:210:1 (Table 1). Dietary cholesterol increased the bile acid mass in liver as well as serum (but not gallbladder) up to 1.6-fold (P<0.001) and 1.5-fold (P<0.001) respectively (Table 1). Since biliary bile acid comprised the bulk of bile acid, total (liver+serum+gall-bladder) bile acid mass was not significantly altered by cholesterol-rich diet. L-FABP gene ablation alone increased the bile acid mass (but not the relative proportion 11000:220:1) of liver, serum and gall-bladder bile 1.6-fold (P<0.01), 1.5-fold (P<0.001) and 1.5-fold (P<0.001) respectively, as compared with control-fed wild-type L-FABP (+/+) mice (Table 1). Total (liver+serum+gall-bladder) bile acid mass was increased 1.5-fold in control-fed L-FABP (−/−) mice as compared with control-fed wild-type L-FABP (+/+) littermates. While the biliary phenotype of cholesterol-fed wild-type L-FABP (+/+) mice was increased slightly or unaltered, cholesterol-fed L-FABP (−/−) mice exhibited 1.4–4-fold reduced bile acid mass in liver, serum and gall-bladder as compared with control-fed L-FABP (−/−) littermates or cholesterol-fed wild-type L-FABP (+/+) littermates (Table 1). The relative bile acid mass distribution in gall-bladder ≫ liver ≫ serum was in the relative proportion 7100:400:1, significantly reduced from that of control-fed L-FABP (−/−) or cholesterol-fed L-FABP (+/+) littermates (Table 1). Thus L-FABP gene ablation alone significantly increased the bile acid mass in liver, serum and gall-bladder of control-fed mice. Furthermore, L-FABP gene ablation significantly altered the response to dietary cholesterol such that bile acid mass distributions were all dramatically decreased, especially in gall-bladder bile.
Table 1. Biliary lipid mass distribution in L-FABP wild-type (+/+) and L-FABP (−/−) mice.
Lipid masses are given in nmol. Liver 105K supernatant and serum bile acid masses were calculated from the bile acid concentration (nmol/mg of protein) and the respective total protein content in liver 105K supernatant and serum. Gall-bladder masses of bile acid, cholesterol and phospholipids were determined from the respective concentration (mM) and the biliary volume. Gall-bladder total lipid refers to the sum of bile acid, phospholipids, and cholesterol masses in gall-bladder bile. Cholesterol/phospholipid (C/PL), cholesterol/bile acid (C/BA) and phospholipid/bile acid (PL/BA) in gall-bladder bile are molar ratios. The data represent means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): **, P<0.01; ***, P<0.001. For cholesterol diet versus control diet: ##, P<0.01; ###, P<0.001.
| L-FABP (+/+) | L-FABP (−/−) | |||||
|---|---|---|---|---|---|---|
| Lipid | Source | Diet… | Control | Cholesterol | Control | Cholesterol |
| Bile acid | Total | 2800±300 | 2500±100 | 4200±500** | 1100±200**,### | |
| Liver | 51±4 | 82±4### | 81±6*** | 56±2**,### | ||
| Serum | 0.238±0.004 | 0.36±0.01### | 0.36±0.03*** | 0.14±0.02***,### | ||
| Gall-bladder | 2700±300 | 2400±100 | 4100±500** | 1000±200**,### | ||
| Cholesterol | Gall-bladder | 160±10 | 200±10## | 240±20*** | 450±30***,### | |
| Phospholipid | Gall-bladder | 440±20 | 580±40## | 630±40*** | 760±20**,## | |
| Total lipid | Gall-bladder | 3300±300 | 3210±60 | 4900±500** | 2200±200***,### | |
| C/PL (mol/mol) | Gall-bladder | 0.38±0.04 | 0.36±0.09 | 0.39±0.09 | 0.59±0.05**,## | |
| C/BA (mol/mol) | Gall-bladder | 0.07±0.01 | 0.088±0.003 | 0.056±0.009 | 0.42±0.04***,### | |
| PL/BA (mol/mol) | Gall-bladder | 0.17±0.03 | 0.23±0.03 | 0.146±0.008 | 0.8±0.1***,### | |
Effect of L-FABP gene ablation on masses of other lipids in gall-bladder bile
Biliary masses of cholesterol and phospholipid were 17- and 6-fold lower than bile acids in control-fed wild-type L-FABP (+/+) mice (Table 1). Cholesterol-rich diet increased biliary masses of cholesterol and phospholipid (but not bile acid) slightly in L-FABP (+/+) mice, but without significantly altering the overall molar ratios of cholesterol/phospholipid (C/PL), cholesterol/bile acid (C/BA), and phospholipid/bile acid (P/BA) (Table 1). Because biliary bile acid mass represents >80% of the total lipid (i.e. bile acids+cholesterol+phospholipid) in gall-bladder bile in wild-type L-FABP (+/+) mice, the biliary bile acid mass as percentage of total lipid was unaltered by cholesterol-rich diet (Table 1). L-FABP gene ablation alone increased the biliary masses of cholesterol, phospholipids and bile acid 1.5-, 1.4- and 1.5-fold respectively, while total biliary lipid mass increased 1.5-fold (Table 1). Since each lipid class in bile was increased proportionally, relative ratios of C/PL, C/BA and PL/BA were unaltered in gall-bladder bile of control-fed L-FABP (−/−) mice (Table 1). In contrast with cholesterol-fed L-FABP (+/+) mice, cholesterol-fed L-FABP (−/−) mice had 2.2- and 1.3-fold increased biliary mass of cholesterol and phospholipid respectively, and molar ratios of C/PL and C/BA were increased 1.6- and 4.8-fold respectively (Table 1). Finally, due to markedly reduced biliary bile acid content, the total biliary lipid mass of cholesterol-fed L-FABP (−/−) mice was decreased by 55 and 31% as compared with that in control-fed L-FABP (−/−) and cholesterol-fed L-FABP (+/+) mice respectively (Table 1).
To determine if L-FABP gene ablation and/or dietary cholesterol altered the biliary cholesterol saturation, the above lipid parameters of bile were used to calculate the biliary cholesterol saturation index as established by Carey [25] and described in the Experimental section. Cholesterol-rich diet did not significantly alter either the ratio of phospholipid/(bile acid+phospholipid) or the cholesterol saturation index in bile of wild-type L-FABP (+/+) mice (Table 2). Likewise, L-FABP gene ablation alone also did not significantly alter either the ratio of phospholipid/(bile acid+phospholipid) or the cholesterol saturation index (Table 2). In contrast, cholesterol-fed L-FABP (−/−) mice exhibited a >2.4-fold increase in phospholipid/(bile acid+phospholipid) ratio versus L-FABP gene ablation alone or cholesterol feeding alone (Table 2). Further, cholesterol-fed L-FABP (−/−) mice had a >2.8-fold higher mol% cholesterol (19±1%) than either cholesterol-fed wild-type L-FABP (+/+) mice (6.7±0.3%) or control-fed L-FABP (−/−) mice (4.7±0.7%, Table 2). The mol% cholesterol measured in gall-bladder bile from the cholesterol-fed L-FABP gene-ablated (−/−) mice was 2.6-fold higher (P<0.001) than the value determined from the cholesterol saturation index (7.2±0.2 mol%, Table 2) as described by Carey [25].
Table 2. Biliary cholesterol saturation index in L-FABP wild-type (+/+) and L-FABP gene-ablated (−/−) mice.
Biliary total lipid (bile acid, cholesterol and phospholipid) concentrations were calculated as described in the Experimental section and are expressed as g/dl. Phospholipid/(bile acid+phospholipid) data are expressed as molar ratios. Cholesterol is expressed in mol% and was determined from the relationship mol% cholesterol=[cholesterol/(cholesterol+bile acid+phospholipid)]×100. The cholesterol saturation index was determined from the critical tables for calculating cholesterol saturation in bile as described in the Experimental section. The data represent the means±S.E.M. **, P<0.01 for L-FABP (−/−) versus L-FABP (+/+); ***, P<0.001 for L-FABP (−/−) versus L-FABP (+/+); ###, P<0.001 for cholesterol diet versus control diet; ‡‡‡, P<0.001 for cholesterol (mol%) versus cholesterol saturation index.
| L-FABP (+/+) | L-FABP (−/−) | ||||
|---|---|---|---|---|---|
| Parameter | Diet… | Control | Cholesterol | Control | Cholesterol |
| Total lipid (g/dl) | 7.5±0.7 | 7.2±0.1 | 11±1** | 5.1±0.4***,### | |
| Phospholipid/(bile acid+phospholipid) (mol/mol) | 0.14±0.02 | 0.18±0.02 | 0.13±0.01 | 0.43±0.05***,### | |
| Cholesterol (mol%) | 5.6±0.7 | 6.7±0.3 | 4.7±0.7 | 19±1***,###,‡‡‡ | |
| Cholesterol saturation index (mol%) | 4.8±0.4 | 5.7±0.4 | 4.8±0.1 | 7.2±0.2**,### | |
In summary, the proportional increases in biliary cholesterol, phospholipid and bile acid in control-fed L-FABP (−/−) as compared with wild-type L-FABP (+/+) mice were consistent with L-FABP gene ablation alone increasing bile production but not the relative proportion of biliary lipid constituents. In contrast, L-FABP gene ablation differentially altered the response of bile lipid levels to dietary cholesterol such that the relative proportions of cholesterol increased markedly, phospholipid increased slightly, and bile acid decreased dramatically. Thus the percentage of biliary lipid represented by cholesterol increased 3.4-fold from 5.6 to 19 mol% (P<0.001) and the cholesterol saturation index increased 1.5-fold in bile of cholesterol-fed L-FABP (−/−) mice.
Effect of L-FABP gene ablation and cholesterol on liver levels of key proteins involved in uptake, synthesis and storage of bile acid precursor cholesterol
The possibility was considered that the reduced bile acid levels in cholesterol-fed L-FABP (−/−) mice were associated with down-regulation of key liver proteins involved in cholesterol uptake, efflux, synthesis and storage. In wild-type L-FABP (+/+) mice, cholesterol-rich diet selectively up-regulated liver proteins involved in cholesterol uptake [1.5-fold increased LDL receptor, but not SRB-1) and cholesterol storage (1.16- and 1.24-fold increased ACAT-2 and ACAT-1 respectively, but not ACBP), while concomitantly down-regulating expression of the key enzyme of endogenous cholesterol synthesis (52% decreased HMG-CoA reductase) (Table 3). L-FABP gene ablation alone (control diet) also selectively up-regulated proteins in cholesterol uptake (1.4-fold increased LDL receptor, but not SRB-1), down-regulated HMG-CoA reductase, but did not alter ACAT-2, ACAT-1 or ACBP (Table 3). Cholesterol-rich diet did not lead to further increased LDL receptor level in L-FABP (−/−) mice, but HMG-CoA reductase was decreased 43%, while ACAT-2 and ACAT-1 were increased 1.26- and 1.28-fold respectively (Table 3). The effects of cholesterol-rich diet in L-FABP (−/−) and wild-type L-FABP (+/+) mice were qualitatively and mostly quantitatively similar. Thus the significantly reduced bile acid in cholesterol-fed L-FABP (−/−) mice was not due to the reduced cholesterol substrate available for bile acid synthesis in the liver.
Table 3. Effect of L-FABP gene ablation on liver levels of key proteins involved in uptake, synthesis and storage of bile acid precursor cholesterol.
Liver homogenates of male mice were separated by SDS/PAGE and electroblotted on to nitrocellulose membranes as described in the Experimental section. The membranes were incubated with polyclonal antisera directed against proteins involved in cholesterol uptake (LDL receptor), efflux (SRB-1), synthesis (HMG-CoA reductase) and storage as cholesteryl esters (ACBP, ACAT-2 and ACAT-1). The amount of protein is expressed in arbitrary units (a.u.) with that present in the control-fed L-FABP (+/+) liver homogenates defined to be 1. The data represent the means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): *, P<0.05; **, P<0.01. For cholesterol diet versus control diet: #, P<0.05; ###, P<0.001.
| L-FABP (+/+) | L-FABP (−/−) | ||||
|---|---|---|---|---|---|
| Protein | Diet… | Control | Cholesterol | Control | Cholesterol |
| LDL receptor | 1.00±0.03 | 1.5±0.2# | 1.4±0.2* | 1.3±0.1 | |
| SRB-I | 1.0±0.1 | 1.1±0.1 | 1.15±0.02 | 0.97±0.06 | |
| HMG-CoA reductase | 1.0±0.1 | 0.48±0.04### | 0.74±0.03** | 0.57±0.03# | |
| ACBP | 1.0±0.1 | 0.85±0.08 | 0.97±0.08 | 0.94±0.03 | |
| ACAT-2 | 1.00±0.03 | 1.16±0.01### | 1.03±0.02 | 1.26±0.03**,### | |
| ACAT-1 | 1.00±0.06 | 1.24±0.04### | 0.98±0.03 | 1.28±0.04### | |
Response of key enzymes in bile acid synthesis in livers of L-FABP wild-type (+/+) and L-FABP (−/−) mice
To determine whether the lowered level of bile acids in cholesterol-fed L-FABP (−/−) was due to impaired bile acid synthesis, the expression of key enzymes in the bile acid synthesis pathway (CYP7A1, CYP27A1 and SCP-x) was examined by Western blotting. (i) In wild-type L-FABP (+/+) mice, cholesterol-rich diet increased 1.24-fold (P<0.05) (Figure 1A) the expression of CYP7A1, the rate-limiting endoplasmic reticulum enzyme initiating bile acid synthesis (reviewed in [2]). L-FABP gene ablation alone increased the expression of CYP7A1 even more, by 1.7-fold. (ii) In contrast, cholesterol-fed L-FABP (−/−) mice exhibited 2.5-fold reduced, rather than increased, CYP7A1 expression as compared with control-fed L-FABP (−/−) mice (Figure 1A). Cholesterol-rich diet did not alter the expression of CYP27A1, the key mitochondrial enzyme initiating the secondary (alternative) bile acid synthesis pathway (reviewed in [2]), in wild-type L-FABP (+/+) mice (Figure 1B). L-FABP gene ablation alone increased the level of CYP27A1 1.24-fold (P<0.01) as compared with control-fed L-FABP (+/+) mice. In contrast, cholesterol-fed L-FABP (−/−) mice exhibited 2.4-fold reduced CYP27A1 expression as compared with control-fed L-FABP (−/−) mice and 2-fold reduced expression as compared with cholesterol-fed or control-fed wild-type L-FABP (+/+) mice (Figure 1B). (iii) In wild-type L-FABP (+/+) mice, cholesterol-rich diet slightly decreased (∼20%, Figure 1C) the expression of SCP-x, the key peroxisomal enzyme for oxidizing the branched acyl side chain of sterols which is essential for synthesis of conjugated bile acids that comprise the majority of bile acids in mammals (reviewed in [2]). L-FABP gene ablation alone had no effect on SCP-x expression. Cholesterol-fed L-FABP (−/−) littermates exhibited 23% decreased (P<0.05) level of SCP-x (Figure 1C). Thus the rate-limiting enzyme of bile acid synthesis (CYP7A1) was moderately up-regulated in livers of cholesterol-fed L-FABP (+/+) and control-fed L-FABP (−/−) mice. Conversely, the dramatically lowered bile acid in cholesterol-fed L-FABP (−/−) mice was associated with extensive down-regulation not only of CYP7A1, but also of the other key enzymes of bile acid synthesis examined. This accounts at least in part for the dramatically lower mass of bile acids in serum, liver and gall-bladder bile reported above.
Figure 1. Levels of key rate-limiting enzymes in bile acid synthesis in livers of L-FABP wild-type (+/+) and L-FABP (−/−) mice.
Proteins from liver homogenates of male mice were separated by SDS/PAGE, electroblotted on to nitrocellulose membranes, and incubated with polyclonal antisera directed against CYP7A1 (A), CYP27A1 (B) or SCP-x (C) as described in the Experimental section. The amount of protein is expressed in arbitrary units with that present in the control-fed L-FABP (+/+) liver homogenates defined to be 1. The data represent the means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): **, P<0.01; ***, P<0.001. For cholesterol diet versus control diet: #, P<0.05; and ###, P<0.001.
Effect of L-FABP gene ablation and cholesterol on liver proteins that bind/transport bile acids and bile acid precursor cholesterol
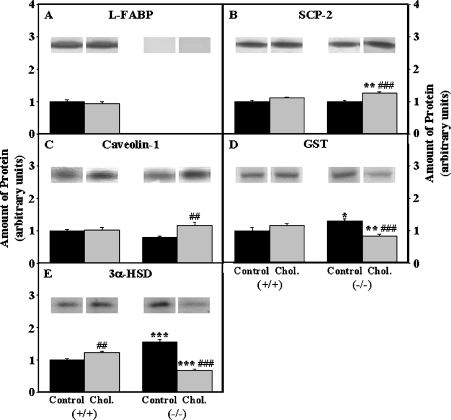
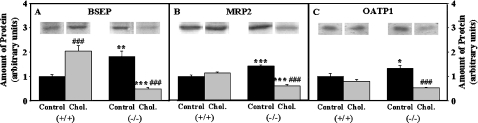
The driving force for biliary lipid secretion is the active transport of bile salts within the hepatocyte and across the hepatocyte canalicular plasma membrane [27]. (i) Cholesterol-rich diet did not alter expression of some cytosolic bile acid-binding/transport proteins such as L-FABP (Figure 2A), GST (Figure 2D), but increased that of others (3α-HSD) moderately (1.2-fold, Figure 2E). L-FABP gene ablation alone increased the expression of GST by 1.3-fold (P<0.05, Figure 2D) and 3α-HSD by 1.6-fold (P<0.001, Figure 2E), likely compensating for the reduced bile acid binding ability due to loss of L-FABP (Figure 2A). In contrast, cholesterol-fed L-FABP (−/−) mice exhibited decreased expression of both GST (35%, P<0.01, Figure 2D) and 3α-HSD (55% P<0.001, Figure 2E) as compared with control-fed L-FABP (−/−) mice. (ii) With regard to key canalicular proteins involved in transporting bile acids into the gall-bladder bile, cholesterol-rich diet doubled the expression of BSEP (Figure 3A) without significantly changing that of MRP2 (Figure 3B) or OATP-1 (Figure 3C) in wild-type L-FABP (+/+) mice. L-FABP gene ablation alone dramatically increased the expression of BSEP (Figure 3A), MRP2 (Figure 3B) and OATP-1 (Figure 3C) 1.8-, 1.4- and 1.3-fold respectively. In contrast, L-FABP gene ablation elicited the opposite response such that the cholesterol-fed L-FABP (−/−) littermates had nearly 2-fold reduced BSEP (Figure 3A), MRP2 (Figure 3B) and OATP-1 (Figure 3C).
Figure 2. Proteins that bind/transport bile acids and the bile acid precursor cholesterol in liver cytoplasm of L-FABP wild-type (+/+) and L-FABP (−/−) mice.
Proteins from liver homogenates of male mice were separated by SDS/PAGE, electroblotted on to nitrocellulose membranes, and incubated with polyclonal antisera directed against cytosolic cholesterol/bile acid transport proteins such as L-FABP (A), SCP-2 (B), caveolin-1 (C), GST (D), or 3α-HSD (E) as described in the Experimental section. The amount of protein was expressed in arbitrary units with that present in the control-fed L-FABP (+/+) liver homogenates defined to be 1. The data represent the means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): *, P<0.05; **, P<0.01; ***, P<0.001. For cholesterol diet versus control diet: ##, P<0.01; and ###, P<0.001.
Figure 3. Proteins that bind/transport bile acids across bile canaliculus and basolateral membranes of L-FABP wild-type (+/+) and L-FABP (−/−) mice.
Proteins from liver homogenates of male mice were separated by SDS/PAGE, electroblotted on to nitrocellulose membranes, and incubated with polyclonal antisera directed against canalicular bile salt translocase proteins such as BSEP (A) and MRP2 (B), or against basolateral/serosal bile salt translocase protein such as OATP-1 (C) as described in the Experimental section. The amount of protein is expressed in arbitrary units with that present in the control-fed L-FABP (+/+) liver homogenates defined to be 1. The data represent the means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): *, P<0.05; **, P<0.01; ***, P<0.001. For cholesterol diet versus control diet, ###, P<0.001.
Within the liver, cholesterol is co-secreted along with bile acids into bile. Liver contains at least three proteins that bind and/or transfer cholesterol: L-FABP, SCP-2 and caveolin-1. With regard to L-FABP: (i) although direct binding of cholesterol by L-FABP in vitro is controversial (reviewed in [28,29]), L-FABP enhances cholesterol transfer from plasma membranes in vitro (reviewed in [28,29]), in transfected cells overexpressing L-FABP [28], and in vivo (reviewed in [30]). Cholesterol-rich diet did not alter L-FABP levels (Figure 2A). (ii) While direct binding of cholesterol by SCP-2 in vitro also appears highly dependent on the type of binding assay used (reviewed in [29,31–33]), SCP-2 dramatically enhances cholesterol transfer from plasma membranes and endoplasmic reticulum in vitro (reviewed in [29,32]), from plasma membranes and endoplasmic reticulum of transfected cells overexpressing SCP-2 [34,35], and in vivo [36]. Cholesterol-rich diet only slightly increased SCP-2 level by 10% (Figure 2B). While L-FABP gene ablation alone did not alter the expression of SCP-2 (Figure 2B), cholesterol-fed L-FABP (−/−) mice exhibited 1.3-fold increased expression of SCP-2 (Figure 2B), significantly more than that shown by cholesterol-fed wild-type L-FABP (+/+) littermates. (iii) Caveolin-1 enhances intracellular cholesterol transport either by directly binding cholesterol and/or by caveolar vesicles (reviewed in [29]). L-FABP gene ablation alone slightly decreased caveolin-1 (Figure 2C). Cholesterol-fed L-FABP (−/−) mice exhibited 1.2-fold increased expression of caveolin-1 (Figure 2C), significantly more than that shown by cholesterol-fed wild-type L-FABP (+/+) littermates.
In summary, the significant increase in bile acid levels in cholesterol-fed wild-type L-FABP (+/+) as well as in control-fed L-FABP gene-ablated mice was associated with moderate increases in levels of other soluble bile acid-binding proteins (GST and 3α-HSD) and much larger increases in the levels of canalicular (but not basolateral/serosal) bile acid transport pumps. The marked decrease in bile acid levels in cholesterol-fed L-FABP (−/−) mice was associated with: markedly reduced expression of cytosolic bile acid-binding/transport proteins (loss of L-FABP; reduced GST and 3α-HSD); reduced expression of bile canalicular bile acid transport proteins (BSEP and MRP2); and reduced level of basolateral/serosal bile acid transport protein (OATP-1). Increased SCP-2 may account in part for the increased biliary cholesterol and phospholipid in cholesterol-fed L-FABP (−/−) mice. SCP-2 binds and efficiently transfers cholesterol and less so phospholipids in vitro (reviewed in [37]), and increased SCP-2 expression is associated with enhanced biliary free cholesterol and phospholipid secretion [15,36].
Effect of L-FABP gene ablation and cholesterol on nuclear proteins regulating cholesterol and bile acid metabolism
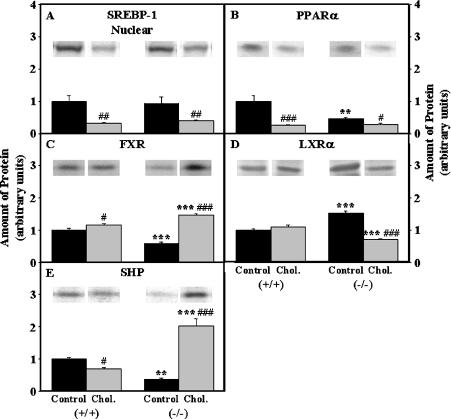
At the nuclear level SREBP-1 and PPARα are involved in regulating transcription of multiple genes involved in cholesterol uptake/metabolism (e.g. HMG-CoA reductase). SREBP-1 exists in two forms, one of which (i.e. the smaller N-form) represents the active SREBP-1 form entering the nucleus and regulating transcription of HMG-CoA reductase (reviewed in [38]). Neither cholesterol-rich diet nor L-FABP gene ablation alone significantly altered the level of the expression of the inactive membrane-bound P-form of SREBP-1 in male mice (results not shown). In contrast, the active N-form of SREBP-1 was decreased 2–3-fold by cholesterol-rich diet in wild-type L-FABP (+/+) (Figure 4A). Although L-FABP gene ablation alone did not alter the expression of the active N-form of SREBP-1 in control-fed mice (Figure 4A), the N-form was reduced in cholesterol-fed L-FABP (−/−) mice (Figure 4A), consistent with the observed reduced levels of HMG-CoA reductase (Table 3). PPARα is co-regulated with SREBP-1 by dietary fat [39]. Cholesterol-rich diet decreased PPARα expression 4-fold (P<0.001) in wild-type L-FABP (+/+) (Figure 4B). L-FABP gene ablation alone also decreased PPARα 2.1-fold (P<0.01) (Figure 4B). Finally, in L-FABP (−/−) mice, a cholesterol-rich diet did not further reduce the expression of PPARα compared with that seen in cholesterol-fed wild-type L-FABP (+/+) littermates (Figure 4B). Therefore the dramatic decrease in bile acid levels in cholesterol-fed L-FABP (−/−) mice as compared with their control-fed wild-type LFABP (+/+) littermates was not associated with differential reduction in expression of nuclear receptors involved in cholesterol metabolism (SREBP-1 and PPARα).
Figure 4. Effect of L-FABP gene ablation and cholesterol on nuclear receptors regulating cholesterol and bile acid metabolism.
Liver homogenates of male mice were separated by SDS/PAGE, electroblotted on to nitrocellulose membranes, and incubated with polyclonal antisera directed against nuclear regulatory proteins SREBP-1 (A), PPARα (B), FXR (C), LXRα (D) or SHP (E) as described in the Experimental section. The amount of protein is expressed in arbitrary units with that present in the control-fed L-FABP (+/+) liver homogenates defined to be 1. The data represent the means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): **, P<0.01, ***P<0.001. For cholesterol diet versus control diet: #, P<0.05; ##, P<0.01; and ###, P<0.001.
Multiple nuclear hormone receptors are involved in an intricate cycle involving positive (LXRα) and negative (FXR and SHP) regulators of transcription of the rate-limiting bile acid synthetic enzyme CYP7A1 as well as other enzymes of bile acid synthesis (reviewed in [2]). Cholesterol-rich diet increased the level of positive nuclear hormone receptor (LXRα) only slightly in wild-type L-FABP (+/+) mice (Figure 4D), while that of the negative receptor SHP, but not FXR (Figure 4C), was reduced by 29% (Figure 4E), consistent with the increased CYP7A1 in cholesterol-fed wild-type L-FABP (+/+) mice. In contrast, cholesterol-rich diet oppositely altered the expression of these nuclear receptors in L-FABP (−/−) mice (Figures 4C–4E): the positive regulator (LXRα) was decreased 2.4-fold (Figure 4D); the negative regulator (FXR) was increased more than 2-fold (Figure 4C), while the negative regulator SHP was increased 5.3-fold (Figure 4E). This predominance of negative regulatory effects of SHP in the cholesterol-fed L-FABP (−/−) male mice was consistent with reduced CYP7A1 (Figure 1A) and 4-fold reduced levels of bile acids (Table 1). Thus altered regulation of the bile acid nuclear receptors contributed significantly to the observed biliary phenotype of cholesterol-fed L-FABP (−/−) male mice.
Effect of L-FABP gene ablation and cholesterol on liver lipid phenotype: total lipid content and fatty-acid distribution
Since mice genetically deficient in the rate-limiting enzymes (CYP7A1) as well as other enzymes (CYP27A1) of the bile acid synthesis pathway exhibit altered liver morphology and liver lipid phenotype (reviewed in [2]), the effect of reduced bile acid levels observed in cholesterol-fed L-FABP (−/−) mice on these parameters was examined. Cholesterol-rich diet, L-FABP gene ablation or both together did not significantly alter liver weight or liver histology (results not shown). Very little fatty vacuolation was evident in livers of control fed male wild-type L-FABP (+/+) mice. The level of fatty vacuolation was not altered by L-FABP gene ablation or by cholesterol feeding. Livers of cholesterol-fed wild-type L-FABP (+/+) mice exhibited nearly 2-fold (P<0.001) increased total lipid content, primarily due to 2.8-fold (P<0.001) and 2.7-fold (P<0.01) increased liver phospholipid and cholesteryl ester (Table 4). While L-FABP gene ablation alone did not alter liver total lipid content (Table 4), its distribution into individual lipid classes was moderately altered: >3-fold (P<0.01) decrease in triacylglycerol concomitant with 2.1-fold (P<0.05) and 1.4-fold increase in non-esterified fatty acid and phospholipid respectively (Table 4). Livers of cholesterol-fed L-FABP (−/−) mice exhibited increased total lipid due to large increases in nearly all esterified lipid (3.2-, 4.4- and 1.8-fold increased triacylglycerol, cholesteryl ester and phospholipid respectively) classes as well as 1.4-fold increased non-esterified fatty acid as compared with control fed L-FABP (−/−) littermates (Table 4). This was in contrast with the cholesterol-fed wild-type L-FABP (+/+) mice where the increase was restricted to phospholipids and cholesteryl ester (Table 4). Thus L-FABP gene ablation significantly altered the pattern of fatty acid-containing lipids in the cholesterol-fed versus control-fed mice. Nevertheless, this did not result in markedly altered gross liver morphology or total lipid as compared with cholesterol-fed L-FABP (+/+) mice.
Table 4. Effect of altered biliary phenotype on liver lipids in L-FABP gene-ablated mice.
Lipid was extracted from liver homogenates of male mice as described in the Experimental section. Lipids were quantified by comparison with a standard curve generated for each lipid class. Lipid concentrations are expressed in units of nmol of lipid per mg of liver homogenate protein. The data represent the means±S.E.M. For L-FABP (−/−) versus L-FABP (+/+): *, P<0.05; **, P<0.01. ***, P<0.001. For cholesterol diet versus control diet: ##, P<0.01; ###, P<0.001.
| L-FABP (+/+) | L-FABP (−/−) | ||||
|---|---|---|---|---|---|
| Lipid class | Diet… | Control | Cholesterol | Control | Cholesterol |
| Cholesterol | 24±4 | 24±3 | 21±2 | 33±2*,## | |
| Cholesteryl ester | 10±1 | 27±4## | 10±3 | 44±6**,### | |
| Non-esterified fatty acid | 20±3 | 21±3 | 42±7* | 57±8*** | |
| Triacylglycerol | 90±10 | 90±10 | 28±6** | 90±10### | |
| Phospholipid | 100±10 | 280±40### | 140±20 | 260±20## | |
| Total cholesterol | 34±5 | 51±6## | 31±5 | 78±8**,### | |
| Total lipid | 250±20 | 440±60### | 240±20 | 480±30### | |
Effect of L-FABP gene ablation and cholesterol on liver lipid phenotype: liver cholesterol content and distribution
Because ablation of key enzymes of bile acid synthesis (e.g. CYP7A1 and CYP27A1) alters the cholesterol phenotype of mice (reviewed in [2]), the effect of reduced bile acid levels in cholesterol-fed L-FABP (−/−) mice on liver cholesterol content and distribution was examined. Livers of cholesterol-fed wild-type L-FABP (+/+) mice were hypercholesterolaemic, exhibiting 2.7- and 1.5-fold (P<0.01) higher levels of cholesteryl ester and total cholesterol as compared with control-fed L-FABP (+/+) littermates (Table 4). Non-esterified cholesterol was not altered in cholesterol-fed wild-type L-FABP (+/+) mice (Table 4). Although L-FABP gene ablation alone had no effect on the liver free, esterified or total cholesterol (Table 4), L-FABP gene ablation exacerbated the liver cholesterol accumulation in cholesterol-fed mice. Free, esterified and total cholesterol were increased 1.6-, 4.4- and 2.5-fold respectively, in cholesterol-fed L-FABP (−/−) mice versus control-fed L-FABP (−/−) littermates (Table 4). Taken together these results indicate that liver cholesterol accumulation was greatest in mice with the lowest bile acid levels, i.e. cholesterol-fed L-FABP (−/−) mice.
Effect of L-FABP gene ablation and cholesterol on serum lipid phenotype: apolipoproteins, fatty acids and cholesterol
Because ablation of key enzymes of bile acid synthesis (e.g. CYP7A1 and CYP27A1) increases serum cholesterol and/or triacylglycerol in mice (reviewed in [2]), the effect of L-FABP gene ablation and cholesterol diet on serum apolipoprotein and lipid phenotype was examined. Neither dietary cholesterol nor L-FABP gene ablation alone altered serum apo A1 (apolipoprotein A1) or apo B levels in control-fed L-FABP (+/+) mice (results not shown). Likewise, high dietary cholesterol together with L-FABP gene ablation only moderately (∼20%) increased serum apo A1 and apo B levels (results not shown). Serum lipid phenotype of male wild-type L-FABP (+/+) and L-FABP gene-ablated mice exhibited no major changes in the levels of total lipid, total fatty acid or total cholesterol (results not shown). Furthermore, resolution of serum lipids into individual lipid classes revealed that with the exception of non-esterified fatty acid (increased 1.9-fold, results not shown) there were only minor changes in the relative distribution of most lipid classes (cholesterol, cholesteryl esters, triacylglycerols and phospholipids) in cholesterol-fed wild-type L-FABP (+/+) mice. Basically similar observations were made in cholesterol-fed L-FABP (−/−) mice. As compared with control-fed L-FABP (−/−) littermates, the levels of non-esterified fatty acid increased 2.3-fold along with moderate increases in triacylglycerol and cholesterol, but not phospholipid or cholesteryl ester (results not shown). Thus L-FABP (−/−), but not wild-type L-FABP (+/+) littermates, became slightly hyper-lipidaemic (increased serum cholesterol and triacylglycerol) in response to dietary cholesterol, consistent with known resistance of rodent serum lipids to dietary cholesterol [19].
Effect of L-FABP gene ablation and cholesterol on whole body phenotype
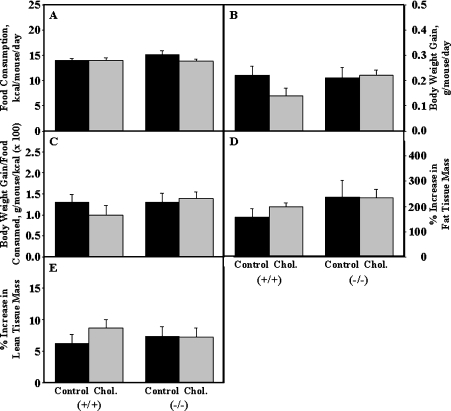
Since the 4-fold decreased level of bile acids in cholesterol-fed L-FABP (−/−) was associated with altered hepatic cholesterol and lipid phenotype, it was important to determine if these changes were correlated with altered food consumption and/or whole body phenotype. Food consumption, measured every 2 days during the 5-week dietary study, was not significantly altered by either cholesterol-rich diet or L-FABP gene ablation (Figure 5A). Neither cholesterol-rich diet nor L-FABP gene ablation significantly altered the gross body weight (Figure 5B) or body weight gain/food consumed (Figure 5C). Neither cholesterol-rich diet, L-FABP gene ablation nor both together significantly altered the relative proportion of whole body FTM (Figure 5D) and bone-free LTM (Figure 5E) of male mice determined by DEXA at the beginning and end of the dietary study as described in the Experimental section.
Figure 5. Effect of L-FABP gene ablation and cholesterol on whole body phenotype in male mice.
Age- and litter-matched male mice were fed on an isocaloric control or 1.25% cholesterol diet as described in the Experimental section. Mouse body weight and food consumed were determined every 2 days during the feeding study. Food consumption (A), daily body weight gain (B) and daily body weight gain/kcal of food consumed (C) were calculated for each individual from the aforementioned parameters. At the beginning and ending of the feeding study, each mouse was also DEXA scanned with a Lunar PIXImus. The PIXImus data obtained at the beginning and end of the feeding study were used to determine the percentage increase in FTM (D) and bone-free lean tissue mass (LTM) (E) for each animal as described in the Experimental section. The data represent the means±S.E.M. 1 kcal=4.184 kJ.
DISCUSSION
Cholesterol homoeostasis is an intricate interplay of multiple pathways for cholesterol uptake, intracellular transport, oxidation and secretion (reviewed in [2,3,5,40–42]). Since accumulation of excess cholesterol is toxic, mammals have evolved bile acids as a mechanism for net removal of excess cholesterol. Human spontaneous mutants or mouse ablations of genes encoding key enzymes of bile acid synthesis (CYP7A1 and CYP27A1) involve severe (70–80%) reductions in bile acids and in multiple phenotypes including hypercholesterolaemia, hypertriglyceridaemia, hepatic cholesterol accumulation, cholestasis, steatorrhea, and/or central nervous system abnormalities (reviewed in [2]). However, despite the fact that hepatocellular transport and active secretion across the canalicular membrane represent the driving force for biliary lipid secretion, relatively little is known regarding the mechanism(s) of intracellular bile acid transport or the effects of mutations/gene ablations in the intracellular bile acid-binding proteins such as L-FABP (reviewed in [2,3,5]). L-FABP is highly expressed in liver hepatocytes (site of bile acid production and co-secretion with cholesterol into bile) and in intestinal mucosal cells (site of bile acid reabsorption and cholesterol absorption) (reviewed in [7]). Although L-FABP in both sites is expected to have an impact on bile acid and cholesterol metabolism, there is no compensatory up-regulation of intestinal I-FABP or other members of the FABP family in liver or intestine of L-FABP (−/−) mice [43]. The recent availability of L-FABP gene-ablated mice [17,18] provides a new means for directly assessing the effect of L-FABP gene ablation and cholesterol-responsiveness of bile acid and lipid phenotype in male mice. The studies presented herein provide new insights on the physiological role of L-FABP in liver and biliary lipid phenotype of male mice.
First, the phenotype of L-FABP (−/−) male mice showed that ablation of the major bile acid-binding protein in liver significantly altered bile acid and cholesterol metabolism and regulation. Although lack of the major bile acid-binding protein (i.e. L-FABP) was expected to decrease bile acid transfer to the bile canaliculus for secretion into the gall-bladder, decreased biliary bile acid was not observed. Instead, the livers of 12 h fasted L-FABP (−/−) male mice exhibited increased biliary bile acid, cholesterol and phospholipid. Interestingly, in response to more prolonged 48 h fasting, the gall-bladder bile levels of cholesterol and phospholipid are no longer increased [43]. In the present study, the elevated biliary lipid in L-FABP (−/−) mice fasted for 12 h was associated with concomitant up-regulation of the other cytosolic bile acid-binding proteins (GST and 3α-HSD) as well as canalicular bile acid transporters (BSEP and MRP2) that more than compensated for the loss of the primary intrahepatic bile acid-binding protein L-FABP. These factors, along with up-regulation of surface receptors involved in the uptake of exogenous cholesterol (i.e. LDL receptor, less so SRB-1), the primary substrate for bile acid synthesis [27], help to explain the increased levels of biliary bile acids, which in turn enhance the co-transport of cholesterol and appearance of phospholipid in bile. Taken together, these compensatory changes were sufficient not only to prevent reduction of biliary lipid levels, but also to significantly increase bile acid levels in all compartments (serum, liver and gall-bladder) such that total biliary lipids were increased in control-fed L-FABP (−/−) mice. This compensation for loss of the primary bile acid-binding protein (i.e. L-FABP), unlike the CYP7A1 and CYP27A1 mutations/ablations, resulted in a normal whole body and liver phenotype. Finally, the finding of increased CYP7A1 and increased bile acid pool size in L-FABP (−/−) mice was opposite to that observed in mice wherein L-FABP is up-regulated (i.e. SCP-x/SCP-2 (−/−) mice) [9], suggesting that increased level of L-FABP as well as loss of SCP-x and SCP-2 together may contribute to the markedly reduced bile acid pool size in the latter mice. Taken together, these results show that genetic ablation of an intracellular bile acid-binding/transport protein (i.e. L-FABP) elicits a unique bile acid and biliary lipid phenotype as compared with that of genetic mutations in key enzymes of bile acid synthesis.
Secondly, feeding a high-cholesterol diet reduced the bile acid levels substantially in liver, serum and gall-bladder of L-FABP (−/−) male mice, whereas cholesterol increased proportionally in the gall-bladder. As a result, the cholesterol saturation index was significantly increased in cholesterol-fed L-FABP (−/−) mice. Elevated cholesterol saturation index may result in the formation of biliary crystals and gallstones [25]. In contrast, bile acid pool size and cholesterol saturation index in cholesterol-fed wild-type L-FABP (+/+) male mice described herein and elsewhere [23] are basically unaltered by dietary cholesterol. Thus, in the cholesterol-replete state, the loss of the major cytosolic bile acid-binding protein (i.e. L-FABP) was not compensated by up-regulation of other bile acid-binding proteins (e.g. GST and 3α-HSD). On the contrary, nearly all other key enzymes/proteins involved in bile acid synthesis and secretion were decreased, while positive nuclear regulators were decreased and negative nuclear regulators were increased. The latter effects may be mediated through increased availability of unbound bile acids that up-regulate the expression of the negative regulatory pathway of bile acid synthesis (i.e. FXR/SHP), which in turn down-regulates LXRα and CYP7A1 production (reviewed in [2]). For example, L-FABP binds bile acids and thus may compete with FXR for these ligands. Although L-FABP is primarily cytosolic, low levels are detectable in the nucleus [44]. In the cholesterol-replete (cholesterol-fed) dietary state in wild-type L-FABP (+/+) mice, the L-FABP may compete efficiently with FXR for the bile acid ligand. In contrast, in the cholesterol-replete state, the L-FABP (−/−) mice are deficient in L-FABP and, in the absence of concomitant up-regulation of other cytosolic bile acid-binding/transport proteins, the excess unbound bile acids are available for interaction with FXR and consequent down-regulation of CYP7A1 and other aspects of bile acid production. This possibility is based on the finding that, depending on the molar ratio of L-FABP/ligand, L-FABP either inhibits or facilitates presentation of another bound ligand [45,46]. Finally, it should be noted that the decreased bile acid pool size and increased hepatic cholesterol in cholesterol-fed male L-FABP (−/−) mice differed significantly from that of mice wherein a key enzyme (e.g. CYP7A1) of bile acid synthesis was ablated; both biliary bile acid and cholesterol content were decreased regardless of whether the mice were fed on a low- or high-cholesterol diet [23,47].
Taken together, these results show for the first time that, especially in the presence of high dietary cholesterol, L-FABP may be an important determinant regulating bile acid synthesis, biliary cholesterol saturation, and hepatic cholesterol accumulation in male mice. Interestingly, as shown herein and earlier, male wild-type L-FABP (+/+) mice are much more resistant than their female wild-type L-FABP (+/+) littermates to the effects of dietary branched-chain lipids such as cholesterol [2,19,23]. This has been attributed in part to the fact that male and female mice normally differ markedly in the activities of key bile acid synthetic enzymes (SCP-x, CYP7A1 and CYP27A1) [12,23]. Although L-FABP gene ablation confers sensitivity to dietary branched-chain lipids such as cholesterol in male mice, it would be interesting in studies beyond the scope of the present study to determine the sensitivity of the biliary phenotype in female L-FABP (−/−) mice to the dietary branched-chain lipid cholesterol. In conclusion, although it was previously known that several cytosolic proteins can bind bile acids (L-FABP, GST and 3α-HSD), the physiological significance of these observations had not previously been demonstrated (reviewed in [3,5]). Herein, L-FABP gene-ablated mice were used to show that at least one of these intracellular bile acid-binding/transport proteins, L-FABP, may be an important contributor to determining biliary phenotype and hepatic cholesterol phenotype in male mice, with specific effects exerted being highly dependent on the level of dietary cholesterol. The phenotype of male L-FABP (−/−) mice, missing the major bile acid-binding protein in hepatic cytosol, was unique as compared with that of other gene-targeted mice wherein individual enzymes of bile acid synthesis were ablated (reviewed in [2]).
Acknowledgments
This work was supported in part by the U.S. Public Health Service National Institutes of Health DK41402 and GM31651.
References
- 1.Carey M. C., Small D. M., Bliss C. M. Lipid digestion and absorption. Annu. Rev. Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- 2.Russell D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 3.Meier P. J., Stieger B. Bile acid transporters. Annu. Rev. Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 4.Nervi F., Marinovic I., Rigotti A., Ulloa N. Regulation of biliary cholesterol secretion. J. Clin. Invest. 1988;82:1818–1825. doi: 10.1172/JCI113797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agellon L. B., Torchia E. C. Intracellular transport of bile acids. Biochim. Biophys. Acta. 2000;1486:198–209. doi: 10.1016/s1388-1981(00)00057-3. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich A., Dieminger W., Fuchte K., Stoll G. H., Schlitz E., Gerok W., Kurz G. Functional significance of interaction of hepatic FABP with sulfated and nonsulfated taurine-conjugated bile salts in rat liver. J. Lipid Res. 1995;36:1745–1755. [PubMed] [Google Scholar]
- 7.McArthur M. J., Atshaves B. P., Frolov A., Foxworth W. D., Kier A. B., Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 8.Takikawa H., Kaplowitz N. Binding of bile acids, oleic acid, and organic anions by rat and human hepatic Z protein. Arch. Biochem. Biophys. 1986;251:385–392. doi: 10.1016/0003-9861(86)90086-x. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs M., Hafer A., Muench C., Kannenberg F., Teichmann S., Scheibner J., Stange E. F., Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J. Biol. Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- 10.Kannenberg F., Ellinghaus P., Assmann G., Seedorf U. Aberrant oxidation of the cholesterol side chain in bile acid synthesis of sterol carrier protein-2/sterol carrier protein-x knockout mice. J. Biol. Chem. 1999;274:35455–35460. doi: 10.1074/jbc.274.50.35455. [DOI] [PubMed] [Google Scholar]
- 11.Seedorf U., Raabe M., Ellinghaus P., Kannenberg F., Fobker M., Engel T., Denis S., Wouters F., Wirtz K. W. A., Wanders R. J. A., et al. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atshaves B. P., Payne H. R., McIntosh A. L., Tichy S. E., Russell D., Kier A. B., Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J. Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Wanders R. J., Denis S., van Berkel E., Wouters F., Wirtz K. W. A., Seedorf U. Identification of the newly discovered 58 kDa peroxisomal thiolase SCP-x as the main thiolase involved in both pristanic acid and trihydroyxycholestanoic acid oxidation: implications for peroxisomal beta-oxidation disorders. J. Inherit. Metab. Dis. 1998;21:302–305. doi: 10.1023/a:1005349028853. [DOI] [PubMed] [Google Scholar]
- 14.Kawata S., Imai Y., Inada M., Inui M., Kakimoto H., Fukuda K., Maeda Y., Tarui S. Modulation of cholesterol 7α hydroxylase activity by nsLTP in human liver – possible altered regulation of its cytosolic level in patients with gallstones. Clin. Chim. Acta. 1991;197:201–208. doi: 10.1016/0009-8981(91)90140-8. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs M., Lammert F., Wang D. Q. H., Paigen B., Carey M. C., Cohen D. E. Sterol carrier protein-2 participates in hypersecretion of biliary cholesterol during cholesterol gallstone formation in genetically gallstone susceptible mice. Biochem. J. 1998;336:33–37. doi: 10.1042/bj3360033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atshaves B. P., McIntosh A. L., Payne H. R., Mackie J., Kier A. B., Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am. J. Physiol. 2005;288:C543–C558. doi: 10.1152/ajpcell.00359.2004. [DOI] [PubMed] [Google Scholar]
- 17.Martin G. G., Huang H., Atshaves B. P., Binas B., Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochemistry. 2003;42:11520–11532. doi: 10.1021/bi0346749. [DOI] [PubMed] [Google Scholar]
- 18.Atshaves B. P., McIntosh A. L., Lyuksyutova O. I., Zipfel W. R., Webb W. W., Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J. Biol. Chem. 2004;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- 19.Turley S. D., Schwarz M., Spady D. K., Dietschy J. M. Gender related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low and high cholesterol diets. Hepatology. 1998;28:1088–1094. doi: 10.1002/hep.510280425. [DOI] [PubMed] [Google Scholar]
- 20.Atshaves B. P., Petrescu A., Starodub O., Roths J., Kier A. B., Schroeder F. Expression and intracellular processing of the 58 kDa sterol carrier protein 2/3-oxoacylCoA thiolase in transfected mouse L-cell fibroblasts. J. Lipid Res. 1999;40:610–622. [PubMed] [Google Scholar]
- 21.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Martin G. G., Danneberg H., Kumar L. S., Atshaves B. P., Erol E., Bader M., Schroeder F., Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J. Biol. Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz M., Russell D. W., Dietschy J. M., Turley S. D. Alternate pathways of bile acid synthesis in the cholesterol 7 alpha hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J. Lipid Res. 2001;42:1594–1603. [PubMed] [Google Scholar]
- 24.Lammert F., Wang D. Q. H., Hillebrandt S., Geier A., Fickert P., Trauner M., Matern S., Paigen B., Carey M. C. Spontaneous cholecysto- and hepatolithiasis in Mdr2−/− mice: a model for low phospholipid-associated cholelithiasis. Hepatology. 2004;39:117–128. doi: 10.1002/hep.20022. [DOI] [PubMed] [Google Scholar]
- 25.Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J. Lipid Res. 1978;19:945–955. [PubMed] [Google Scholar]
- 26.Puglielli L., Rigotti A., Amigo L., Nunez L., Greco A. V., Santos M. J., Nervi F. Modulation on intrahepatic cholesterol trafficking: evidence by in vivo antisense treatment for the involvement of sterol carrier protein-2 in newly synthesized cholesterol transfer into bile. Biochem. J. 1996;317:681–687. doi: 10.1042/bj3170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen D. E. Hepatocellular transport and secretion of biliary lipids. Curr. Opin. Lipidol. 1999;10:295–302. doi: 10.1097/00041433-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder F., Jefferson J. R., Kier A. B., Knittell J., Scallen T. J., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc. Soc. Exp. Biol. Med. 1991;196:235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder F., Frolov A., Schoer J., Gallegos A., Atshaves B. P., Stolowich N. J., Scott A. I., Kier A. B. Intracellular sterol binding proteins: cholesterol transport and membrane domains. In: Chang T. Y., Freeman D. A., editors. Intracellular Cholesterol Trafficking. Boston: Kluwer Academic Publishers; 1998. pp. 213–234. [Google Scholar]
- 30.Hafer A., Katzberg N., Muench C., Scheibner J., Stange E. F., Seedorf U., Fuchs M. Studies with sterol carrier protein-2 (SCP-2) gene knockout mice identify liver fatty acid binding protein (FABP1) as intracellular cholesterol transporter contributing to biliary cholesterol hypersecretion and gallstone formation. Gastroenterology. 2000;118(Part 1, Suppl. 2):A926. [Google Scholar]
- 31.Stolowich N. J., Frolov A., Petrescu A. D., Scott A. I., Billheimer J. T., Schroeder F. Holo-sterol carrier protein-2: 13C-NMR investigation of cholesterol and fatty acid binding sites. J. Biol. Chem. 1999;274:35425–35433. doi: 10.1074/jbc.274.50.35425. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder F., Frolov A., Starodub O., Russell W., Atshaves B. P., Petrescu A. D., Huang H., Gallegos A., McIntosh A., Tahotna D., et al. Pro-sterol carrier protein-2: role of the N-terminal presequence in structure, function, and peroxisomal targeting. J. Biol. Chem. 2000;275:25547–25555. doi: 10.1074/jbc.M000431200. [DOI] [PubMed] [Google Scholar]
- 33.Stolowich N. J., Petrescu A. D., Huang H., Martin G., Scott A. I., Schroeder F. Sterol carrier protein-2: structure reveals function. Cell. Mol. Life Sci. 2002;59:193–212. doi: 10.1007/s00018-002-8416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy E. J., Schroeder F. Sterol carrier protein-2 mediated cholesterol esterification in transfected L-cell fibroblasts. Biochim. Biophys. Acta. 1997;1345:283–292. doi: 10.1016/s0005-2760(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 35.Puglielli L., Rigotti A., Greco A. V., Santos M. J., Nervi F. Sterol carrier protein-2 is involved in cholesterol transfer from the endoplasmic reticulum to the plasma membrane in human fibroblasts. J. Biol. Chem. 1995;270:18723–18726. doi: 10.1074/jbc.270.32.18723. [DOI] [PubMed] [Google Scholar]
- 36.Zanlungo S., Amigo L., Mendoza H., Glick J., Rodriguez A., Kozarsky K., Miquel J. F., Rigotti A., Nervi F. Overexpression of sterol carrier protein-2 in mice leads to increased hepatic cholesterol content and enterohepatic circulation of bile acids. Gastroenterology. 2000;118(Part 1, Suppl. 2):A997. doi: 10.1053/gast.2000.20198. [DOI] [PubMed] [Google Scholar]
- 37.Gallegos A. M., Atshaves B. P., Storey S. M., Starodub O., Petrescu A. D., Huang H., McIntosh A., Martin G., Chao H., Kier A. B., et al. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog. Lipid Res. 2001;40:498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 38.Brown M. S., Goldstein J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell (Cambridge, Mass.) 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 39.Osborne T. F. Sterol regulatory element binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000;275:25537–25540. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- 40.Fielding C. J., Fielding P. E. Cellular cholesterol efflux. Biochim. Biophys. Acta. 2001;1533:175–189. doi: 10.1016/s1388-1981(01)00162-7. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder F., Gallegos A. M., Atshaves B. P., McIntosh A., Petrescu A. D., Huang H., Chao H., Yang H., Frolov A., Kier A. B. Recent advances in membrane microdomains: rafts, caveolae and intracellular cholesterol trafficking. Exp. Biol. Med. 2001;226:873–890. doi: 10.1177/153537020122601002. [DOI] [PubMed] [Google Scholar]
- 42.Paz Marzolo M., Rigotti A., Nervi F. Secretion of biliary lipids from the hepatocyte. Hepatology. 1990;12:134S–142S. [PubMed] [Google Scholar]
- 43.Newberry E. P., Xie Y., Kennedy S., Buhman K. K., Luo J., Gross R. W., Davidson N. O. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J. Biol. Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 44.Huang H., Starodub O., McIntosh A., Atshaves B. P., Woldegiorgis G., Kier A. B., Schroeder F. Liver fatty acid binding protein colocalizes with peroxisome proliferator receptor alpha and enhances ligand distribution to nuclei of living cells. Biochemistry. 2004;43:2484–2500. doi: 10.1021/bi0352318. [DOI] [PubMed] [Google Scholar]
- 45.Bhuiyan A. K., Pande S. V. Carnitine palmitoyltransferase activities: effects of serum albumin, ACBP, and FABP. Mol. Cell. Biochem. 1994;139:109–116. doi: 10.1007/BF01081733. [DOI] [PubMed] [Google Scholar]
- 46.Jolly C. A., Wilton D. A., Schroeder F. Microsomal fatty acyl CoA transacylation and hydrolysis: fatty acyl CoA species dependent modulation by liver fatty acyl CoA binding proteins. Biochim. Biophys. Acta. 2000;1483:185–197. doi: 10.1016/s1388-1981(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz M., Russell D. W., Dietschy J. M., Turley S. D. Marked reduction in bile acid synthesis in cholesterol 7 alpha hydroxylase deficient mice does not lead to diminished tissue cholesterol turnover or hypercholesterolemia. J. Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]