Abstract
We have observed that human neutrophils (polymorphonuclear leukocytes [PMNs]) have an increased growth-inhibitory and killing effect on a strain of Candida albicans with a deletion of CHK1, a gene encoding a putative histidine kinase. The PMN effect was not due to increased phagocytosis of the null strain. This observation may partially explain the reduced virulence in a hematogenously disseminated murine model of candidiasis.
Candida albicans is the fourth leading cause of nosocomial disease in the United States, and a mortality of approximately 35% has been reported (18). While primarily a pathogen of the immunocompromised patient, the organism also expresses virulence attributes (adhesins, morphogenesis, digestive enzymes, and phenotypic switching) (3). Morphogenesis, or the reversible change from a unicellular form (yeast) to a hyphal or pseudohyphal growth form, is regulated by several signal transduction pathways, including the two-component Hog1 (for hyperosmotic glycerol) pathway (3, 15). Two-component signal transduction is transmitted via phosphorylation of histidine and aspartate residues of component proteins, an activity that has not been demonstrated in mammalian cells (14). We and others have shown that two-component histidine kinase signaling is critical to morphogenesis and virulence (3-6, 19). For example, strains of C. albicans with a deletion in either SLN1, CHK1, CSSK1, or NIK1-COS1 have morphogenesis defects and are attenuated in their virulence (3, 5, 19). Importantly, the nik1-cosi/sln1 double deletion is lethal in C. albicans (19). Further, chk1-deleted strains exhibited a change in cell surface properties, suggested by the extensive flocculation of hyphae when cells were grown in m-199 broth (pH 7.5) at 37°C (5).
We used the “urablaster” technique (12) to construct C. albicans strains CHK11, CHK21, and CHK23 as previously described (reference 5 and Table 1). The CHK1 null strain was avirulent in a murine model of hematogenously disseminated candidiasis but was still capable of causing vaginitis in a rat model, suggesting a site-specific requirement for this gene in virulence (6). Sections from the kidneys of animals hematogenously infected with CHK21 revealed an intense cellular infiltrate (mostly neutrophilic) and sparse hyphal development compared to the extensive hyphal growth in the kidney of animals infected with the parental strain (CAF2). This observation suggested that CHK21 was readily cleared by host cells (including neutrophils) compared to parental cells. Because of this, and knowing the critical role of polymorphonuclear leukocytes (PMNs) in controlling invasive candidiasis, we initiated studies on the effect of human PMNs on the growth of C. albicans CHK21. Growth inhibition of this strain was compared to that of CAF2 (parental) as well as single-gene constructs (CHK11 and CHK23).
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| CAF2 | URA3/Δura3::imm434 | 12 |
| CHK11 | CHK1/Δchk1::hisG-URA3-hisGΔura3::imm434/Δura3::imm434 | 5 |
| CHK21 | Δchk1::hisG-URA3-hisg/Δchk1::hisGΔura3::imm434/Δura3::imm434 | 5 |
| CHK23 | CHK1::URA3-hisG/Δchk1::hisGΔura3::imm434/Δura3::imm434 | 5 |
Human PMNs were isolated essentially as reported previously (17). The susceptibility of CAF2 and the strains mentioned above to PMNs was first measured by a growth inhibition assay, as already described (16). Briefly, PMNs were suspended to different cell densities (6 × 105, 3 × 105, and 1.5 × 105 ml−1) in complete medium (CM) and were cultured (50 μl/well) with yeast cells (50 μl of a 104 ml−1 cell suspension) in 96-well plates in the presence or in the absence of 500 ng of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D System, Minneapolis, Minn.)/ml so as to obtain effector-to-target ratios (E:T) of 60:1, 30:1, and 15:1. Cells in each condition was assayed in triplicate. Control, triplicate wells with C. albicans alone were also included in the experiment. After 18 h of incubation at 37°C, PMNs were lysed in water and 5 μCi of d-(5,6)-[3H]glucose (specific activity, 70 Ci/mmol; NEN, Boston, Mass.) was added. The plates were incubated at 37°C for an additional 3 h, after which the Candida cells were harvested. Incorporated radiolabel was used to determine the percent growth inhibition of fungal cells at each E:T ratio as follows: percent growth inhibition = [(cpm of candida alone − cpm of candida plus PMN)/(cpm of candida alone)] × 100 (16).
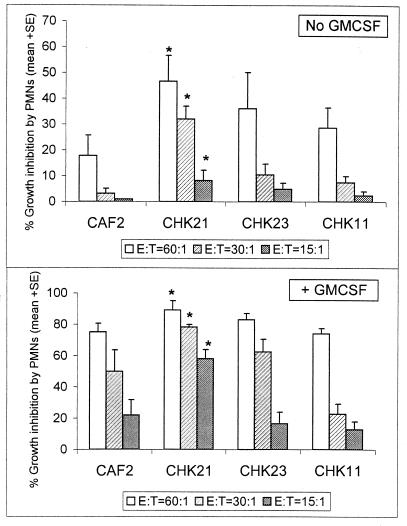
At each of the E:T ratios, we observed that in the absence of GM-CSF, CHK21 was growth inhibited to a significantly greater extent than CAF2 by PMN (P < 0.05; Fig. 1, upper). When cocultured with GM-CSF, the amount of growth inhibition increased significantly, but CHK21 was again inhibited to the greatest extent by the PMNs (P < 0.05; Fig. 1, lower). The data were also analyzed by analysis of variance (ANOVA) and Bonferroni multiple comparison tests. Differences among various strains were not observed except for that described above, i.e., CAF2 versus CHK21. For the experiments described in Fig. 1, the data were derived with PMNs from healthy donors and from two independent experiments. Strains CHK11 and CHK23 were not significantly growth inhibited compared to CAF2 at any of the E:T ratios used. This observation implies that the presence of a single copy of CHK1 is sufficient to protect these strains from the inhibitory activity of the PMNs.
FIG. 1.
Susceptibility of C. albicans strains to the growth-inhibitory activity of PMNs. Cells of the parental strain (CAF2), single-gene-deleted strains (CHK11 and CHK23), or chk1 null strain (CHK21) were cocultured with purified human PMNs at different E:T ratios (60:1, 30:1, and 15:1) in the absence (top) or presence (bottom) of GM-CSF. Growth inhibition was measured by the [3H]glucose assay described in the text. The bars represent the mean percent growth inhibition obtained from triplicate determinations in two independent experiments ± standard error. ∗, a statistically significant difference (P < 0.05), as assessed by Student's t test with respect to values obtained for strain CAF2 as well as ANOVA and the Bonferroni multiple comparison test.
Killing of the C. albicans cells by PMNs was evaluated by mixing freshly isolated PMNs in RPMI 1640 plus 10% fetal calf serum (107 cells ml−1) with 5 × 106 yeast cells ml−1 in a total volume of 0.2 ml in flat-bottom 96-well microtiter plates (E:T ratio of 5:1), with or without GM-CSF (500 ng/ml), for 30 min. For this study, only one single-gene-copy strain (CHK23) was used. All PMN-yeast mixtures were incubated for 3 h at 37°C in 5% CO2 and treated with Triton-X (2%), and serial dilutions were plated on Sabouraud agar. The CFU were determined after a 48-h incubation, and the percent killing was determined as follows: [(CFU of candida alone − CFU of candida plus PMN)/CFU of candida alone] × 100 (Table 2). In the absence of GM-CSF, CHK21 cells were killed to a greater extent than CAF2 cells (P < 0.05). GM-CSF increased the killing of CAF2 so that the difference between CAF2 and CHK21 was not statistically significant. The percent killing of CHK23 was not statistically different from CAF2 killing either in the presence or absence of GM-CSF.
TABLE 2.
Percent killing of C. albicans strains by human PMNs
| Strain | % Killing (mean ± SD)a
|
|
|---|---|---|
| Without Gm-CSF | With GM-CSF | |
| CAF2 | 36.9 ± 29.6 | 65 ± 23 |
| CHK21 | 80.9 ± 28 (P < 0.05) | 89.9 ± 6.1 (NS) |
| CHK23 | 69.3 ± 33.3 (NS) | 52.5 ± 4.8 (NS) |
The percent killing values are averages ± standard deviations of duplicate determinations from three independent experiments with PMNs from different donors. P values were assessed by Student's t test, comparing parental CAF2 with either CHK21 (chk1/chk1) or CHK23 (chk1/CHK1). NS, not significant. Statistical analysis by ANOVA and the Bonferroni multiple comparison test revealed similar observations.
We presumed that the augmented susceptibility of strain CHK21 to growth inhibition and killing by PMNs might be associated with a greater susceptibility to phagocytosis of this strain compared to CAF2. To assess this possibility, phagocytosis assays (8) were performed at E:T ratios of 1:2 and 1:4 of PMN-C. albicans cocultures. Triplicate PMN-Candida cocultures in CM (PMN, 2 × 106, 106, or 5 × 105/μl, 100 μl/well; C. albicans, 4 × 106, 100 μl/well) were prepared in 96-well plates and incubated for 30 min at 37°C under a 5% CO2 atmosphere. Incorporation of [3H]glucose by fungal cells was measured as described above. Percent phagocytosis was calculated by comparing [3H]glucose incorporation by fungal cells cocultured with PMNs with incorporation of control fungal cells in the absence of PMNs. Our results indicated no apparent differences in the percent phagocytosis of CAF2, CHK21, or CHK23 by PMNs at E:T ratios of 1:2 and 1:4 (data not shown).
Strain CHK21 (chk1/chk1) is avirulent in a murine model of hematogenously disseminated candidiasis when compared to CAF2 (6). The kidneys of animals infected with the mutant appeared to have a more extensive cellular infiltrate consisting of mostly neutrophilic cells. Therefore, we determined if growth inhibition or killing by PMNs differed among these strains. We found that CHK21 is more sensitive to the antifungal effects of PMNs in vitro, in terms of both growth inhibition and a true candidacidal activity.
The host factors that are associated with protection in the vascular system appear to be different from the protective factors in the vaginal canal, a conclusion based in part upon data from animal models (7-11, 13). In accordance with overwhelming clinical observations (2), experimental studies have provided convincing evidence for the role of PMNs in protection against invasive candidiasis, whereas even forced recruitment of PMNs in the vaginal canal had no effect on clearance of the organism (1). This suggests that innate immunity, including an intact neutrophil population, is an essential component of resistance to invasive candidiasis but not to vaginal candidiasis. However, anti-candida antibody has been shown to be protective not only against vaginal disease but in systemic disease (13). Our data can be interpreted to support the models that have been used to explain vaginal immunity to C. albicans, since the chk1 mutant survives quite readily within the vaginal canal, a site which does not develop a neutrophil-mediated immunity. Two-component signaling is critical to the adaptation of microorganisms against environmental insult. Thus, it is possible that CHK21 is more sensitive to the stress conditions that exist within a phagocytic cell.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH-NIAID AI47047 and AI43465 to R.C.) and the National AIDS Program (Istituto Superiore di Sanita, Rome, Italy, contract no. 50 C/B).
Giusy Mandarino helped in the preparation of the manuscript. The research derived from the use of human subjects has complied with all relevant institutional guidelines of the Istituto Superiore di Sanita.
Editor: T. R. Kozel
REFERENCES
- 1.Black, C. A., F. M. Eyers, A. Russel, M. L. Dunkley, R. L. Clancy, and K. W. Beagley. 1998. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect. Immun. 66:1273-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodey, C. A., M. Buckley, Y. S. Sathe, and E. J. Freirich. 1986. Quantitative relationship between circulating leukocytes and infections in patients with acute leukemia. Ann. Intern. Med. 64:328-340. [DOI] [PubMed] [Google Scholar]
- 3.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 4.Calera, J. A., G. Cho, and R. A. Calderone. 1998. Identification of a putative histidine kinase two-component phosphorelay gene (CaCHK1) in Candida albicans. Yeast 14:665-674. [DOI] [PubMed] [Google Scholar]
- 5.Calera, J. A., and R. A. Calderone. 1999. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology 145:1431-1442. [DOI] [PubMed] [Google Scholar]
- 6.Calera, J. A., X-J. Zhao, M. Sheridan, and R. A. Calderone. 1999. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect. Immun. 67:4280-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantorna, M. T., and E. Balish. 1991. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect. Immun. 59:2447-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiani, P., C. Bromuro, and A. Torosantucci. 2000. Defective induction of interleukin-12 in human monocytes by germ-tube forms of Candida albicans. Infect. Immun. 68:5628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bernardis, F. A., G. Santoni, M. Boccanera, E. Spreghini, D. Adriani, L. Morelli, and A. Cassone. 2000. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect. Immun. 68:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1993. Candida-specific Th-1 type responsiveness in mice with experimental vaginal candidiasis. Infect. Immun. 61:4202-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel, P. L., Jr., W. Luo, C. Steele, J. Chaban, M. Baker, and F. Wormley, Jr. 1999. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect. Immun. 67:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koretke, K. K., A. N. Lupas, P. V. Warren, M. Rosenberg, and J. R. Brown. 2000. Evolution of two-component signal transduction. Mol. Biol. E vol. 17:1956-1970. [DOI] [PubMed] [Google Scholar]
- 15.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W-C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palma, C., D. Serbousek, A. Torosantucci, A. Cassone, and J. Y. Djeu. 1992. Identification of a mannoprotein fraction from Candida albicans that enhances human polymorphonuclear leukocyte (PMNL) functions and stimulates lactoferrin in PMNL inhibition of candidal growth. J. Infect. Dis. 166: 1103-1112. [DOI] [PubMed] [Google Scholar]
- 17.Torosantucci, A., P. Chiani, I. Quinti, C. M. Ausiello, I. Mezzaroma, and A. Cassone. 1997. Responsiveness of human polymorphonuclear cells (PMNL) to stimulation by a mannoprotein fraction (MP-F2) of Candida albicans; enhanced production of IL-6 and tumour necrosis factor-alpha (TNF-α) by MP-F2-stimulated PMNL from HIV-infected subjects. Clin. Exp. Immunol. 107:451-457. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel, R. P. 1995. Nosocomial candidiasis: risk factors and attributable mortality. Clin. Infect. Dis. 20: 1531-1534. [DOI] [PubMed] [Google Scholar]
- 19.Yamada-Okabe, T., T. Mio, T. Ono, Y. Kashima, M. Arisawa, and Y. Yamada-Okabe. 1999. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]