Abstract
The A2A adenosine receptor (A2AAR) mediates anti-inflammatory actions of adenosine in a variety of cell types. LPS (lipopolysaccharide) was reported to induce a small (<2-fold) increase in the expression of A2AAR mRNA in human monocytes and monocytic cell lines. We investigated the effects of LPS on the expression of adenosine receptor mRNAs in primary mouse IPMΦ (intraperitoneal macrophages), human macrophages and Wehi-3 cells. Treatment with 10 ng/ml LPS for 4 h produced a >100-fold increase in A2AAR mRNA. LPS-induced increases in mRNA for A2AAR and TNFα (tumour necrosis factor α) are reduced by 90% in IPMΦ pretreated with the NF-κB (nuclear factor κB) inhibitor, BAY 11-7082 {(E)3-[(4-methylphenyl)sulphonyl]-2-propenenitrile; 10 μM}. In Wehi-3 cells exposed to LPS, A2AAR and A2BAR transcripts are elevated by 290- and 10-fold respectively, the A1AR transcript is unchanged and the A3AR transcript is decreased by 67%. The induction of A2AAR mRNA by LPS is detectable after 1 h, reaches a peak at 6 h at 600 times control and remains elevated beyond 24 h. The ED50 (effective dose) of LPS is 2.3 ng/ml. A2AAR receptor number, measured by 125I-ZM241385 binding to whole cells, is undetectable in naïve cells and increases linearly at a rate of 23 receptors·cell−1·min−1 to a Bmax of 348 fmol/mg (28000 receptors/cell) in 20 h. The increase in receptor number is correlated with an increase in the potency of an A2A agonist (4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester; referred to as ATL146e) to stimulate cAMP in these cells. After LPS pretreatment, the potency of the A2A agonist, ATL146e, to reduce TNFα release from IPMΦ was increased by 200-fold. The results support the hypothesis that regulation of adenosine receptor expression, especially up-regulation of the A2AAR, is part of a delayed feedback mechanism initiated through NF-κB to terminate the activation of human and mouse macrophages.
Keywords: adenosine receptor, ATL146e, inflammation, lipopolysaccharide, macrophage, tumour necrosis factor α (TNFα)
Abbreviations: AR, adenosine receptor; ADA, adenosine deaminase; ATL146e, 4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester; BAY 11-7082, (E)3-[(4-methylphenyl)sulphonyl]-2-propenenitrile; EIA, enzyme immunoassay; FBS, foetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 125I-ZM241385, 4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]-triazin-5-yl amino]ethyl)-3-[125I]iodophenol; IPMΦ, intraperitoneal macrophages; LPS, lipopolysaccharide; NECA, 5′-N-ethylcarboxamidoadenosine; NF-κB, nuclear factor κB; TLR, Toll-like receptor; TNFα, tumour necrosis factor α
INTRODUCTION
Adenosine released from stressed cells can act as a physiological inhibitor of inflammation [1]. Utilization of ATP during periods of high metabolic activity leads to an increased concentration of intracellular adenosine that can be transported across the cell membrane by nucleoside transporters. Adenine nucleotides also are released from stressed cells or necrotic cells or by degranulation of nerves, mast cells or platelets and are dephosphorylated to form adenosine by extracellular nucleotidases [2]. Adenosine acts at the cell surface through four G-protein-coupled adenosine receptors (ARs): A1, A2A, A2B and A3. These receptors each have a unique pharmacological and physiological profile that enables adenosine to stimulate a variety of effects depending on its concentration and the distribution of receptors in a given tissue [3]. The Gs-coupled high-affinity A2AAR mediates many anti-inflammatory actions of adenosine in a variety of cell types, including inhibition of neutrophil [4], monocyte [5], platelet [6], and T-cell activation [7,8]. In animal models, A2AAR agonists can prevent death from bacterial LPS (lipopolysaccharide) or sepsis [9]. The Gs/Gq-coupled low-affinity A2BAR is thought to contribute an anti-inflammatory action of adenosine in macrophages [10–12]. The role of the Gi/o-coupled A1AR and the A3AR in macrophages is not clear.
Bacterial LPS and inflammatory cytokines have been reported to induce a small (<2-fold) increase in the expression of A2AAR mRNA in human monocytic cell lines [13,14]. Maturation of monocytes to macrophages is associated with increased expression and secretion of TNFα (tumour necrosis factor α) in response to inflammatory stimuli [15]. Macrophages pretreated with LPS have been noted to have an exaggerated response to A2AAR agonists [16,17]. In mouse IPMΦ (peritoneal macrophages), LPS was found to elicit a 15-fold increase in mRNA for the A2BAR [18]. Although macrophages provide an important defence against bacterial pathogens, their overactivation can cause damage to inflamed host tissues, and such overactivation may be prevented by adenosine. In order to further examine the regulation of adenosine receptors in macrophages, we have investigated the effects of LPS on the expression of mouse and human adenosine receptor mRNAs and receptor number. LPS causes a very strong induction of A2AAR mRNA in macrophages and corresponding increases in A2AAR density and potency to inhibit macrophage activation.
EXPERIMENTAL
Materials
ZM241385 {4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a]-[1,3,5]triazin-5-yl amino]ethyl)phenol} [19] was purchased from Tocris Cookson (Ellisville, MO, U.S.A.). Carrier-free 125I-ZM241385 was synthesized and HPLC-purified as described in [20,21]. ATL146e (4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester) [22] was a gift from Dr J. Rieger of Adenosine Therapeutics (Charlottesville, VA, U.S.A.). ADA (adenosine deaminase) was purchased from Boehringer Mannheim Biochemicals (Indianapolis, IN, U.S.A.). BAY 11-7082 {(E)3-[(4-methylphenyl)sulphonyl]-2-propenenitrile} was purchased from Calbiochem (San Diego, CA, U.S.A.). Cell-culture media and reagents were purchased from Gibco BRL (Grand Island, NY, U.S.A.). The following reagents were purchased from Sigma (St. Louis, MO, U.S.A.): NECA (5′-N-ethylcarboxamidoadenosine), HCl, PMSF, LPS, leupeptin, pepstatin and aprotinin.
Cell culture
Wehi-3 cells (A.T.C.C.) were grown in suspension at 0.2–1.0×106 cells/ml in Iscove's medium supplemented with 10% (v/v) FBS (foetal bovine serum) and 5 mM 2-mercaptoethanol. Cultures were kept at 37 °C in a humidified incubator with 5% CO2. All experiments used cells below passage 20.
IPMΦ harvest
Balb/c mice injected with 2 ml of 39.8 g/l sterile thioglycolate solution were killed after 7 days. The peritoneal cavity was washed twice with 10 ml of PBS+2 mM EDTA to make a cell suspension containing 3–6×105 cells/ml. After centrifugation at 300 g for 8 min, the cells were resuspended in medium and added to tissue-culture plates. After 3 h, non-adherent cells were removed and the adherent cells were washed with PBS. The resulting macrophage preparations were cultured in Dulbecco's modified Eagle's medium with high glucose, 10% heat-inactivated FBS and penicillin/streptomycin for up to 48 h.
Human monocyte-derived macrophages
Peripheral venous blood was obtained from healthy adult volunteers with informed written consent. The blood was anti-coagulated with heparin (10 units/ml), and the monocytes were enriched with Rosettesep™ according to the manufacturer's instructions. This isolation yielded approx. 5×106 monocytes (>80%)/10 ml of blood. By FACS, the cells had a phenotype of CD14+, CD24− and CD3−. The monocytes (1–2×106 cells/well) were cultured for 3 days in 24-well tissue-culture-treated plates (37 °C and 5% CO2) in RPMI 1640 growth medium containing penicillin/streptomycin, 5% (v/v) autologous serum and 10 ng/ml recombinant human macrophage colony-stimulating factor, resulting in macrophages expressing high non-specific esterase staining, high CD14 and low MHC Class II, consistent with a macrophage phenotype.
Quantitative PCR
Using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, U.S.A.), cDNA was made from 1 μg of RNA, using mixed random and oligo-dT primers, following the manufacturer's instructions. The reaction mixture was then diluted to the equivalent of 5 μg/ml RNA, and 5 μl of the diluted mixture was incorporated into the quantitative-PCR reaction mixture. Quantitative PCR was performed using the Quantitect SYBR® Green PCR kit (Qiagen, Valencia, CA, U.S.A.). A typical reaction contained 25 μl of the kit reaction mixture, 17 μl of molecular biology grade water, 1.5 μl each of 10 μM primer stocks and 5 μl of cDNA or plasmid standard. Standard curves were produced using diluted plasmids with known copy numbers of the gene of interest. Real-time PCR was performed using the iCycler iQ Real-Time PCR Detection System from Bio-Rad using the supplied software. The thermal cycler tracks fluorescence levels over 40 amplification cycles. A melt curve was performed at the end of each run to verify that there was a single amplification product and a lack of primer dimers. All samples were normalized to the amount of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) or β-actin (human cells) mRNAs present in the sample. The relative amount of a given mRNA of interest was determined using the ΔΔCT method [23].
Primers for the real-time PCR were designed from sequences in GenBank® database. The PCR primers were validated by sequencing the reaction products after TA-cloning (Invitrogen, Carlsbad, CA, U.S.A.). The following primers were used (forward, reverse): (i) mouse: A1AR (5′-CCTGAGTGAGGTGGAACAAG-3′, 5′-ACCAGAGGAGGCTGACAC-3′); A2AAR (5′-TGGCTTGGTGACGGGTATG-3′, 5′-CGCAGGTCTTTGTGGAGTTC-3′); A2BAR (5′-CTGGGACACGAGCGAGAG-3′, 5′-CTGGGACACGAGCGAGAG-3′; A3AR (5′-GAGACGGACTGGCTGAACATC-3′, 5′-GAGACGGACTGGCTGAACATC-3′); TNFα (5′-CCTCCCTCTCATCAGTTCTATGG-3, 5′-CGTGGGCTACAGGC TTGTC-3′); and GAPDH (5′-TTCACCACCATGGAGAAGGC-3′, 5′-GGCATGGACTGTGGTCATGA-3′). (ii) Human: A2AR (5′-AGTTCCGCCAGACCTTCC-3′, 5′-ACCTGCTCTCCGTCACTG-3′) and human β-actin (5′-CCCTGGCACCCAGCAC-3′, 5′-GCCGATCCACACGGAGTA-3′).
Radioligand binding
Wehi-3 or IPMΦ cells were resuspended in PBS with 1.5 units/ml ADA at a concentration of 2×106 cells/ml. Aliquots (100 μl) of the cell suspensions were placed into wells of a 96-well Millipore Multiscreen® GF/C filter plate. Various concentrations of the radiolabelled antagonist, 125I-ZM241385 were added in 50 μl of PBS with 2 units/ml ADA. After incubating the assays at 4 °C for 2–3 h, binding reactions were terminated by rapid filtration on a cell harvester (Brandel, Gaithersburg, MD, U.S.A.) followed by 4×150 μl washes for 30 s with ice-cold 10 mM Tris/HCl (pH 7.4) and 10 mM MgCl2. Non-specific binding was measured in the presence of 50 μM NECA.
cAMP assays
Cells were removed from their media, washed twice by centrifugation with PBS and resuspended in PBS supplemented with 2 units/ml ADA at a concentration of 1×106 cells/ml. Aliquots (200 μl) of the cell suspension were added to 75 mm polypropylene tubes with 50 μl of 5× ATL146e in various concentrations. All tubes received 50 μM rolipram. After incubation for 15 min at 37 °C, the reaction was stopped by the addition of 0.5 ml of 0.15 M HCl. The cells were pelleted by centrifugation and the supernatants were frozen for cAMP analysis by EIA (enzyme immunoassay; Assay Designs, Ann Arbor, MI, U.S.A.).
Measurement of TNFα concentration
IPMΦ were resuspended in PBS supplemented with 2 units/ml ADA at a concentration of 0.5×106 cells/ml. Cells were stimulated to produce TNFα by the addition of 10 ng/ml LPS with various concentrations of ATL146e. After 4 h, the supernatant was removed and assayed for TNFα by ELISA (eBioscience, San Diego, CA, U.S.A.).
RESULTS
LPS rapidly changes adenosine receptor mRNAs in macrophages
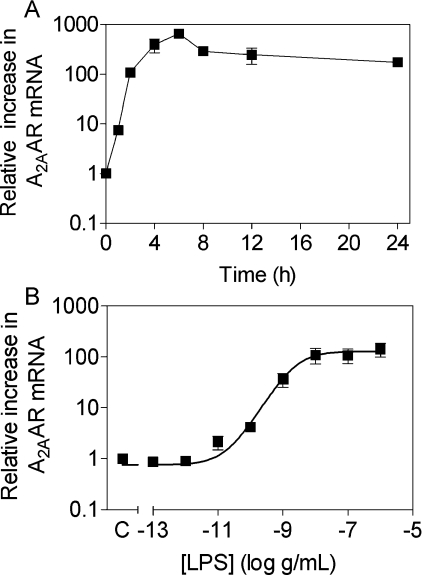
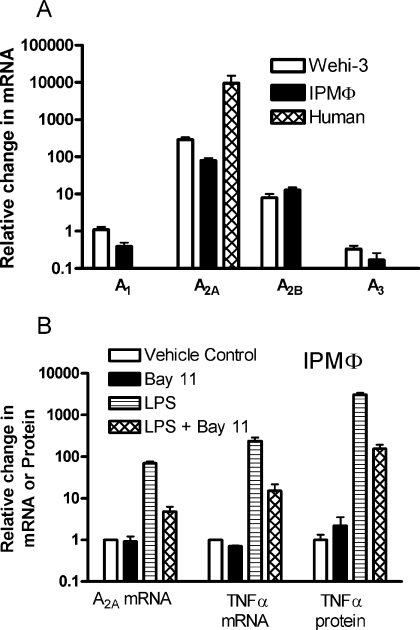
Treatment of Wehi-3 cells, mouse macrophages or human macrophages with LPS evoked rapid and large changes in A2AAR mRNA and smaller changes in mRNAs for the other adenosine receptor subtypes. Figure 1 shows the time-course and dose–response curve for A2AAR mRNA induction in Wehi-3 cells. A2AAR mRNA was increased by over 8-fold within 1 h and reached a peak above 600-fold in 6 h. LPS increased A2AAR mRNA with an ED50 of 2.3 ng/ml, with significant induction noted by 10 pg/ml (Figure 1B). This up-regulation was almost completely inhibited by 30 μg/ml polymyxin B (results not shown). LPS elicited a smaller increase in A2BAR transcript and a small decrease in A3AR transcript in both Wehi-3 cells and mouse IPMΦ (Figure 2A). A1AR mRNA expression did not change from the initial low-level expression in Wehi-3 cells and it was decreased in IPMΦ. In human macrophages, mRNA in unstimulated cells was very low and strongly induced by LPS, resulting in a very high fold stimulation of A2AAR mRNA.
Figure 1. Effect of LPS treatment on expression of A2AAR mRNA in Wehi-3 cells as determined by real-time, quantitative PCR.
(A) Time course of A2AAR expression upon stimulation with 100 ng/ml LPS. (B) Dose–response of A2AAR expression as measured after 4 h of LPS treatment. Transcript levels were normalized to GAPDH levels and all data points are the means±S.E.M. for at least three independent experiments performed in duplicate and quantitative PCR performed in triplicate.
Figure 2. Effects of LPS and BAY 11-7082 on mRNA and protein expression in macrophages.
(A) Fold changes in transcripts following treatment of various cells with 10 ng/ml LPS for 4 h. (B) Effect of pretreating IPMΦ with 10 μM BAY 11-7082 (BAY 11) on the transcription of A2AR, TNFα mRNAs and TNFα protein. Control TNFα levels are 3.6±1.2 pmol/ml. Data are pooled for three to seven experiments.
Role of NF-κB (nuclear factor κB) in A2AAR mRNA induction
NF-κB has been implicated as a mediator of cytokine induction in macrophages. We investigated the effects of the selective NF-κB inhibitor BAY 11-7082 on induction of A2AAR mRNA and, as a positive control, on the induction of TNFα mRNA and protein in IPMΦ. As shown in Figure 2(B), inhibition of NF-κB resulted in a >90% decrease in A2AAR and TNFα mRNAs and TNFα protein.
Increased 125I-ZM241385 binding to LPS-treated Wehi-3 cells
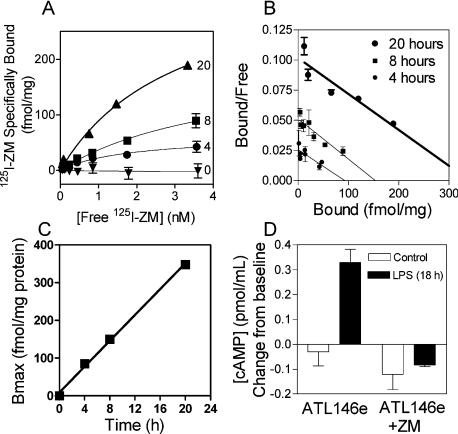
In order to determine if increased A2AAR mRNA levels result in increased A2AAR expression, we measured receptor number in Wehi-3 cells using the specific A2AAR antagonist, 125I-ZM241385. As seen in Figure 3(A), there is no significant specific radioligand binding to vehicle-treated control cells. After treatment with 100 ng/ml LPS, the cells specifically bind 125I-ZM241385 with a Bmax of 85 fmol/mg after 4 h, 150 fmol/mg after 8 h and 348 fmol/mg after 20 h. Figure 3(B) shows Scatchard transformations of these data, indicating binding to a single saturable site that does not change affinity as a result of receptor up-regulation. We determined that 1 μg of protein corresponds to 7500 Wehi-3 cells. On the basis of this calculation, the number of receptors/cell at 20 h is 28000 and the receptor density increases at a linear rate of 23 receptors·cell−1·min−1 for at least 20 h (Figure 3C). In order to determine whether the newly made A2AARs on Wehi-3 cells function normally, we measured cAMP levels in cells pretreated with or without 100 ng/ml LPS. Figure 3(D) shows that control cells did not have a significant cAMP response to the A2A agonist ATL146e. In contrast, cells that had been treated overnight with LPS made significant amounts of cAMP in the presence of 10 nM ATL146e. This effect was inhibited by treatment with 50 nM ZM241385.
Figure 3. Effect of LPS treatment on A2AAR receptor expression and function in Wehi-3 cells.
(A) Saturation binding isotherms for control cells and cells treated for 4–20 h with LPS (n=3; where n is the number of replicates). (B) Scatchard transformations of the data in (A) fitted with linear regressions demonstrating binding to a single site. (C) Change over time in the number of A2AARs (125I-ZM241385 binding sites) after treatment with LPS. The data are fitted to a straight line (coefficient of determination r2=0.998). (D) Change in cAMP (TNFα-treated – basal) in response to A2AAR agonist stimulation. After stimulation with or without 10 nM ATL146e and 50 nM ZM241385 (125I-ZM) (in four combinations shown in D), cells were incubated for 20 min at 37 °C in the presence of 50 μM rolipram. The cAMP concentrations in the supernatant were measured by EIA. The baseline level of cAMP was 0.2 pmol/ml.
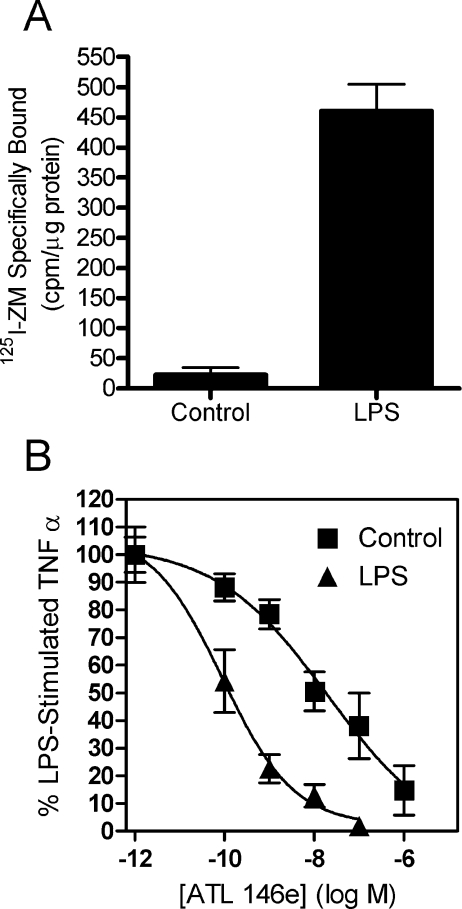
LPS-pretreated IPMΦs are more sensitive to an A2AAR agonist
We also examined the effect of LPS on A2AAR number on IPMΦ. Since LPS has no effect on 125I-ZM241385 affinity, we used a single concentration of radioligand. Figure 4(A) shows that cells that had been pretreated with LPS overnight displayed over 20 times higher radioligand binding than control cells. In order to confirm the functional significance of A2AAR up-regulation, we examined the sensitivity of these cells to A2AAR-mediated inhibition of TNFα release in response to acute LPS treatment. Cells were treated overnight with or without (±) 10 ng/ml LPS, washed with PBS, then rechallenged for 4 h with 10 ng/ml LPS and ATL146e at various doses. As seen in Figure 4(B), the EC50 for ATL146e in LPS-pretreated cells was 0.1 nM, approx. 200-fold more potent than the EC50 in control cells (20 nM).
Figure 4. Effect of LPS treatment on whole-cell radioligand binding and functional potency of ATL146e in IPMΦ.
Macrophages were harvested from Balb/c mice as described in the Experimental section. (A) Cells treated with or without 10 ng/ml LPS for 20 h were removed from the tissue-culture plates with PBS +10 mM EDTA and gentle scraping. After washing in PBS, cells were resuspended and radioligand binding was performed with approx. 0.3 nM 125I-ZM241385 for 3 h at 4 °C. Non-specific binding was measured in the presence of 100 nM ZM241385. Data represent the means±S.E.M., n=3. (B) Cells were pretreated with or without 10 ng/ml LPS for 18 h, rinsed twice with PBS, and then restimulated with 10 ng/ml LPS±ATL146e in various concentrations. Cell supernatants were harvested after 4 h and assayed for TNFα. The IC50 of ATL146e for control and LPS-pretreated cells was 20 and 0.1 nM respectively. Each point represents the mean±S.E.M. for three independent experiments.
DISCUSSION
TLRs (Toll-like receptors) on macrophages recognize microbial products (e.g. lipoproteins, peptidoglycan, LPS, flagellin and bacterial DNA) and initiate the transcription of many cytokines that promote phagocytosis in response to bacterial pathogens. The present study demonstrates that activation of macrophages with LPS also initiates a mechanism to terminate inflammation after a delay, by causing the induction of anti-inflammatory adenosine receptors A2B and principally A2A. There is also a reduction of Gi-coupled A1 and A3 mRNAs that may also contribute to inactivation of macrophages exposed to adenosine after LPS. Changes in the macrophage response to adenosine may also be modulated by altered expression of the β-subunits of heterotrimeric G-proteins [24–26]. The magnitude of A2AAR mRNA induction, >100-fold in Wehi-3 cells, IPMΦ and human macrophages, is far greater than the induction noted in the human monocyte-like THP-1 cell line and mouse endothelial cells [14,26]. The large A2AAR mRNA and protein induction may be an important adaptation to limit collateral damage to host tissues during infection.
The activation of the Gs-coupled A2AAR in a variety of cell types leads to inhibition of pro-inflammatory pathways. A2AAR activation inhibits aggregation in platelets [28–30], inhibits adhesion and oxidative burst in neutrophils [4,31] and decreases pro-inflammatory cytokines in monocytes [5,32,33] and macrophages [34,35]. Genetic deletion of the A2AAR gene, adora2a, leads to a hyperactive immune response after chemical or ischaemic liver injury, suggesting that the A2AAR is an important endogenous anti-inflammatory pathway [1,36]. Previous work from our group has shown that A2AAR activation improves survival in murine models of endotoxaemia and sepsis [9].
It is noteworthy that our findings demonstrate for the first time that the anti-inflammatory potency of A2AAR activation is enhanced after inflammation due to the large induction of radio-ligand-binding sites and functional A2A receptors. Hence, A2AAR or A2BAR agonists or adenosine are probably most effective as anti-inflammatory agents when administered during infection or other inflammatory conditions that provokes the induction of these receptors. The increase in A2AAR mRNA and functional potency was associated with an increase in receptor density measured by radioligand binding. Leibovich and colleagues [16,17] did not observe an increase in A2AAR immunoreactivity by Western blotting following treatment of macrophages with LPS. Our experience is that radioligand binding is more sensitive and less prone to non-specific binding than Western blotting as a means to detect the A2AAR. For example, some antibodies detect immunoreactive proteins near the molecular mass of the A2AAR, even in tissues from mice in which the A2AAR gene has been deleted [38]. No specific radioligand binding is detected in such mice. The results of the present study clearly demonstrate an increase in A2AAR receptor density over time after exposure of Wehi-3 cells, IPMΦ or human macrophages to LPS.
Pretreatment of IPMΦ with the NF-κB inhibitor BAY 11-7082 inhibited the induction of A2AAR and TNFα mRNAs. A role for NF-κB in the induction of cytokines, along with inhibition by BAY 11-7082, has been previously demonstrated in human macrophages [39]. The AP-1 family of transcription factors comprising the Jun, Fos, Maf and ATF subfamilies can be activated by LPS in macrophages to stimulate cytokine production [40,41]. Analysis of the 5′-end of the adora2a gene by Fredholm et al. [42] shows a putative AP-1 binding site approx. 1 kb upstream of the transcriptional start site, suggesting that transcription of cytokines and A2AARs may be regulated by some of the same transcription factors.
In summary, we have shown that LPS rapidly and markedly increases the expression of A2AAR mRNA in human and mouse macrophages. The newly formed receptors are functional as evidenced by A2AAR agonist-stimulated cAMP accumulation, enhanced radioligand binding and an increased potency of an agonist to inhibit TNFα release from these cells. LPS causes a smaller increase in A2BAR mRNAs and decreases A1 and A3 mRNA in mouse macrophages. The up-regulation of the A2AAR may provide an endogenous and inducible anti-inflammatory pathway to limit the activity of the immune system in response to bacterial infection and other inflammatory stimuli.
Acknowledgments
This work was supported by NIH grant number HL37942 and the Falk Medical Research Trust. We are grateful to Dr J. Rieger for his gift of ATL146e. J. L. and G. W. S. are shareholders in Adenosine Therapeutics.
References
- 1.Ohta A., Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature (London) 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 3.Fredholm B. B., Ijzerman A. P., Jacobson K. A., Klotz K. N., Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan G. W., Rieger J. M., Scheld W. M., MacDonald T. L., Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2A receptor agonists. Br. J. Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link A. A., Kino T., Worth J. A., McGuire J. L., Crane M. L., Chrousos G. P., Wilder R. L., Elenkov I. J. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J. Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 6.Cooper J. A., Hill S. J., Alexander S. P., Rubin P. C., Horn E. H. Adenosine receptor-induced cyclic AMP generation and inhibition of 5-hydroxytryptamine release in human platelets. Br. J. Clin. Pharmacol. 1995;40:43–50. doi: 10.1111/j.1365-2125.1995.tb04533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S., Apasov S., Koshiba M., Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 8.Koshiba M., Kojima H., Huang S., Apasov S., Sitkovsky M. V. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J. Biol. Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan G. W., Fang G., Linden J., Scheld W. M. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J. Infect. Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 10.Nemeth Z. H., Leibovich S. J., Deitch E. A., Vizi E. S., Szabo C., Hasko G. cDNA microarray analysis reveals a nuclear factor-κB-independent regulation of macrophage function by adenosine. J. Pharmacol. Exp. Ther. 2003;306:1042–1049. doi: 10.1124/jpet.103.052944. [DOI] [PubMed] [Google Scholar]
- 11.Xaus J., Mirabet M., Lloberas J., Soler C., Lluis C., Franco R., Celada A. IFN-γ up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J. Immunol. 1999;162:3607–3614. [PubMed] [Google Scholar]
- 12.Xaus J., Valledor A. F., Cardo M., Marques L., Beleta J., Palacios J. M., Celada A. Adenosine inhibits macrophage colony-stimulating factor-dependent proliferation of macrophages through the induction of p27kip-1 expression. J. Immunol. 1999;163:4140–4149. [PubMed] [Google Scholar]
- 13.Bshesh K., Zhao B., Spight D., Biaggioni I., Feokistov I., Denenberg A., Wong H. R., Shanley T. P. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J. Leukocyte Biol. 2002;72:1027–1036. [PubMed] [Google Scholar]
- 14.Khoa N. D., Montesinos M. C., Reiss A. B., Delano D., Awadallah N., Cronstein B. N. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J. Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 15.Kelsey S. M., Allen P. D., Razak K., Macey M. G., Newland A. C. Induction of surface tumor necrosis factor (TNF) expression and possible facilitation of surface TNF release from human monocytic cells by granulocyte-macrophage colony-stimulating factor or γ interferon in combination with 1,25-dihydroxyvitamin D3. Exp. Hematol. 1993;21:864–869. [PubMed] [Google Scholar]
- 16.Leibovich S. J., Chen J. F., Pinhal-Enfield G., Belem P. C., Elson G., Rosania A., Ramanathan M., Montesinos C., Jacobson M., Schwarzschild M. A., et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am. J. Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinhal-Enfield G., Ramanathan M., Hasko G., Vogel S. N., Salzman A. L., Boons G. J., Leibovich S. J. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am. J. Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang R., Patel D., Morris J. J., Rutschman R. L., Murray P. J. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 19.Poucher S. M., Keddie J. R., Singh P., Stoggall S. M., Caulkett P. W., Jones G., Coll M. G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden J., Patel A., Spanier A. M., Weglicki W. B. Rapid agonist-induced decrease of 125I-pindolol binding to β-adrenergic receptors: relationship to desensitization of cyclic AMP accumulation in intact heart cells. J. Biol. Chem. 1984;259:15115–15122. [PubMed] [Google Scholar]
- 21.Sullivan G. W., Linden J., Buster B. L., Scheld W. M. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J. Infect. Dis. 1999;180:1550–1560. doi: 10.1086/315084. [DOI] [PubMed] [Google Scholar]
- 22.Rieger J. M., Brown M. L., Sullivan G. W., Linden J., MacDonald T. L. Design, synthesis, and evaluation of novel adenosine A2A receptor agonists. J. Med. Chem. 2001;44:531–539. doi: 10.1021/jm0003642. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntire W. E., MacCleery G., Garrison J. C. The G protein β subunit is a determinant in the coupling of Gs to the β1-adrenergic and A2a adenosine receptors. J. Biol. Chem. 2001;276:15801–15809. doi: 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- 25.Murphree L. J., Marshall M. A., Rieger J. M., MacDonald T. L., Linden J. Human A2A adenosine receptors: high-affinity agonist binding to receptor-G protein complexes containing Gβ4. Mol. Pharmacol. 2002;61:455–462. doi: 10.1124/mol.61.2.455. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen D. K., Montesinos M. C., Williams A. J., Kelly M., Cronstein B. N. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J. Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 27. Reference deleted.
- 28.Dionisotti S., Zocchi C., Varani K., Borea P. A., Ongini E. Effects of adenosine derivatives on human and rabbit platelet-aggregation – correlation of adenosine receptor affinities and antiaggregatory activity. Naunyn-Schmiedebergs Arch. Pharmacol. 1992;346:673–676. doi: 10.1007/BF00168741. [DOI] [PubMed] [Google Scholar]
- 29.Paul S., Feoktistov I., Hollister A. S., Robertson D., Biaggioni I. Adenosine inhibits the rise in intracellular calcium and platelet-aggregation produced by thrombin – evidence that both effects are coupled to adenylate-cyclase. Mol. Pharmacol. 1990;37:870–875. [PubMed] [Google Scholar]
- 30.Varani K., Gessi S., Merighi S., Ongini E., Borea P. A. Adenosine A2a receptors of human circulating blood elements. Drug Dev. Res. 1998;45:253–260. [Google Scholar]
- 31.Sullivan G. W., Lee D. D., Ross W. G., DiVietro J. A., Lappas C. M., Lawrence M. B., Linden J. Activation of A2A adenosine receptors inhibits expression of α4/β1 integrin (very late antigen-4) on stimulated human neutrophils. J. Leukocyte Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- 32.Bouma M. G., Stad R. K., van den Wildenberg F. A., Buurman W. A. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J. Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- 33.Sullivan G. W., Linden J. Role of A2A adenosine receptors in inflammation. Drug Dev. Res. 1998;45:103–112. [Google Scholar]
- 34.Hasko G., Kuhel D. G., Chen J. F., Schwarzschild M. A., Deitch E. A., Mabley J. G., Marton A., Szabo C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 35.Hasko G., Szabo C., Nemeth Z. H., Kvetan V., Pastores S. M., Vizi E. S. Adenosine receptor agonists differentially regulate IL-10, TNF-α and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 36.Day Y. J., Marshall M. A., Huang L., McDuffie M. J., Okusa M. D., Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 37. Reference deleted.
- 38.Robeva A. S., Woodard R. L., Jin X. W., Gao Z. H., Bhattacharya S., Taylor H. E., Rosin D. L., Linden J. Molecular characterization of recombinant human adenosine receptors. Drug Dev. Res. 1996;39:243–252. [Google Scholar]
- 39.Devadas K., Hardegen N. J., Wahl L. M., Hewlett I. K., Clouse K. A., Yamada K. M., Dhawan S. Mechanisms for macrophage-mediated HIV-1 induction. J. Immunol. 2004;173:6735–6744. doi: 10.4049/jimmunol.173.11.6735. [DOI] [PubMed] [Google Scholar]
- 40.Proffitt J., Crabtree G., Grove M., Daubersies P., Bailleul B., Wright E., Plumb M. An ATF/CREB-binding site is essential for cell-specific and inducible transcription of the murine MIP-1β cytokine gene. Gene. 1995;152:173–179. doi: 10.1016/0378-1119(94)00701-s. [DOI] [PubMed] [Google Scholar]
- 41.Shin H. S., Drysdale B. E., Shin M. L., Noble P. W., Fisher S. N., Paznekas W. A. Definition of a lipopolysaccharide-responsive element in the 5′-flanking regions of MuRantes and crg-2. Mol. Cell. Biol. 1994;14:2914–2925. doi: 10.1128/mcb.14.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fredholm B. B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]