Abstract
Cyclo-oxygenases (COXs) catalyse the synthesis of PGH2 (prostaglandin H2), which serves as the common substrate for the production of PGE2, PGD2, PGF2α, prostacyclin (or PGI2) and TXs (thromboxanes). While COX-1 is the major isoform responsible for prostanoid synthesis in healthy tissues, little information is available on the contribution of constitutive COX-2 to the various prostanoid synthetic pathways under non-inflammatory conditions. To evaluate further the role of COX-2 in prostanoid biosynthesis, rats were acutely treated with the selective COX-1 inhibitor SC-560 [5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole] or the selective COX-2 inhibitors MF tricyclic [3-(3,4-difluorophenyl)-4-(4-(methylsulphonyl)phenyl)-2-(5H)-furanone] and DFU [5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2-(5H)-furanone]. Selected tissues were then processed for a complete analysis of their prostanoid content by liquid chromatography MS. Whereas the treatment with SC-560 caused a 60–70% inhibition in the total prostanoid content of most tissues examined, a significant decrease (35–50%) in total prostanoid content following selective COX-2 inhibition was solely detected for kidney and brain tissues. Analysis of the individual prostanoids reveals significant inhibition of 6-oxo-PGF1α, PGE2, PGD2, PGF2α and TXB2 in the kidney and inhibition of all these prostanoids with the exception of PGD2 in the forebrain. These results demonstrate that constitutively expressed COX-2 contributes to the production of prostanoids in kidney and brain for each of the PGE2, PGI2 and TXB2 pathways under non-inflammatory conditions. Approaches to modulate inflammation through specific inhibition of terminal synthases, such as mPGES-1 (microsomal PGE2 synthase-1), thus have the potential to differ from COX-2 inhibitors and non-selective non-steroidal anti-inflammatory drugs with regard to effects on constitutive prostanoid synthesis and on renal function.
Keywords: brain, cyclo-oxygenase-2 inhibitor, cyclo-oxygenase (COX), kidney, prostaglandin, thromboxane
Abbreviations: COX, cyclo-oxygenase; DFU, 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2-(5H)-furanone; ED50, effective dose; LC-MS, liquid chromatography MS; MF tricyclic, 3-(3,4-difluorophenyl)-4-(4-(methylsulphonyl)phenyl)-2-(5H)-furanone; NSAID, non-steroidal anti-inflammatory drug; PG, prostaglandin; mPGES-1, microsomal PGE2 synthase-1; SC-560, 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole; TX, thromboxane
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their effects by inhibiting the enzyme cyclo-oxygenase (COX) [1], which exists as two major isoforms, COX-1 and COX-2. Most, if not all, of the efficacy of NSAIDs as anti-inflammatory agents is due to inhibition of COX-2 [2]. The COX-2 enzyme is generally undetectable or present at low levels in resting state but is induced by various stimuli like bacterial endotoxins, inflammatory cytokines and growth factors, as opposed to COX-1, which is constitutively expressed in most of the tissues [3]. These enzymes catalyse the first step in the conversion of arachidonic acid into prostanoids, including PGs (prostaglandins) (PGE2, PGI2, PGD2 and PGF2α) and TXs (thromboxanes) (TXA2) [4]. Prostanoids are a family of paracrine and autocrine mediators that possess multiple biological functions [5]. For example, PGE2 is implicated in the maintenance of gastrointestinal integrity, in fever generation, in allodynia and hyperpalgaesia, as well as in inflammation [6].
Although it is mainly induced by pro-inflammatory stimuli, COX-2 has been found to be constitutively expressed in some tissues including brain [7], kidney [8], lung [9] and reproductive organs [10]. Particularly, in the central nervous system, COX-2 has been detected predominantly in cortex, hypothalamus, hippocampus, amygdala and spinal cord [7,11]. The role of the constitutive COX-2 in the brain has not been defined, but it is speculated to play a role in nerve transmission, as COX-2 expression is up-regulated by nerve activity [12,13]. Also, COX-2 is induced in the brain after inflammatory stimuli and it is implicated in fever generation and hyperalgaesia [12]. As for the kidney, COX-2 protein expression has been detected in the macula densa and associated cortical thick ascending limb of Henle's loop, in medullary interstitial cells, and to a lower level in the inner medullary collecting duct [8,14,15]. COX-2 is thought to play a role in regulation of renal perfusion, since high-salt diets induce COX-2 expression in renal medulla [14], as well as in salt and water ex-cretion, since low-salt diet or loop diuretics increase COX-2 cortical expression in the macula densa [16,17]. Moreover, selective COX-2 inhibitors and NSAIDs tend to cause acute salt and water retention in healthy subjects [18].
COXs act in concert with terminal synthases for the production of the various prostanoids. For example, mPGES-1 (microsomal PGE2 synthase-1) has been shown to preferentially couple with COX-2, as compared with COX-1, in stably transfected cells [19]. Up-regulation of COX-2 by interleukin-1β in human umbilical vein endothelial cells leads to selective increases in PGE2 and 6-oxo-PGF1α production as compared with TXB2 [20]. These apparent couplings of terminal synthases with COXs could be explained by physical coupling, different kinetic properties of terminal synthases or simply co-ordinate up-regulation of the COX and a specific terminal synthase. In animal models of carrageenan-induced paw oedema and lipopolysaccharide-induced pyresis, the co-induction of mPGES-1 and COX-2 is responsible for the observed elevated PGE2 production in the central nervous system [21,22].
As indicated above, data from in vitro studies raise the possibility that COX-2 could preferably couple with terminal synthases for the production of PGI2 or PGE2. In addition, COX-2 slightly differs from COX-1 in its sensitivity to activation by exogenous arachidonic acid [23], in its response to peroxide tone [24], and in its coupling with different phospholipases [25]. It is thus difficult to estimate relative activities of COX-1 and COX-2 from in vitro or ex vivo assays since the conditions of activation of both isoforms might not reflect the in vivo situation. The aim of the present study was to determine the contribution of COX-2 to the synthesis of prostanoids under conditions where the activity of COX-2 is dependent on physiological conditions for its supply of the arachidonic acid substrate. Our results demonstrate that constitutively expressed COX-2 in rat kidney and forebrain contributes significantly to the production of prostanoids for each of the PG, prostacyclin and TX pathways.
EXPERIMENTAL
Materials
The PG standards (6-oxo-PGF1α, PGE2, PGD2, PGF2α and TXB2) used for LC (liquid chromatography)-MS quantification were purchased as solids from Cayman Chemicals (Ann Arbor, MI, U.S.A.) and were reweighed using a microbalance. Corresponding deuterated PGs and indomethacin were also purchased from Cayman Chemicals. The selective COX inhibitors MF tricyclic [3-(3,4-difluorophenyl)-4-(4-(methylsulphonyl)phenyl)-2-(5H)-furanone] [26], SC-560 [5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole] [27] and DFU [5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2-(5H)-furanone] [28] were synthesized at Merck Frosst. Complete® Protease Inhibitor tablets were purchased from Roche Diagnostics (Mannheim, Germany).
Animal procedures
All animal procedures were approved by the Animal Care Committee at the Merck Frosst Centre for therapeutic research (Kirkland, QC, Canada) and the guidelines established by the Canadian Council on Animal Care were followed. Male Sprague–Dawley rats (Charles River, St. Constant, QC, Canada) weighing 225–275 g were deprived of food but had free access to water for 16 h before administration of vehicle or inhibitors. Compounds were administered orally in a 0.5% methylcellulose vehicle (10 ml/kg) for a period of 4 h. The inhibitor doses were selected in order to obtain potent inhibition of COX-1 with SC-560 (10 mg/kg) [27] or COX-2 with MF tricyclic (5 mg/kg) and DFU (10 mg/kg) [28] but also at an appropriate dose to ensure selectivity towards the other COX isoforms.
Preparation of tissues extracts
Animals were killed by pentobarbital injection (120 mg/kg, intraperitoneal) and tissues were collected as fast as possible, rinsed in PBS buffer containing 10 μM indomethacin and frozen in liquid nitrogen. Samples were kept at −80 °C until analysis. Frozen tissues were pulverized in liquid nitrogen using a mortar and pestle until a fine powder was obtained. This powder was then homogenized in 7–10 vol. of ice-cold PBS (pH 7.4; supplemented with 2 mM dithiothreitol, 10 μM indomethacin and 1×Complete® Protease Inhibitor mixture) using a tissue tearer (Polytron PRO 200). The homogenates were subsequently sonicated on ice for 10–30 s (Cole-Parmer ultrasonic homogenizer, 50% output) before being subjected to centrifugation for 10 min at 1000 g (4 °C). Supernatants were collected for LC-MS analysis, quantification of MF tricyclic concentration by HPLC, as well as protein quantification by a modified Bradford method (Bio-Rad protein assay).
Quantification of prostanoid content in tissue homogenates by LC-MS
6-Oxo-PGF1α (stable breakdown product of PGI2), PGE2, PGD2, PGF2α and TXB2 (stable breakdown product of TXA2) were detected in each sample by LC-MS. Sample aliquots (100 μl) were protein-precipitated by the addition of 150 μl of acetonitrile containing 2 ng/ml deuterated prostanoids that served as internal standards for quantification (d4-PGE2 was used as internal standard for both PGE2 and PGD2). Samples were vortex-mixed and centrifuged at 1200 g for 10 min (4 °C) and the supernatants were transferred to a new 96-well plate. Samples (50 μl) were analysed by LC-MS. Details of the procedure as well as optimized parent mass and fragment mass sensitivity for each PG have been reported previously [21]. For the whole kidney samples in the DFU experiment, prostanoids were concentrated by solid-phase extraction before LC-MS in order to increase the detection limit. Briefly, 0.9 ml aliquots of whole kidney homogenates were protein-precipitated with 1.5 ml of acetonitrile containing deuterated internal standards. Samples were vortex-mixed, centrifuged at 1200 g (4 °C) for 10 min and supernatants (2 ml) were added to 4 vol. of water. The pH was then adjusted to 3–3.5 with dilute HCl and samples were applied on SEP-PAK C18 cartridges (Waters, Milford, MA, U.S.A.) that had been prerinsed with methanol and water. Cartridges were then washed with hexane and prostanoids were eluted with ethyl acetate. The eluate was evaporated under N2 and resuspended in 150 μl of acetonitrile/water (60:40, v/v) before prostanoid quantification by LC-MS.
Measurement of plasma and tissue levels of MF tricyclic
Aliquots of plasma or tissue extracts were protein-precipitated with 1 vol. of acetonitrile, vortex-mixed and centrifuged at 9000 g (4 °C) for 20 min. Supernatants were recovered and injected (100 μl) on to a 4.6 mm×150 mm Zorbax RX-C18 column (Agilent Technologies, Stockport, Cheshire, U.K.) using an Agilent 1100 HPLC system with UV detection at 275 nm. MF tricyclic was eluted at 1 ml/min using a linear gradient from 30 to 50% acetonitrile versus 20 mM ammonium acetate/methanol (90:10, v/v) for 15 min. Quantification was achieved by comparison with a standard curve prepared with vehicle-treated samples to which were added known amounts of MF tricyclic.
Statistical analysis
The data were log-scaled so that underlying assumptions of equal variance and normality were better satisfied and standard one-way ANOVA was performed to compare the different treatment groups. Fisher's LSD (least significant difference) method was used as post-hoc analysis, and results were considered statistically significant at the P<0.05 level. All data were back-transformed to original scale and are expressed as geometric means±S.E.M., which was determined by analysis of the variance of all treatment groups.
RESULTS
Prostanoid levels in brain and kidney are selectively affected by COX-2 inhibitors
In order to evaluate the relative importance of each COX isoform on the basal generation of prostanoids, Sprague–Dawley rats were administered SC-560 (selective COX-1 inhibitor), MF tricyclic (selective COX-2 inhibitor) or vehicle for 4 h. The different tissues were then collected and the major prostanoids were quantified by LC-MS. Table 1 summarizes the data for the total amount of prostanoids measured in each tissue of vehicle-treated animals. Vena cava and lung tissues contained the highest level of total prostanoid content, while liver and kidney showed the lowest amounts. Furthermore, analysis of prostanoid profiles revealed differences among tissues. 6-Oxo-PGF1α was the main prostanoid found in lung, lymph nodes, bladder and aorta. In the ileum, PGD2 and 6-oxo-PGF1α were the major products; the kidney contained predominantly PGF2α and, in the forebrain and liver, the main PGs detected were PGF2α and PGD2. As for vena cava, the prostanoids were almost evenly distributed among 6-oxo-PGF1α, PGD2 and TXB2.
Table 1. Relative abundance of each prostanoid measured in different rat tissues.
Total prostanoid content (6-oxo-PGF1α+PGE2+PGD2+PGF2α+TXB2) in various tissues of vehicle-treated animals (n=8–10) was quantified by LC-MS. Values are expressed as percentage of each prostanoid compared with the total concentration of measured prostanoids. nd, not detected.
| Tissue | Total prostanoid (ng/mg of protien) | 6-Oxo-PGF1α (%) | PGE2 (%) | PGD2 (%) | PGF2α (%) | TXB2 (%) |
|---|---|---|---|---|---|---|
| Vena cava | 2.17±0.41 | 38 | 8.4 | 30 | nd | 24 |
| Lung | 2.16±0.30 | 75 | 2.4 | 4.6 | 5.8 | 12 |
| Lymph nodes | 1.58±0.26 | 58 | 7.0 | 17 | 6.7 | 11 |
| Ileum | 1.51±0.26 | 30 | 15 | 36 | 7.7 | 11 |
| Bladder | 1.18±0.15 | 59 | 7.5 | 8.3 | 21 | 4.8 |
| Aorta | 0.86±0.16 | 58 | 3.3 | 13 | nd | 26 |
| Forebrain | 0.83±0.07 | 14 | 7.5 | 42 | 37 | nd |
| Liver | 0.21±0.02 | 3.5 | 18 | 43 | 30 | 6.7 |
| Kidney | 0.20±0.02 | 18 | 14 | 11 | 49 | 8.6 |
Figure 1 shows that treatment of the animals with the selective COX-1 inhibitor SC-560 (10 mg/kg) resulted in a significant decrease in total prostanoid levels in ileum, lymph nodes, bladder, aorta and kidney (60–75% inhibition) and a more moderate inhibition in the lung (36%). On the other hand, selective COX-2 inhibition with MF tricyclic (5 mg/kg) did not significantly affect total prostanoid content in any of these tissues with the exception of kidney where a 49% inhibition was observed (Figure 1). In this experiment, MF tricyclic was administered at a dose 2–5-fold higher than the ED50 (effective dose) for anti-inflammatory effects in COX-2-dependent models (ED50 of 1 mg/kg in the rat model of adjuvant-induced arthritis and 2.8 mg/kg in the carrageenan-paw oedema assay; C.-C. Chan, personal communication). In addition, the plasma concentration of MF tricyclic (3.1 μM) was in the range of efficacious doses [26] and was sufficient to inhibit COX-2 (IC50=0.5 μM), but lower than that causing inhibition of COX-1 (IC50≥15 μM) as determined by human whole blood assays in vitro [29] or using a cell assay with CHO (Chinese-hamster ovary) cells transfected with rat COX-1 (IC50>50 μM; results not shown). Furthermore, tissue concentrations of MF tricyclic were also measured (Table 2), which indicate that MF tricyclic has a very good distribution in all tissues examined.
Figure 1. Effect of treatment with the selective COX-1 inhibitor SC-560 or the selective COX-2 inhibitor MF tricyclic on total prostanoid content of various rat tissues.
Animals were administered vehicle (0.5% methylcellulose), SC-560 (10 mg/kg) or MF tricyclic (5 mg/kg) for 4 h and levels of total prostanoids (6-oxo-PGF1α+PGE2+PGD2+PGF2α+TXB2) were determined for the indicated tissues. Error bars represent S.E.M. for n=8–10 animals, and significantly different levels compared with matching vehicles are depicted above the corresponding bars as follows: *P<0.05, **P<0.001.
Table 2. Tissue concentrations of MF tricyclic.
Tissue concentrations of MF tricyclic were determined by HPLC as described in the Experimental section. MF tricyclic was administered at 5 mg/kg and tissues were collected 4 h post-dose. Molar concentrations were calculated by approximating tissue density to 1 mg/ml. Values are means±S.E.M. (n=5–10).
| Tissue | MF tricyclic concentration (μM) |
|---|---|
| Lung | 7.8±1.1 |
| Ileum | 5.5±0.7 |
| Lymph nodes | 2.3±0.2 |
| Bladder | 2.8±0.2 |
| Aorta | 4.1±1.0 |
| Liver | 8.3±0.8 |
| Kidney | 7.8±0.4 |
| Plasma | 3.1±0.3 |
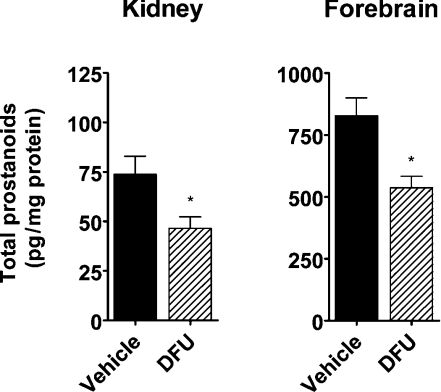
In order to independently confirm the effect of COX-2 inhibition on renal prostanoids, another selective COX-2 inhibitor (DFU) was used (SC-560 was not repeated in this experiment). DFU has been shown to be efficacious as a COX-2 inhibitor in various models of inflammation in the rat at 1 mg/kg and to possess a very high degree of selectivity for COX-2 inhibition [28]. Administration of DFU (10 mg/kg) resulted in a significant 37% inhibition (P<0.05) of total renal prostanoids, confirming the effect observed with MF tricyclic (Figure 2). In this experiment, the forebrain was also collected for analysis. A significant 35% inhibition (P<0.05) of prostanoid content in the forebrain was observed following DFU treatment (Figure 2).
Figure 2. Effect of selective COX-2 inhibition on total prostanoid content of rat kidney and forebrain.
Vehicle (0.5% methylcellulose) or DFU (10 mg/kg) was administered to rats for 4 h and total prostanoid levels were determined (6-oxo-PGF1α+PGE2+PGD2+PGF2α+TXB2) by LC-MS. Error bars represent S.E.M. for n=5–10 animals, and statistical significance is indicated above the corresponding bars as follows: *P<0.05.
In the present experiments, the doses of SC-560 and MF tricyclic were carefully chosen to achieve efficient inhibition of each COX isoform while maintaining selectivity. To evaluate further the extent of COX inhibition caused by these inhibitors, an additional experiment was performed to compare the effect of the combined administration of SC-560 and MF tricyclic with that of the non-selective indomethacin (5 mg/kg). Total prostanoid content was measured 4 h post-administration and found to be strongly inhibited by the combination of inhibitors in the aorta, brain and kidney (Table 3). The extent of inhibition (73–83%) with the combination was similar to that of indomethacin for the aorta and brain, and slightly lower than indomethacin for the kidney (71 versus 86%). In the lung, the combination was ineffective whereas indomethacin caused inhibition comparable with that measured in the other tissues (77%). These data indicate that efficient inhibition of total COX activity can be achieved in aorta, brain and kidney tissues, but not in the lung where SC-560 and MF tricyclic, administered alone or in combination, have slight or no effects on tissue prostanoid content.
Table 3. Inhibition of total prostanoid content in selected rat tissues by co-administration of selective COX inhibitors (SC-560+MF tricyclic) and indomethacin.
Animals were administered vehicle (0.5% methylocellulose), SC-560 (10 mg/kg)+MF tricyclic (5 mg/kg) or indomethacin (5 mg/kg) for 4 h and levels of total prostanoids (6-oxo-PGF1α+PGE2+PGD2+PGF2α+TXB2) were determined for the indicated tissues. Data are reported as percentage of inhibition±S.E.M. compared with vehicle-treated animals (n=8).
| Inhibition (%) | ||
|---|---|---|
| SC-560+MF tricyclic | Indomethacin | |
| Aorta | 73±7* | 84±4* |
| Forebrain | 83±3* | 76±4* |
| Lung | −13±29 | 77±6* |
| Kidney | 71±7* | 86±3* |
*Significantly different (P<0.05) from vehicle.
Tissue processing can stimulate ex vivo prostanoid production
In all studies on the measurement of prostanoid levels, the tissues were flash-frozen in the presence of indomethacin to minimize the possibility of prostanoid production after tissue collection. Tissue dissection and preparation of subfractions were found to result in stimulation of prostanoid release and did not permit to enrich for fractions that might have been more sensitive to COX-2 inhibition. For example, dissection of one of the kidneys from a vehicle-treated group into cortex and medulla revealed that the total prostanoid content in each of the dissected tissues was higher than that observed in the whole kidney (Table 4). The observed increase, once normalized for mass, corresponded to a 6.5-fold induction of total prostanoid content in dissected cortex and medulla compared with whole kidney homogenates. Interestingly, the observed increase was larger for PGE2 and 6-oxo-PGF1α as compared with the other prostanoids. Presumably, activation of phospholipases following tissue damage can stimulate arachidonic acid release and COX activity, leading to an increased prostanoid production in fractionated kidney extracts.
Table 4. Increased prostanoid content in dissected kidney cortex and medulla as compared with whole kidney extracts.
Results are means±S.E.M. for n=5. nd, not detected.
| Whole kidney (pg/mg of tissue) | Cortex (pg/mg of tissue) | Medulla (pg/mg of tissue) | Fold induction* | |
|---|---|---|---|---|
| 6-Oxo-PGF1α | 0.7±0.2 | 3.2±1.0† | 5.2±1.7† | 13 |
| PGE2 | 0.9±0.3 | 5.1±1.4† | 7.2±2.0† | 13 |
| PGD2 | 0.6±0.1 | 2.2±0.4† | 0.6±0.1 | 5.0 |
| PGF2α | 1.7±0.3 | nd | 2.1±0.4 | 1.2 |
| TXB2 | 0.21±0.07 | 1.1±0.4† | 0.4±0.1 | 7.5 |
| Total prostanoids | 4.4±1.0 | 12±2.7† | 16±3.5† | 6.5 |
*Fold induction of cortex+medulla over whole kidney homogenates. The value for (cortex+medulla) is obtained by normalizing the detected prostanoid concentrations for mass (cortex × percentage mass of total kidney)+(medulla×percentage mass of total kidney).
†Statistically different (P<0.05) from corresponding values of whole kidney level.
Profile of prostanoid content in kidney and brain following selective COX inhibition
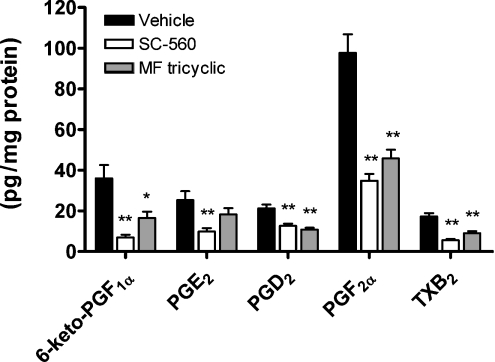
To evaluate the individual importance of COX-1 and COX-2 in the different prostanoid pathways, the effect of selective COX inhibitors on the prostanoid profile was examined. Interestingly, in the kidney, a significant decrease in all individual prostanoids was observed following administration of either SC-560 or MF tricyclic (Figure 3). The percentage inhibition caused by SC-560 was 81% for 6-oxo-PGF1α, 61% for PGE2, 41% for PGD2, 64% for PGF2α and 68% for TXB2. The inhibition caused by MF tricyclic was 54% for 6-oxo-PGF1α, 28% for PGE2, 49% for PGD2, 53% for PGF2α and 47% for TXB2. Statistical significance was obtained for every prostanoid, except PGE2. However, in two other independent experiments, PGE2 levels were significantly inhibited in the kidney following COX-2 inhibition (60% with MF tricyclic, P<0.001 and 44% with DFU, P<0.05; results not shown).
Figure 3. Profile of prostanoid content in rat kidney after selective COX-1 and COX-2 inhibition.
Rats were treated for 4 h with vehicle (0.5% methylocellulose), the selective COX-1 inhibitor SC-560 (10 mg/kg) or the selective COX-2 inhibitor MF tricyclic (5 mg/kg). Kidneys were collected and analysed for prostanoid content. Error bars represent S.E.M. for n=10 animals, and statistically significant differences from matching vehicles are indicated above the corresponding bars as follows: *P<0.05, **P<0.001.
In the forebrain, selective COX-2 inhibition caused a significant decrease in all individual prostanoid levels, with the exception of PGD2 (Figure 4). The inhibition caused by DFU was 41% for 6-oxo-PGF1α, 29% for PGE2, 17% for PGD2 (not significant) and 59% for PGF2α. In summary, these data demonstrate that the inhibition of constitutively expressed COX-2 results in decreased levels of several major prostanoids of kidney and brain, including those of the major PGI2 and PGE2 pathways.
Figure 4. Effect of selective COX-2 inhibition on individual prostanoid levels in rat forebrain.
Rats were treated for 4 h with vehicle (0.5% methylcellulose) or the selective COX-2 inhibitor DFU (10 mg/kg). The forebrain was collected and analysed for prostanoid content. Error bars represent S.E.M. for n=10 animals, and statistically significant differences from matching vehicles are indicated above the corresponding bars as follows: *P<0.05, **P<0.001. nd, not detected.
DISCUSSION
The present study focused on the role of constitutive COX-2 in the production of PGs and TX. By analysing the effects of selective COX-2 inhibitors on tissue prostanoid levels in rats, we show that constitutive COX-2 is catalytically active in kidney and forebrain under non-inflammatory conditions. No significant contribution of COX-2 to prostanoid synthesis in any other tissue examined was detected using whole tissue extracts. Moreover, the results of the present study suggest that COX-2 can be limiting for the constitutive synthesis of each of the PGE2, PGI2 and TX pathways in kidney and forebrain.
Production of prostanoids during processing of tissues
By measuring the tissue levels rather than the ex vivo or in vitro production of prostanoids, our objective was to obtain a more accurate estimation of COX-2-mediated prostanoid production under conditions where enzyme activity is dependent on endogenous release of the arachidonic acid substrate. To minimize post-harvesting production of prostanoids, especially in tissues where COX-2 is expressed at low levels compared with COX-1, tissues were harvested rapidly, flash-frozen, ground using liquid nitrogen and homogenized in a buffer containing indomethacin. The results obtained from the dissection of the kidney indicate that levels of prostanoids can be induced easily by tissue manipulation and that special care needs to be taken in order to measure levels that accurately reflect the basal levels in vivo. Although it is virtually impossible to ascertain that the measured levels correspond to the in vivo levels and that the extent of inhibition might be underestimated, the contribution of constitutive COX-2 to the production of prostanoids in kidney and brain, as well as the particular sensitivity of these tissues to selective COX-2 inhibition, was demonstrated in the present experiments.
Relative contribution of COX-1 and COX-2 to prostanoid synthesis
The results obtained with the selective inhibitor SC-560 confirmed the predominant role of COX-1 in the synthesis of prostanoids in most tissues [12]. Inhibition of COX-2 with the selective inhibitors did not affect the prostanoid content in any of the selected tissues, with the exception of brain and kidney.
The reduction of renal prostanoid levels by selective COX-2 inhibition was confirmed with two different inhibitors (DFU and MF tricyclic). These results are in line with previous experiments in which ex vivo brain PGF2α production along with kidney PGE2 and 6-oxo-PGF1α production was found to be slightly inhibited by the selective COX-2 inhibitor NS-398 administered at 3 and 9 mg/kg (28% inhibition of brain PGF2α, P<0.05, and a non-significant 20% inhibition for kidney PGE2 and 6-oxo-PGF1α) [30]. However, the results on the inhibition of renal COX-2 are in contrast with those observed in kidney extracts using added arachidonic acid for which PGE2 production is COX-1-dependent [31]. The lack of inhibition of prostanoid synthesis following selective COX-2 inhibition in ex vivo or in vitro assays where exogenous arachidonic acid is added, as opposed to the results presented here, suggests that the availability of arachidonic acid might be more limiting for the activity of COX-1 as compared with COX-2 in the kidney under basal conditions.
The lung constitutively expresses both COX-1 and COX-2; however, this tissue was found to be rather insensitive to inhibition by SC-560 and MF tricyclic (a low level of inhibition was detected with SC-560 and was not confirmed in the combination experiment). A complication for analysis of the lung tissue is that euthanasia (by barbiturates and other methods) has been shown to affect the pulmonary vasculature [32], which could result in a stimulation of prostacyclin production (6-oxo-PGF1α was the major product detected in lung extracts), therefore masking the inhibitory effects of the selective inhibitors. In this case, the very slow off-rate of indomethacin from both forms of COXs [33] might have contributed to the maintenance of enzyme inhibition, which could explain the higher level of inhibition compared with that of SC-560, which is more rapidly reversible [34]. It is also possible that the inhibitors used do not reach the site of active COX; more work will be required to define the contribution of COX-1 and COX-2 to prostanoid synthesis in the lung.
Kidney and brain prostanoid profiles
In the kidney and forebrain, COX-2 inhibition caused a decrease in the concentration of all the individual prostanoids measured, except brain PGD2. This suggests that COX-2 can be limiting for the synthesis of a fraction of the prostanoids in these tissues. Of the two major PGs of the forebrain, PGF2α was inhibited, while PGD2 was not significantly affected by selective COX-2 inhibition. These results can be interpreted in many ways, one of which could be preferential coupling of COX-1 with PGD2 synthase in this tissue under normal conditions. Alternatively, PGD2 might have a slower turnover than the other prostanoids in the brain and might not have been produced to a significant level during the 4 h treatment period. Conversely, in the kidney, the individual PGs were all inhibited to a similar degree (∼50%); hence the data do not provide any evidence of preferential coupling of COX-2 with a specific terminal synthase pathway at the whole tissue level, although coupling in subcompartments of the kidney [35] cannot be excluded.
It is well known that COX-2 inhibitors and NSAIDs can cause fluid retention and increase blood pressure [36]. The importance of COX-2-derived prostanoids in the regulation of renal physiology is well established [37], but the exact PGs implicated in these phenomena have not been clearly identified. Results obtained from mice lacking the prostacyclin receptor suggest that prostacyclin is the key player for stimulation of renin release in response to renal artery stenosis [38]. However, PGE2 has also been proposed to be important for the stimulation of renin release, as the rate of renin secretion was stimulated by PGE2 in perfused kidneys of mice [39]. This stimulation was less important in kidneys isolated from mice lacking either of the PGE2 receptors EP2 and EP4 [39]. These reports suggest that prostanoids, especially PGI2 and PGE2, can both play a role in regulating renal physiology. Moreover, since COX-2 inhibitors affect both of these prostanoid pathways in the kidney, selective inhibitors of terminal synthases, such as mPGES-1 [40], have the potential to differ from COX-2 inhibitors or NSAIDs with regard to renal side effects.
Acknowledgments
We thank K. Bateman and M. Ouellet (Merck Frosst) for technical advice and support during the LC-MS analysis, D. Normandin, S. Wong and the Comparative Medicine Department staff (Merck Frosst) for animal procedures and the staff of the Medicinal Chemistry Department (Merck Frosst) for the synthesis of SC-560, MF tricyclic and DFU. This work was partially supported by fellowships from the Natural Sciences and Engineering Research Council of Canada and from the Department of Biochemistry at the Université de Montréal to P.-O.H.
References
- 1.Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Hinz B., Brune K. Cyclooxygenase-2 – 10 years later. J. Pharmacol. Exp. Ther. 2002;300:367–375. doi: 10.1124/jpet.300.2.367. [DOI] [PubMed] [Google Scholar]
- 3.Smith W. L., Garavito R. M., DeWitt D. L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 4.Smith W. L., DeWitt D. L., Garavito R. M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Funk C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 6.Warner T. D., Mitchell J. A. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 7.Breder C. D., Dewitt D., Kraig R. P. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris R. C., McKanna J. A., Akai Y., Jacobson H. R., Dubois R. N., Breyer M. D. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J. Clin. Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermert L., Ermert M., Goppelt-Struebe M., Walmrath D., Grimminger F., Steudel W., Ghofrani H. A., Homberger C., Duncker H., Seeger W. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am. J. Respir. Cell Mol. Biol. 1998;18:479–488. doi: 10.1165/ajrcmb.18.4.2939. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus M., Eguchi N., Matsumoto S., Nagata N., Yano T., Killian G. J., Urade Y. Species-specific expression of microsomal prostaglandin E synthase-1 and cyclooxygenases in male monkey reproductive organs. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:233–240. doi: 10.1016/j.plefa.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Beiche F., Scheuerer S., Brune K., Geisslinger G., Goppelt-Struebe M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- 12.Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Yamagata K., Andreasson K. I., Kaufmann W. E., Barnes C. A., Worley P. F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 14.Yang T., Singh I., Pham H., Sun D., Smart A., Schnermann J. B., Briggs J. P. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am. J. Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 15.Nantel F., Meadows E., Denis D., Connolly B., Metters K. M., Giaid A. Immunolocalization of cyclooxygenase-2 in the macula densa of human elderly. FEBS Lett. 1999;457:475–477. doi: 10.1016/s0014-5793(99)01088-1. [DOI] [PubMed] [Google Scholar]
- 16.Catella-Lawson F., McAdam B., Morrison B. W., Kapoor S., Kujubu D., Antes L., Lasseter K. C., Quan H., Gertz B. J., FitzGerald G. A. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J. Pharmacol. Exp. Ther. 1999;289:735–741. [PubMed] [Google Scholar]
- 17.Mann B., Hartner A., Jensen B. L., Kammerl M., Kramer B. K., Kurtz A. Furosemide stimulates macula densa cyclooxygenase-2 expression in rats. Kidney Int. 2001;59:62–68. doi: 10.1046/j.1523-1755.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz J. I., Vandormael K., Malice M. P., Kalyani R. N., Lasseter K. C., Holmes G. B., Gertz B. J., Gottesdiener K. M., Laurenzi M., Redfern K. J., et al. Comparison of rofecoxib, celecoxib, and naproxen on renal function in elderly subjects receiving a normal-salt diet. Clin. Pharmacol. Ther. 2002;72:50–61. doi: 10.1067/mcp.2002.126182. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M., Naraba H., Tanioka T., Semmyo N., Nakatani Y., Kojima F., Ikeda T., Fueki M., Ueno A., Oh S., et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 20.Caughey G. E., Cleland L. G., Penglis P. S., Gamble J. R., James M. J. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J. Immunol. 2001;167:2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 21.Guay J., Bateman K., Gordon R., Mancini J., Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J. Biol. Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- 22.Inoue W., Matsumura K., Yamagata K., Takemiya T., Shiraki T., Kobayashi S. Brain-specific endothelial induction of prostaglandin E2 synthesis enzymes and its temporal relation to fever. Neurosci. Res. 2002;44:51–61. doi: 10.1016/s0168-0102(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 23.Swinney D. C., Mak A. Y., Barnett J., Ramesha C. S. Differential allosteric regulation of prostaglandin H synthase 1 and 2 by arachidonic acid. J. Biol. Chem. 1997;272:12393–12398. doi: 10.1074/jbc.272.19.12393. [DOI] [PubMed] [Google Scholar]
- 24.Chen W., Pawelek T. R., Kulmacz R. J. Hydroperoxide dependence and cooperative cyclooxygenase kinetics in prostaglandin H synthase-1 and -2. J. Biol. Chem. 1999;274:20301–20306. doi: 10.1074/jbc.274.29.20301. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M., Kambe T., Shimbara S., Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 26.Oshima M., Dinchuk J. E., Kargman S. L., Oshima H., Hancock B., Kwong E., Trzaskos J. M., Evans J. F., Taketo M. M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell (Cambridge, Mass.) 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 27.Smith C. J., Zhang Y., Koboldt C. M., Muhammad J., Zweifel B. S., Shaffer A., Talley J. J., Masferrer J. L., Seibert K., Isakson P. C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riendeau D., Percival M. D., Boyce S., Brideau C., Charleson S., Cromlish W., Ethier D., Evans J., Falgueyret J. P., Ford-Hutchinson A. W., et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br. J. Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riendeau D., Salem M., Styhler A., Ouellet M., Mancini J. A., Li C. S. Evaluation of loxoprofen and its alcohol metabolites for potency and selectivity of inhibition of cyclooxygenase-2. Bioorg. Med. Chem. Lett. 2004;14:1201–1203. doi: 10.1016/j.bmcl.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 30.Tegeder I., Neupert W., Guhring H., Geisslinger G. Effects of selective and unselective cyclooxygenase inhibitors on prostanoid release from various rat organs. J. Pharmacol. Exp. Ther. 2000;292:1161–1168. [PubMed] [Google Scholar]
- 31.Chan C. C., Boyce S., Brideau C., Charleson S., Cromlish W., Ethier D., Evans J., Ford-Hutchinson A. W., Forrest M. J., Gauthier J. Y., et al. Rofecoxib [Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J. Pharmacol. Exp. Ther. 1999;290:551–560. [PubMed] [Google Scholar]
- 32.Feldman D. B., Gupta B. N. Histopathologic changes in laboratory animals resulting from various methods of euthanasia. Lab. Anim. Sci. 1976;26:218–221. [PubMed] [Google Scholar]
- 33.Kargman S., Wong E., Greig G. M., Falgueyret J. P., Cromlish W., Ethier D., Yergey J. A., Riendeau D., Evans J. F., Kennedy B., et al. Mechanism of selective inhibition of human prostaglandin G/H synthase-1 and -2 in intact cells. Biochem. Pharmacol. 1996;52:1113–1125. doi: 10.1016/0006-2952(96)00462-5. [DOI] [PubMed] [Google Scholar]
- 34.Walker M. C., Kurumbail R. G., Kiefer J. R., Moreland K. T., Koboldt C. M., Isakson P. C., Seibert K., Gierse J. K. A three-step kinetic mechanism for selective inhibition of cyclo-oxygenase-2 by diarylheterocyclic inhibitors. Biochem. J. 2001;357:709–718. doi: 10.1042/0264-6021:3570709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider A., Zhang Y., Zhang M., Lu W. J., Rao R., Fan X., Redha R., Davis L., Breyer R. M., Harris R., et al. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int. 2004;65:1205–1213. doi: 10.1111/j.1523-1755.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 36.Cheng H. F., Harris R. C. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- 37.Harris R. C., Zhang M. Z., Cheng H. F. Cyclooxygenase-2 and the renal renin-angiotensin system. Acta Physiol. Scand. 2004;181:543–547. doi: 10.1111/j.1365-201X.2004.01329.x. [DOI] [PubMed] [Google Scholar]
- 38.Fujino T., Nakagawa N., Yuhki K., Hara A., Yamada T., Takayama K., Kuriyama S., Hosoki Y., Takahata O., Taniguchi T., et al. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J. Clin. Invest. 2004;114:805–812. doi: 10.1172/JCI21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweda F., Klar J., Narumiya S., Nusing R. M., Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am. J. Physiol. Renal Physiol. 2004;287:F427–F433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 40.Uematsu S., Matsumoto M., Takeda K., Akira S. Lipopolysaccharide-dependent prostaglandin E2 production is regulated by the glutathione-dependent prostaglandin E2 synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J. Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]