Abstract
s-ACE (the somatic form of angiotensin-converting enzyme) consists of two homologous domains (N- and C-domains), each bearing a catalytic site. Negative co-operativity between the two domains has been demonstrated for cow and pig ACEs. However, for the human enzyme there are conflicting reports in the literature: some suggest possible negative co-operativity between the domains, whereas others indicate independent functions of the domains within s-ACE. We demonstrate here that a 1:1 stoichiometry for the binding of the common ACE inhibitors, captopril and lisinopril, to human s-ACE is enough to abolish enzymatic activity towards FA {N-[3-(2-furyl)acryloyl]}-Phe-GlyGly, Cbz (benzyloxycarbonyl)-Phe-His-Leu or Hip (N-benzoylglycyl)-His-Leu. The kinetic parameters for the hydrolysis of seven tripeptide substrates by human s-ACE appeared to represent average values for parameters obtained for the individual N- and C-domains. Kinetic analysis of the simultaneous hydrolysis of two substrates, Hip-His-Leu (S1) and Cbz-Phe-His-Leu (S2), with a common product (His-Leu) by s-ACE at different values for the ratio of the initial concentrations of these substrates (i.e. σ=[S2]0/[S1]0) demonstrated competition of these substrates for binding to the s-ACE molecule, i.e. binding of a substrate at one active site makes the other site unavailable for either the same or a different substrate. Thus the two domains within human s-ACE exhibit strong negative co-operativity upon binding of common inhibitors and in the hydrolysis reactions of tripeptide substrates.
Keywords: active-site titration, angiotensin-converting enzyme, negative co-operativity, simultaneous hydrolysis of two substrates
Abbreviations: ACE, angiotensin-converting enzyme; s-ACE, somatic ACE; C-ACE, C-domain of ACE; N-ACE, N-domain of ACE; FA, N-[3-(2-furyl)acryloyl]; Hip, N-benzoylglycyl; Cbz, benzyloxycarbonyl
INTRODUCTION
ACE (angiotensin-converting enzyme; EC 3.4.15.1) is a zinc-metallopeptidase and a member of the gluzincin family, which is defined by a HEXXH motif and a glutamic acid residue as the third zinc ligand [1]. The enzyme is responsible for the hydrolysis of angiotensin I and bradykinin and, thus, is essential for blood pressure control and water/electrolyte homoeostasis.
Two distinct ACE isoenzymes have been identified in mammalian tissues, and their primary structures have been determined by molecular cloning and sequencing of the cDNA [2,3]. The widely distributed s-ACE (somatic ACE) is composed of two homologous domains, N-ACE (the N-domain) and C-ACE (the C-domain), within a single polypeptide chain, with each domain bearing its own catalytic site [2]. The smaller ACE isoenzyme (t-ACE) is present exclusively in testes [4] and is involved in male fertility [5]. This isoenzyme is identical with C-ACE, except for 36 amino acid residues at its N-terminus [3]. N-ACE has been found in ileal fluid; this form of the enzyme is probably the result of limited proteolysis of the parent intestinal somatic form [6].
Since the discovery that s-ACE has two active sites, there has been much speculation about the significance of the presence of two active sites in the same enzyme. The two active sites both possess peptidyl dipeptidase and endopeptidase activities [1,7,8], but have distinct enzymatic properties with different substrates. Experiments with mutants of human s-ACE that have had one domain suppressed by deletion or one active site inactivated by point mutation have been performed [9]. These experiments have shown that both active sites of s-ACE are active.
The two catalytic sites were long considered to function independently, as the activity of human s-ACE on some substrates (especially angiotensin I) was found to be equal to the sum of the activities of N-ACE and C-ACE [9], and two molecules of inhibitor were required for full inhibition of angiotensin I or bradykinin cleavage in vitro [10]. The use of radiolabelled inhibitors also demonstrated binding of 2 mol of inhibitor per mol of s-ACE [11,12]. The concept of independent functions of the two domains within human s-ACE was given additional support by the discovery of domain-specific inhibitors [13,14] capable of blocking predominantly either N-ACE or C-ACE, while the other domain retained its catalytic activity.
However, other data indicate the possibility of some co-ordination or co-operation between the domains [7,15,16]. Active-site titration with tight-binding inhibitors [17,18], isothermal titration calorimetry [18], and kinetic analysis of the hydrolysis of tripeptide substrates by two-domain and single-domain ACE forms [17] presented evidence for strong negative co-operativity between the domains within s-ACE from bovine and porcine lung. Recently, negative co-operativity between the two domains was also reported for human ACE [19]. However, these reports were based either on the assumption that the enzyme preparations were fully active [19], or on rather contradictory findings that s-ACE demonstrates negative co-operativity between the two active sites upon hydrolysis of angiotensin I or angiotensin-(1–7), but exhibits independent functioning of active sites when titrated with lisinopril or Mca-ASDK-Dpa [(7-methoxycoumarin-4-yl)acetyl-Ala-Ser-Asp-Lys-3-(2,4-dinitrophenyl)-L-2,3-diamino-propionic acid] as substrate [20]. Thus, some uncertainty remains about how the two domains interact within human s-ACE, and the possibility that negative co-operativity is limited to ACEs of certain species requires clarification.
In the present study, by kinetic analysis of the catalytic properties of three forms of human ACE and by analysis of the simultaneous hydrolysis of two substrates by s-ACE, we present evidence that the two active sites in human ACE act in absolutely dependent manner.
MATERIALS AND METHODS
Materials
FA {N-[3-(2-furyl)acryloyl]}-Phe-Gly-Gly, captopril and lisinopril were from Sigma; Hip (N-benzoylglycyl)-His-Leu and Cbz (benzyloxycarbonyl)-Phe-His-Leu were from Bachem; FA-Phe-Ala-Pro and FA-Phe-Phe-Arg were synthesized as described in [21] and [22] respectively; FA-Phe-Ala-Ala and FA-Phe-Ala-Lys were a gift from Dr M. V. Ovchinnikov (Research Center of Cardiology, RAMS, Moscow, Russia).
Enzymes
Human s-ACE was isolated from the membrane fraction of human kidney by extraction with 50 mM phosphate buffer, pH 7.5, containing 150 mM NaCl and 0.1% Triton X-100. Culture medium containing recombinant C-ACE (expressed by CHO cells and described as tACEΔ36NJ in [23]) was a gift from Dr S. Danilov (Department of Anesthesiology, University of Illinois at Chicago, IL, U.S.A.). Both s-ACE and C-ACE were purified by lisinopril affinity chromatography as described previously [24]. N-ACE was obtained by limited proteolysis of the parent s-ACE after partial denaturation of the enzyme in NH4OH solution, as described in [25]. All ACE preparations were proved to be homogeneous according to electrophoresis by the Laemmli method [26] in a polyacrylamide gel in the presence of 0.1% SDS and β-mercaptoethanol. Proteins were stained with Coomassie Brilliant Blue G-250. Protein concentrations were determined according to the modified Lowry method [27,28].
Catalytic properties of the three ACE forms
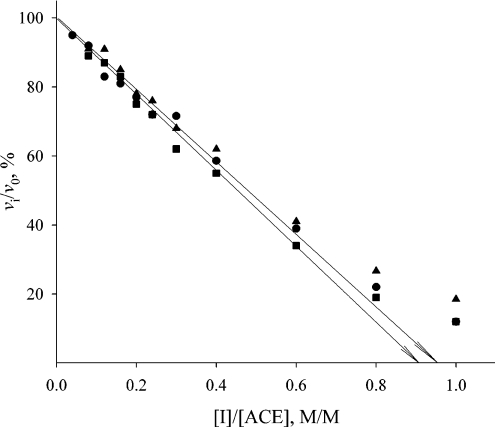
Stoichiometric titration of ACE active sites in solutions of s-ACE, recombinant C-ACE and N-ACE was performed with the specific competitive inhibitors lisinopril and captopril, as described in [17], in the presence of inhibitor and enzyme concentrations much higher than the binding constant. Aliquots of the enzyme containing 20 nM protein were incubated with 0–2.5 equivalents of the inhibitors for 30 min to produce an equilibrium between enzyme and inhibitor. Residual enzyme activities were then determined by measuring the initial rates of hydrolysis of FA-Phe-Gly-Gly, Hip-His-Leu or Cbz-Phe-His-Leu. The partition ratio of the inhibitor/enzyme association was determined by plotting the fractional activity vi/v0, (where vi is the activity in the presence of the inhibitor and v0 is the initial activity) against the inhibitor/enzyme concentration ratio. The linear part of the plot extrapolated to the x-axis gives an intersection point representing mol of inhibitor per mol of ACE required to abolish enzymatic activity [29].
The rates of catalytic hydrolysis of FA-containing substrates were monitored spectrophotometrically as described in [30]. Hydrolysis of Hip-His-Leu and Cbz-Phe-His-Leu was followed fluorimetrically by the release of His-Leu, which was derivatized with o-phthalaldehyde [31]. Initial rates were measured during the first 5% of substrate hydrolysis. The kinetic constants, Km and kcat, were calculated by Cornish-Bowden and Eisenthal analysis [32] from at least three independent experiments. The S.D.s of the Km and kcat values were <10%.
Kinetics of the simultaneous hydrolysis of two substrates
The simultaneous hydrolysis of Cbz-Phe-His-Leu and Hip-His-Leu by s-ACE was followed fluorimetrically by the release of their common product, His-Leu. The ratio, σ=[S2]0/[S1]0, of the initial concentrations of Cbz-Phe-His-Leu ([S2]) to the initial concentrations of Hip-His-Leu ([S1]) was varied in the range 0–0.15. The data were plotted in Lineweaver–Burk co-ordinates, 1/v against 1/[S]0, where [S]0 is Hip-His-Leu concentration.
RESULTS AND DISCUSSION
For all kinetic experiments we chose near-physiological conditions, namely pH 7.5, 150 mM NaCl and 1 μM ZnCl2. Three forms of human ACE were used in a single set of experiments: two-domain s-ACE and the individual domains C-ACE and N-ACE.
Active-site titration
Active-site titration of the three forms of human ACE was performed with the tight-binding inhibitors lisinopril and captopril. Lisinopril possesses similar affinity for both active sites in human ACE, whereas captopril binds more tightly to N-ACE [33,34]. Titration curves of s-ACE with both inhibitors in comparison with the titration curve of N-ACE with captopril are shown in Figure 1. Plots of residual activity (expressed as percentage activity in the presence of the inhibitor) against the ratio [I]/[ACE] gave, in both cases, an intersection point on the x-axis corresponding to approx. 1 mol of inhibitor per mol of ACE. Thus binding of competitive inhibitor (lisinopril or captopril) at 1:1 stoichiometry abolished the activity of both the two-domain and single-domain forms of human ACE. This result is in agreement with previous observations that the FA-Phe-Gly-Gly-hydrolysing activity of human and bovine s-ACE was abolished by 1 equivalent of lisinopril [16,17] and the corresponding activity of ACE from pig lung was abolished by 1 equivalent of lisinopril, captopril or enalaprilat [18]. We obtained similar titration results when other tripeptide substrates (Cbz-Phe-His-Leu or Hip-His-Leu) were used for measuring residual ACE activity (results not shown). Thus, binding of one molecule of the inhibitor (lisinopril or captopril) to any active site in human s-ACE prevented the hydrolysis of a tripeptide substrate by the second active site.
Figure 1. Stoichiometric titration of active sites in human s-ACE with lisinopril (●) and captopril (▲), and in N-ACE with captopril (■).
ACE (20 nM) in 50 mM phosphate buffer, pH 7.5, containing 150 mM NaCl and 1 μM ZnCl2 was incubated with 0–2.5 equivalents of inhibitor at 25 °C for 3 h in 980 μl. Residual enzyme activities were then determined by adding 20 μl of 5 mM FA-Phe-Gly-Gly in the same buffer and by measuring the initial rates of hydrolysis.
Kinetic characterization of the three human ACE forms
In order to reveal a possible influence of each of the two domains in human s-ACE on the other, we determined the kinetic parameters for the hydrolysis of seven tripeptide substrates by s-ACE and by the two single-domain forms, C-ACE and N-ACE. The results are presented in Table 1.
Table 1. Kinetic parameters for the hydrolysis of tripeptide substrates by three human ACE forms.
Assay conditions were 50 mM phosphate buffer, pH 7.5, containing 150 mM NaCl and 1 μM ZnCl2 (25 °C). Theoretical parameters were calculated according to Scheme 1 and eqn (2).
| s-ACE | ||||
|---|---|---|---|---|
| Substrate | Experimental parameters | Theoretical parameters | N-ACE | C-ACE |
| Hip-His-Leu | ||||
| kcat (s−1) | 71±7 | 65 | 49±4 | 97±6 |
| Km (mM) | 0.51±0.07 | 0.375 | 0.60±0.07 | 1.7±0.4 |
| kcat/Km (s−1·mM−1) | 139 | 82 | 57 | |
| Cbz-Phe-His-Leu | ||||
| kcat (s−1) | 116±4 | 108 | 256±17 | 81±6 |
| Km (mM) | 0.055±0.007 | 0.041 | 0.23±0.02 | 0.033±0.013 |
| kcat/Km (s−1·mM−1) | 2109 | 1113 | 2455 | |
| FA-Phe-Gly-Gly | ||||
| kcat (s−1) | 438±19 | 473 | 730±58 | 420±12 |
| Km (mM) | 0.29±0.03 | 0.24 | 1.40±0.13 | 0.29±0.04 |
| kcat/Km (s−1·mM−1) | 1510 | 521 | 1448 | |
| FA-Phe-Ala-Lys | ||||
| kcat (s−1) | 78±4 | 81 | 325±41 | 41±4 |
| Km (mM) | 0.14±0.03 | 0.033 | 0.23±0.8 | 0.038±0.008 |
| kcat/Km (s−1·mM−1) | 557 | 1413 | 1079 | |
| FA-Phe-Ala-Ala | ||||
| kcat (s−1) | 320±3 | 320 | 827±15 | 44±1 |
| Km (mM) | 0.081±0.003 | 0.029 | 0.084±0.009 | 0.045±0.007 |
| kcat/Km (s−1·mM−1) | 3950 | 9845 | 978 | |
| FA-Phe-Ala-Pro | ||||
| kcat (s−1) | 121±8 | 129 | 273±12 | 18±1 |
| Km (mM) | 0.041±0.007 | 0.021 | 0.048±0.005 | 0.037±0.008 |
| kcat/Km (s−1·mM−1) | 2950 | 5688 | 486 | |
| FA-Phe-Phe-Arg | ||||
| kcat (s−1) | 120±4 | 94 | 167±6 | 29±2 |
| Km (mM) | 0.019±0.003 | 0.0067 | 0.011±0.003 | 0.011±0.001 |
| kcat/Km (s−1·mM−1) | 6316 | 15182 | 2636 | |
The substrates widely used in laboratories for testing ACE activity are Hip-His-Leu and Cbz-Phe-His-Leu. The ratio kcat (Cbz-Phe-His-Leu)/kcat (Hip-His-Leu) was proved to be useful for distinguishing the activities of N- and C-ACE in ACE mutants [35]. Our data on the catalytic activities of the three forms of human ACE gave ratios of hydrolysis of Cbz-Phe-His-Leu/Hip-His-Leu equal to 1.6, 5.2 and 0.8 for s-ACE, N-ACE and C-ACE respectively. These data coincide rather well with those published elsewhere [35].
Hip-His-Leu is generally accepted as being more C-ACE-selective [9], while Cbz-Phe-His-Leu does not usually distinguish between the two active sites [35,36]. However, in our study the kcat value for the hydrolysis of Hip-His-Leu by C-ACE was only twice that with N-ACE, whereas the kcat value for the hydrolysis of Cbz-Phe-His-Leu was higher for N-ACE (Table 1). The reason is that we performed all experiments under near-physiological conditions (i.e. pH 7.5 and 150 mM NaCl), whereas the catalytic parameters for the hydrolysis of these substrates were usually determined at pH 8.3 and 300 mM NaCl (optimal conditions for Hip-His-Leu hydrolysis) [9,37,38]. It is known, however, that N- and C-ACE activities show different responses to chloride anions. While the Hip-His-Leu-hydrolysing activity of N-ACE reaches a maximum at 10 mM NaCl, the activity of C-ACE increases up to 800 mM NaCl [9]. Thus N-ACE possesses the same activity at both 150 and 300 mM NaCl, whereas C-ACE exhibits much higher activity at 300 mM NaCl compared with that at 150 mM NaCl.
Comparison of our values of catalytic parameters obtained for human ACE with results reported previously for bovine ACE [17] reveals some difference in substrate specificity between human and bovine ACEs. Whereas apparent Km values for the hydrolysis of all substrates were similar for bovine N-ACE and C-ACE, human C-ACE had a higher affinity for FA-Phe-Ala-Lys and Cbz-Phe-His-Leu than N-ACE (Table 1). Among the seven tripeptides surveyed, only Hip-His-Leu was hydrolysed by human C-ACE with a higher catalytic-centre activity (kcat) than with N-ACE, whereas other substrates were hydrolysed faster by N-ACE (Table 1). For bovine ACE, however, only Cbz-Phe-His-Leu was hydrolysed faster by N-ACE, whereas other substrates were hydrolysed either faster by C-ACE or at equal rates by N-ACE and C-ACE [17]. Comparison of catalytic efficacies (kcat/Km) revealed that FA-Phe-Ala-Ala and FA-Phe-Ala-Pro were hydrolysed preferentially by human N-ACE, but by bovine C-ACE. In contrast, Cbz-Phe-His-Leu was hydrolysed preferentially by human C-ACE, but by bovine N-ACE. Despite relatively high identity between human and bovine ACEs (89.1% between N-ACEs and 82.5% between C-ACEs), the differences in substrate specificity between human and bovine ACE may indicate structural differences in the active-site channels within these ACE forms.
Comparison of the catalytic activities of the three human ACE forms shows that, although both domains in s-ACE are active, the kcat values for the hydrolysis of any substrate by s-ACE never represented the sum of the corresponding kcat values obtained for N-ACE and C-ACE. Thus the two domains within s-ACE cannot be considered as being absolutely independent.
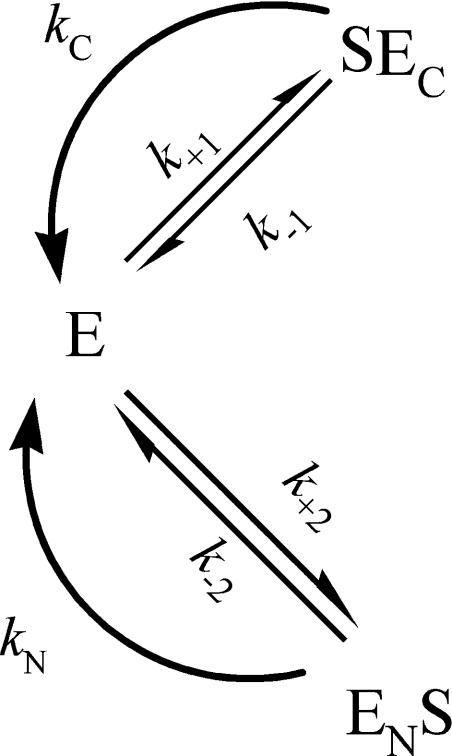
If human N- and C-ACE exhibit strong negative co-operativity, the kinetic mechanism of the hydrolysis of a substrate would be described by Scheme 1. The rate of the enzymatic reaction is represented by:
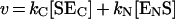 |
(1) |
According to steady-state assumption:
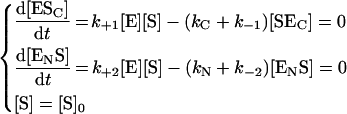 |
(2) |
and the stoichiometric relationship:
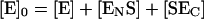 |
(3) |
the rate of the reaction is as follows:
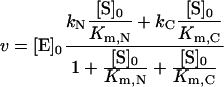 |
(4) |
where kN and kC are the catalytic constants for the hydrolysis of a substrate by N- and C-ACE respectively, and Michaelis constants are:
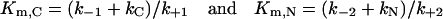 |
(5) |
The kinetic parameters of the hydrolysis of a substrate by s-ACE would be represented, therefore, by:
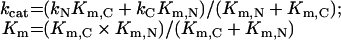 |
(6) |
Scheme 1. Hydrolysis of a substrate by an enzyme with two active sites (indicated by N and C) that exhibit strong negative co-operativity.
Theoretical values of parameters kcat and Km for the two-domain ACE were calculated, in accordance with Scheme 1, from the values of kcat and Km obtained for the single-domain forms. The calculated values are given in Table 1 along with experimental data. Good coincidence between experimental and theoretical values for the catalytic constants (kcat) demonstrates that the two domains within human s-ACE exhibit strong negative co-operativity. Thus, random binding of a substrate molecule to one of the active sites in human s-ACE prohibits or dramatically decreases hydrolysis of another substrate molecule by the second site.
The theoretical and experimental Km values, however, differed for several substrates (Table 1), suggesting that the catalytic parameters of a single domain can differ slightly from those in a two-domain molecule. This suggestion is in agreement with our earlier observation [39] that the rates of dissociation of complexes of a fluorescein-labelled inhibitor with bovine s-ACE were not the same as the rates of dissociation of complexes of this inhibitor with single-domain ACE forms.
Simultaneous hydrolysis by s-ACE of two substrates with a common product
In order to probe inter-domain co-operation in s-ACE further, we investigated the simultaneous hydrolysis by this enzyme of two substrates, Cbz-Phe-His-Leu and Hip-His-Leu, giving the common product His-Leu. Kinetic analysis of the simultaneous hydrolysis of two substrates with a common product by an enzyme with single active site was reported previously [40] to probe competition between these substrates for binding to the enzyme. We applied this approach to an enzyme with two active sites.
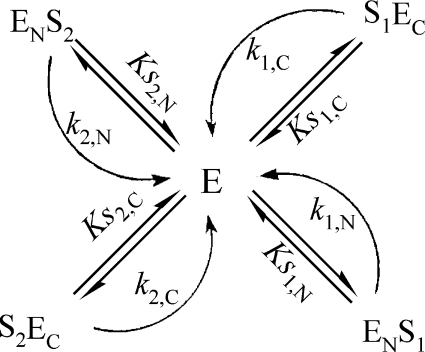
The hydrolysis of two substrates by an enzyme with two active sites (marked as N and C) that exhibit strong negative co-operativity (‘competition’ of substrates for binding to the enzyme) can be described by Scheme 2. The rate of the enzymatic reaction is represented by:
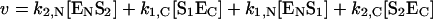 |
(7) |
With steady-state assumption, the final rate equation is very complicated, and thus we used equilibrium approximation:
 |
(8) |
and the stoichiometric relationship:
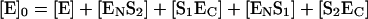 |
(9) |
If the hydrolysis product is common for the two substrates, the initial rate of the simultaneous hydrolysis of these substrates at a constant ratio of initial substrate concentrations (σ=[S2]0/[S1]0) is as follows:
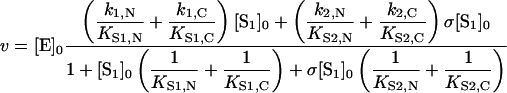 |
(10) |
where KS1,C and KS2,C are binding equilibrium constants of substrates 1 and 2 respectively for the C-ACE active site; KS1,N and KS2,N are binding equilibrium constants of substrates 1 and 2 respectively for the N-ACE active site; k1,N and k1,C are the catalytic constants of the hydrolysis of substrate 1 by the N-ACE and C-ACE active sites respectively; and k2,N and k2,C are the catalytic constants of the hydrolysis of substrate 2 by the N-ACE and C-ACE active sites respectively.
Scheme 2. Hydrolysis of two substrates by an enzyme with two active sites (indicated by N and C) that exhibit strong negative co-operativity.
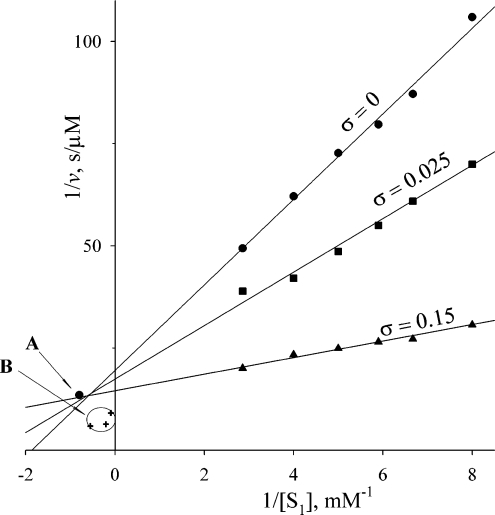
Lineweaver–Burk plots at various σ values yielded, in this case, a set of straight lines with a single intersection point with co-ordinates:
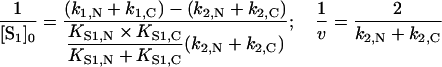 |
(11) |
The hydrolysis of two substrates by an enzyme with independent active sites (absence of substrate ‘competition’ for binding to the enzyme) can be described by Scheme 3. The rate of enzymatic hydrolysis is given by:
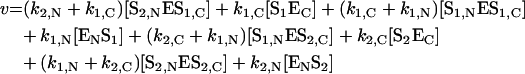 |
(12) |
Under equilibrium conditions, the rate of hydrolysis at a constant ratio of initial substrates concentrations (i.e. σ=[S2]0/[S1]0) is represented by:
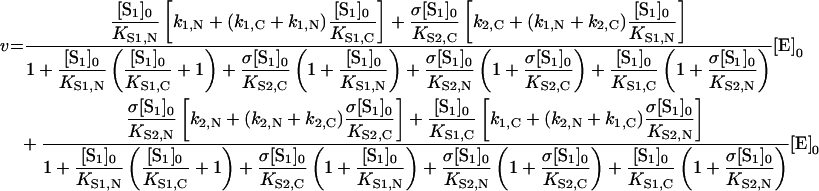 |
(13) |
where all symbols are as in eqn (10).
Scheme 3. Hydrolysis of two substrates by an enzyme with two independent active sites (indicated by N and C).
In general, Lineweaver–Burk plots at various σ values are not linear in this case, and do not yield a single intersection point. However, if Michaelis constants for the hydrolysis of a substrate were equal for both active sites, the rate equation would be much simpler:
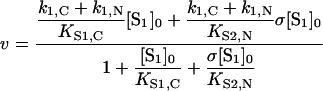 |
(14) |
and Lineweaver–Burk plots at various σ values would yield a set of straight lines with intersection point co-ordinates:
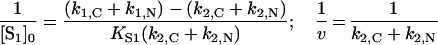 |
(15) |
These co-ordinates, however, differ markedly from co-ordinates given in eqn (11), obtained for ‘competitive’ substrates.
The Michaelis constants for the hydrolysis of either Cbz-Phe-His-Leu or Hip-His-Leu were not equal for N-ACE and C-ACE (Table 1), so we could not expect linear Lineweaver–Burk plots and single intersection point in the case of non-competitive binding of these substrates with s-ACE, in accordance with Scheme 3.
The results for the simultaneous hydrolysis of Cbz-Phe-His-Leu and Hip-His-Leu by human s-ACE showed, however, that Lineweaver–Burk plots were linear over the Hip-His-Leu (S1) concentration range 100 μM–1 mM for various values of parameter σ, and yielded a single intersection point (Figure 2). The position of this point coincides well with the intersection point calculated from the values of kinetic parameters for the hydrolysis of these two substrates by N-ACE and C-ACE (Table 1), in accordance with Scheme 2 and eqns (10) and (11). For comparison, Figure 2 also shows the theoretical intersection points calculated for each pair of σ values (0 and 0.025; 0 and 0.15; 0.025 and 0.15) according to Scheme 3. The area of these points appeared to be quite far from the intersection point obtained experimentally. Thus the present study demonstrates that Cbz-Phe-His-Leu and Hip-His-Leu compete for binding to s-ACE; thus binding of a substrate at one active site makes the other site unavailable for another substrate molecule.
Figure 2. Lineweaver–Burk plots for the simultaneous hydrolysis of two substrates with a common product (His-Leu) by s-ACE (750 pM) at different values of the ratio σ=[S2]0/[S1]0.
The two substrates used were Hip-His-Leu (S1) and Cbz-Phe-His-Leu (S2). Reactions were carried out in 50 mM phosphate buffer, pH 7.5, containing 150 mM NaCl and 1 μM ZnCl2 at 25 °C. Arrow A indicates the intersection point of theoretical plots calculated from kinetic parameters for the hydrolysis of Hip-His-Leu and Cbz-Phe-His-Leu by N-ACE and C-ACE according to an assumption of strong negative co-operativity of the two domains in s-ACE. B indicates the intersection area of theoretical plots calculated from kinetic parameters for the hydrolysis of these substrates by N-ACE and C-ACE according to an assumption of independent domains within s-ACE. Each cross corresponds to the intersection of two plots with different values of σ (0 and 0.025; 0 and 1.15; 0.15 and 0.025).
Conclusion
In conclusion, two domains of human s-ACE exhibit strong negative co-operativity, at least upon binding of common inhibitors, such as captopril and lisinopril, and in the hydrolysis of tripeptide substrates. Thus this property can be considered as a characteristic of s-ACE that suggests a close proximity of the domains, and may be the result of a flexible domain–domain movement upon binding of a ligand.
Acknowledgments
This work was supported by the Russian Foundation for Basic Research, grant 03-04-48821.
References
- 1.Corvol P., Williams T. A., Soubrier F. Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- 2.Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers M. R. W., Fox E. A., Strydom D. J., Riordan J. F. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strittmatter S. M., Thiele E. A., De Souza E. B., Snyder S. H. Angiotensin-converting enzyme in the testis and epididymis: differential development and pituitary regulation of isozymes. Endocrinology (Baltimore) 1985;117:1374–1379. doi: 10.1210/endo-117-4-1374. [DOI] [PubMed] [Google Scholar]
- 5.Kondoh G., Tojo H., Nakatani Y., Komazawa N., Murata C., Yamagata K., Maeda Y., Kinoshita T., Okabe M., Taguchi R., Takeda J. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 2005;11:160–166. doi: 10.1038/nm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deddish P. A., Wang J., Michel B., Morris P. W., Davidson N. O., Skidgel R. A., Erdos E. G. Naturally occurring active N-domain of human angiotensin I-converting enzyme. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7807–7811. doi: 10.1073/pnas.91.16.7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaspard E., Wei L., Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J. Biol. Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 8.Rousseau A., Michaud A., Chauvet M. T., Lenfant M., Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J. Biol. Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 9.Wei L., Alhenc-Gelas F., Corvol P., Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- 10.Georgiadis D., Beau F., Czarny B., Cotton J., Yiotakis A., Dive V. Roles of the two active sites of somatic angiotensin-converting enzyme in the cleavage of angiotensin I and bradykinin: insights from selective inhibitors. Circ. Res. 2003;93:148–154. doi: 10.1161/01.RES.0000081593.33848.FC. [DOI] [PubMed] [Google Scholar]
- 11.Perich R. B., Jackson B., Rogerson F., Mendelsohn F. A., Paxton D., Johnston C. I. Two binding sites on angiotensin-converting enzyme: evidence from radioligand binding studies. Mol. Pharmacol. 1992;42:286–293. [PubMed] [Google Scholar]
- 12.Wei L., Clauser E., Alhenc-Gelas F., Corvol P. The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J. Biol. Chem. 1992;267:13398–13405. [PubMed] [Google Scholar]
- 13.Dive V., Cotton J., Yiotakis A., Michaud A., Vassiliou S., Jiracek J., Vazeux G., Chauvet M. T., Cuniasse P., Corvol P. RXP 407, a phosphinic peptide, is a potent inhibitor of angiotensin I converting enzyme able to differentiate between its two active sites. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4330–4335. doi: 10.1073/pnas.96.8.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotton J., Hayashi M. A., Cuniasse P., Vazeux G., Ianzer D., De Camargo A. C., Dive V. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry. 2002;41:6065–6071. doi: 10.1021/bi012121x. [DOI] [PubMed] [Google Scholar]
- 15.Araujo M. C., Melo R. L., Cesari M. H., Juliano M. A., Juliano L., Carmona A. K. Peptidase specificity characterization of C- and N-terminal catalytic sites of angiotensin I-converting enzyme. Biochemistry. 2000;39:8519–8525. doi: 10.1021/bi9928905. [DOI] [PubMed] [Google Scholar]
- 16.Ehlers M. R., Riordan J. F. Angiotensin-converting enzyme: zinc- and inhibitor-binding stoichiometries of the somatic and testis isozymes. Biochemistry. 1991;30:7118–7126. doi: 10.1021/bi00243a012. [DOI] [PubMed] [Google Scholar]
- 17.Binevski P. V., Sizova E. A., Pozdnev V. F., Kost O. A. Evidence for the negative cooperativity of the two active sites within bovine somatic angiotensin-converting enzyme. FEBS Lett. 2003;550:84–88. doi: 10.1016/s0014-5793(03)00825-1. [DOI] [PubMed] [Google Scholar]
- 18.Andujar-Sanchez M., Camara-Artigas A., Jara-Perez V. A calorimetric study of the binding of lisinopril, enalaprilat and captopril to angiotensin-converting enzyme. Biophys. Chem. 2004;111:183–189. doi: 10.1016/j.bpc.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Woodman Z. L., Schwager S. L. U., Redelinghuys P., Carmona A. K., Ehlers M. R. W., Sturrock E. D. The N domain of somatic angiotensin-converting enzyme negatively egulates ectodomain shedding and catalytic activity. Biochem. J. 2005;389:739–744. doi: 10.1042/BJ20050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice G. I., Thomas D. A., Grant P. J., Turner A. J., Hooper N. M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozdnev V. F. Synthesis of N-furylacryloyl derivatives of aminoacids and peptides – chromogenic substrates of proteolytic enzymes – with the use of anhydride of furylacrylic acid. Zh. Obshch. Khim. 1986;56:690–695. [Google Scholar]
- 22.Grinshtein S. V., Binevski P. V., Gomazkov O. A., Pozdnev V. F., Nikolskaya I. I., Kost O. A. Inhibitor analysis of angiotensin I-converting and kinin-degrading activities of bovine lung and testicular angiotensin-converting enzyme. Biokhimiya (Moscow) 1999;64:938–944. [PubMed] [Google Scholar]
- 23.Gordon K., Redelinghuys P., Schwager S. L. U., Ehlers M. R. W., Paoageorgiou A. C., Natesh R., Acharya K. R., Sturrock E. D. Deglycosylation, processing and crystallization of human testis angiotensin-converting enzyme. Biochem. J. 2003;371:437–442. doi: 10.1042/BJ20021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kost O. A., Bovin N. V., Chemodanova E. E., Nasonov V. V., Ort T. A. New feature of angiotensin-converting enzyme: carbohydrate-recognizing domain. J. Mol. Recog. 2000;13:360–369. doi: 10.1002/1099-1352(200011/12)13:6<360::AID-JMR508>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Binevski P. V., Nikolskaya I. I., Pozdnev V. F., Kost O. A. Isolation and characterization of the N-domain of bovine angiotensin-converting enzyme. Biokhimiya (Moscow) 2000;65:651–658. [PubMed] [Google Scholar]
- 26.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Rodriguez-Vico F. A procedure for eliminating interferences in the Lowry method of protein determination. Anal. Biochem. 1989;183:275–278. doi: 10.1016/0003-2697(89)90479-x. [DOI] [PubMed] [Google Scholar]
- 29.Bieth J. Some kinetic consequences of the tight binding of protein-proteinase-inhibitors to proteolytic enzymes and their application to the determination of dissociation constants. In: Fritz H., Tschesche H., Greene L. J., editors. Proteinase-Inhibitors: Bayer-Symposium V. Berlin, Heidelberg, New York: Springer-Verlag; 1974. pp. 463–469. [Google Scholar]
- 30.Holmquist B., Bünning P., Riordan J. F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal. Biochem. 1979;95:540–548. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- 31.Piquilloud Y., Reinharz A., Roth M. Studies on the angiotensin converting enzyme with different substrates. Biochim. Biophys. Acta. 1970;206:136–142. doi: 10.1016/0005-2744(70)90090-2. [DOI] [PubMed] [Google Scholar]
- 32.Cornish-Bowden A. London: Portland Press Ltd; 1999. Fundamentals of Enzyme Kinetics; pp. 306–307. [Google Scholar]
- 33.Deddish P. A., Wang L.-X., Jackman H. L., Michel B., Wang J., Skidgel R. A., Erdös E. G. Single-domain angiotensin I converting enzyme (kininase II): characterization and properties. J. Pharmacol. Exp. Ther. 1996;279:1582–1589. [PubMed] [Google Scholar]
- 34.Michaud A., Williams T. A., Chauvet M.-T., Corvol P. Substrate dependence of angiotensin I-converting enzyme inhibition: captopril displays a partial selectivity for inhibition of N-acetyl-seryl-aspartyl-lysyl-proline hydrolysis compared with that of angiotensin I. Mol. Pharmacol. 1997;51:1070–1076. doi: 10.1124/mol.51.6.1070. [DOI] [PubMed] [Google Scholar]
- 35.Williams T. A., Danilov S., Alhenc-Gelas F., Soubrier F. A study of chimerads constructed with the two domains of angiotensin I-converting enzyme. Biochem. Pharmacol. 1996;51:11–14. doi: 10.1016/0006-2952(95)02125-6. [DOI] [PubMed] [Google Scholar]
- 36.Danilov S., Jaspard E., Churakova T., Towbin H., Savoie F., Wei L., Alhen-Gelas F. Structure-function analysis of angiotensin I-converting enzyme using monoclonal antibodies. J. Biol. Chem. 1994;269:26806–26814. [PubMed] [Google Scholar]
- 37.Lanzillo J. J., Fanburg B. L. Angiotensin I converting enzyme from human plasma. Biochemistry. 1977;16:5491–5495. doi: 10.1021/bi00644a015. [DOI] [PubMed] [Google Scholar]
- 38.Gordon K., Redelinghuys P., Schwager S. L. U., Ehlers M. R. W., Papageorgiou A. S., Natesh R., Acharya K. V., Sturrock E. D. Deglycosylation, processing and crystallization of human testis angiotensin-converting enzyme. Biochem. J. 2003;371:437–442. doi: 10.1042/BJ20021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voronov S. V., Binevski P. V., Eremin S. A., Kost O. A. Fluorescence polarization studies of different forms of angiotensin-converting enzyme. Biokhimiya (Moscow) 2001;66:788–794. doi: 10.1023/a:1010220930765. [DOI] [PubMed] [Google Scholar]
- 40.Pocklington T., Jeffery J. Competition of two substrates for a single enzyme. A simple kinetic theorem exemplified by a hydroxy steroid dehydrogenase reaction. Biochem. J. 1969;112:331–334. doi: 10.1042/bj1120331. [DOI] [PMC free article] [PubMed] [Google Scholar]