Abstract
Enterococcus faecalis has become one of the most notable nosocomial pathogens in the last decade. Aggregation substance (AS) on the sex pheromone plasmids of E. faecalis has been implicated as a virulence factor in several model systems. We investigated the AS-encoding plasmid pCF10 for its ability to increase virulence in a rabbit endocarditis model. Cells containing pCF10 increased the virulence in the model significantly, as assessed by an increase in aortic valve vegetation size. The results confirmed in vivo induction of the normally tightly controlled AS. In addition to the expression of AS when E. faecalis cells were in contact with plasma, plasmid transfer of the tetracycline resistance-carrying plasmid was also activated in vitro and in vivo. In vivo, plasmid transfer reached remarkable frequencies of 8 × 10−2 to 9 × 10−2. These values are comparable to the highest frequencies ever observed in vitro. Cells harboring pCF10 had a significant survival advantage over plasmid-free cells indicated by pCF10 present in two-thirds of the recipient population. Plasma induction was dependent on the presence of the plasmid-encoded PrgZ protein, indicating the requirement of the pheromone-sensing system in the induction process. The data suggested that the mechanism of in vivo induction may involve interference of plasma with the normal function of the pheromone peptide and its inhibitor.
Aggregation substance (AS) is a 137-kDa surface protein encoded on sex pheromone plasmids of Enterococcus faecalis. This protein mediates strong binding between E. faecalis cells as manifested by the formation of visible cell aggregates preceding the conjugative transfer of a sex pheromone plasmid (14). The plasmid transfer response is initiated by 7- to 8-amino-acid-long hydrophobic peptides secreted by potential recipient cells (16, 32). These peptides are part of signal sequences of chromosomally encoded lipoproteins (10) and lead to induction of AS expression.
Asc10, the AS of the sex pheromone plasmid pCF10 (encoding tetracycline resistance) (17), is induced by the 7-amino-acid peptide cCF10 (32) encoded by the ccfA gene. The donor cell binds the peptide (37), employing a specific plasmid-encoded receptor, PrgZ, an OppA homologue (Fig. 1). The peptide is then transported into the cell via the oligopeptide permease system (29), leading to the expression of AS and subsequent plasmid transfer. Since expression of AS and the other plasmid transfer machinery is presumably a very energy-consuming process, it is not surprising that expression of AS is tightly regulated. This ensures that the nonmotile enterococcal cells expressing transfer functions are in close proximity for cell contact to occur between mating partners.
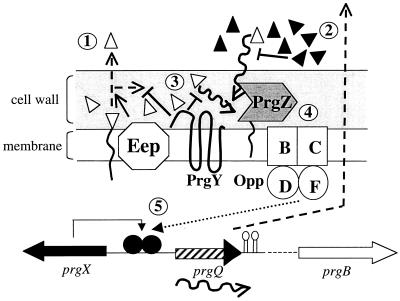
FIG. 1.
Schematic overview of an uninduced E. faecalis cell containing pCF10. AS is not expressed. Cells containing pCF10 secrete unaltered amounts of cCF10 (no. 1). The cells' own secreted cCF10 (open triangles) is neutralized by the inhibitor peptide iCF10 (solid triangles) (no. 2). Induction by cCF10 contained in the cell wall is prevented by PrgY (no. 3). Induction occurs if nearby recipient cells tip the balance in favor of cCF10, which is bound by PrgZ, imported by the Opp system (no. 4), and then interacts with PrgX to allow AS expression (no. 5). PrgX controls transcription of the prgQ promoter, allowing production of iCF10 (solid arrowhead). The protein Eep is involved in the processing of the sex pheromone peptides (1). Opp, oligopeptide permease. Parts of the figure were adapted from reference 5.
pCF10 donor cells are faced with the dilemma that they secrete unaltered amounts of cCF10 (33), necessitating a complex scheme to prevent autoinduction by their own pheromones. A schematic overview of the events controlling AS expression is given in Fig. 1. Secreted cCF10 may accumulate to approximately 8 pg/ml (33). To counteract this inducing activity, donor cells secrete the 7-amino-acid peptide iCF10, which acts as a competitive inhibitor of cCF10. The inhibitor peptide prevents self-induction by autogenous cCF10 and also provides the threshold that allows only recipients in immediate proximity to the donor to overcome the inhibitor activity with their secreted cCF10. The inhibitor is secreted at an 80-fold excess to cCF10 (33). The amino acid sequence of iCF10 is encoded by the last 7 amino acids of the prgQ gene product on pCF10, preceded by a regular signal sequence. Cells unable to produce iCF10 show a slight increase in expression of Asc10, as would be expected with autoinduction by cCF10 (4). In addition, the cell wall of E. faecalis contains a considerable amount of cCF10 activity (5). The membrane protein PrgY was recently shown to reduce the level of cCF10 activity in the cell wall (5), therefore preventing induction by cell wall-associated pheromone. Insertions in prgY result in constitutive expression of Asc10 (22), confirming its involvement in the negative regulation of the system. Binding and transport of cCF10 by PrgZ and the Opp system into the cell would lead to interaction with PrgX, presumably altering the conformation of this negative regulator (3) and allowing AS expression.
AS of E. faecalis is highly conserved among sex pheromone plasmids (25) and was assumed to be a contributor to virulence of this important nosocomial pathogen after sequencing studies revealed the presence of two RGD motifs encoded in the AS protein (20, 21, 27). Kreft and coworkers demonstrated increased adhesion of AS-expressing cells to cultured renal tubular cells (28), which could be inhibited somewhat by the addition of RGD-containing peptides. Increased uptake of AS-expressing cells into intestinal epithelial cells was also demonstrated (34), suggesting a possible involvement of AS in the dissemination of E. faecalis from its natural environment in the intestine into the bloodstream. Recently, increased uptake of AS-expressing cells by a colon cell line was reported (26).
E. faecalis is a major contributor to cases of infective endocarditis (31). This illness is manifested by a mass of fibrin and platelets—vegetation (30)—caused mostly by injury to the endocardium that becomes infected by various species of bacteria, with gram-positive cocci being the majority of causative organisms (2). Chow and coworkers demonstrated the contribution of AS to endocarditis in a rabbit endocarditis model (6). AS increased the size of vegetations, whereas the encoded cytolysin on the plasmid pAD1 contributed to lethality. Recently, Schlievert et al. (38) demonstrated that AS of the plasmid pCF10 has the same effect. In addition to AS, the receptor for AS on E. faecalis, enterococcal binding substance, was also required for a maximal virulence phenotype.
Studies demonstrating AS involvement in virulence were performed with mostly constitutive AS-expressing cells in both the pAD1 and pCF10 systems (26, 28, 34-36, 38). The tight regulation of AS expression in vitro led us to investigate if AS on the plasmid pCF10 plays a role in a rabbit endocarditis model and therefore if AS on pCF10 is induced in vivo. We demonstrate in this communication that AS of pCF10 was induced in vivo and increased the size of vegetations significantly. In addition, the expression of AS conferred a survival advantage to cells harboring the plasmid and led to highly efficient plasmid transfer in vivo. The involvement of the pheromone-sensing system in AS expression in plasma was indicated by the absence of AS induction in a mutant lacking the pheromone-sensing protein PrgZ. The data suggest that at least part of the effect of plasma components on mating behavior of E. faecalis donor cells was via an interaction with the inhibitor peptide iCF10.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Strains used in this study are listed in Table 1. Stocks were maintained frozen at −80°C until used. Overnight cultures not older than 15 h were used for the experiments. E. faecalis was grown in Todd-Hewitt broth (THB; Difco Laboratories, Detroit, Mich.) or fresh human plasma without shaking at 37°C. For the endocarditis experiments, strains were grown 4 h at 37°C. After 1/100 dilution of an overnight culture, a 10-ml sample was sedimented and resuspended in the same volume of 0.9% NaCl prior to injection. For cell extracts, cells were diluted 1/10 in 5 ml of THB, with addition of the inducing factors, and allowed to grow for 4 h, pelleted, and washed three times in phosphate-buffered saline (PBS; 10 mM NaPO4 [pH 7.2], 0.9% NaCl) before extraction. For the test of induction with the strains MSP4301 and MSP4901, respectively, cells were diluted 1/10 in THB plus cCF10 in the amounts described above or 100% human plasma and incubated for 4 h at 37°C prior to extraction. The plasmids pMSP6043, pMSP6049, and pINY1801 were isolated from Escherichia coli DH5α (32) and transformed into E. faecalis by electroporation (12). Petri plates contained 1.5% agar, and antibiotics were added in the following concentrations when needed: streptomycin, 1,000 μg/ml; kanamycin, 1,000 μg/ml; spectinomycin, 1,000 μg/ml; rifampin, 200 μg/ml; chloramphenicol, 20 μg/ml; and tetracycline, 10 μg/ml (Sigma Chemical Co., St. Louis, Mo.).
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| OG1SSp | Strr Spcr | 14 |
| OG1SSp(pCF10) | Wild-type plasmid | 11 |
| OG1SSp(pCF121) | prgB::Tn 917 insertion | 7 |
| OG1RF | Rifr Fusr | 14 |
| OG1RF(pCF10) | Wild-type plasmid | 37 |
| MSP4301 [OG1RF(pMSP6043/pINY1801)] | Negative regulatory region (pMSP6043), positive regulatory region, and prgB gene (pINY1801) | This study; pMSP6043 (22), pINY1801 (7) |
| MSP4901 [OG1RF(pMSP6049/pINY1801)] | Insertion (Specr) in the prgZ gene of pMSP6043 | This study; pMSP6049 (22) |
Experimental endocarditis.
New Zealand White Rabbits (Birchwood Farms, Red Wing, Minn.) were catheterized over the aortic valve as described previously (23, 38). The catheters were removed after 2 h, and 2 ml of the bacterial strains (2 × 109) in 0.9% NaCl was injected in the right marginal ear vein. After three days, the animals were sacrificed, the formed vegetations were aseptically removed, and the wet weight was determined. The vegetations were homogenized and assessed for bacterial counts. In the plasmid transfer experiment, donor and recipient strains were injected simultaneously in the right and left ear vein with a combined cell number of 2 × 109. Animals were treated according to the University of Minnesota Animal Care and Use Committee requirements.
Plasmid transfer experiments.
The wild-type strain OG1SSp(pCF10) served as the donor strain, and OG1RF served as the recipient. Cultures were diluted 1/10 from overnight cultures in 2 ml of THB or 2 ml of fresh human or rabbit plasma for the donor and in 2 ml of THB for the recipient. The donor cultures were induced by adding 10 ng of cCF10 per ml when needed; iCF10 was added at the concentrations indicated. The peptides were synthesized at the Microchemical Facility, University of Minnesota. The cultures were allowed to grow separately for 2 h at 37°C, and then the recipient cultures were inoculated with a 1/10 volume of the induced donor culture. The mating was performed for 10 min, and the culture was then vortexed, diluted in 0.9% NaCl, and spread on the appropriate antibiotic plates, selecting for the donor and transconjugants, respectively.
Preparation of human and rabbit plasma.
Blood was drawn from healthy human volunteers or New Zealand White rabbits. Heparin (Pharmacia, Piscataway, N.J.) was added to a final concentration of 100 U/ml. The blood was centrifuged for 10 min at 2,500 rpm in a standard clinical centrifuge, and the plasma was immediately used for experiments.
Cell extracts, SDS-PAGE, and Western blotting.
Cells, grown in 5-ml cultures, were treated with 50 μl of extraction buffer as described previously (21) for 1 h at room temperature on a shaker. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were performed as previously described (41) and with use of the ECL (enhanced chemiluminescence) system (Pierce, Rockford, Ill.) according to the manufacturer's recommendations. The polyclonal antibody QSD 3.7, raised against whole AS Asa1, was kindly provided by A. Muscholl (Universität Regensburg, Regensburg, Germany) and used in a 1:10,000 dilution. Protein concentration was determined with a bicinchoninic acid protein assay (Pierce).
Electron microscopy.
Cells were grown in fresh human plasma for 4 h. For controls, cells were grown in THB. The positive control culture for AS expression was induced with 10 ng of cCF10 per ml. No peptide was added to plasma. Cells (1 ml) were pelleted, washed with PBS, and resuspended in 500 μl of PBS with 5% goat serum and incubated with a monoclonal antibody against AS (40) at 20 μg/ml for 2 h, washed three times with PBS-goat serum, incubated for 1 h with goat anti-mouse immunoglobulin G conjugated with 12-nm-diameter colloidal gold (Jackson ImmunoResearch Laboratories, West Grove, Pa.) diluted 1:50, and subsequently washed with PBS three times. The cells were concentrated by centrifugation at low speed, resuspended in 50 μl of PBS, and then placed on poly-l-lysine (Sigma)-covered glass supports (5 by 10 mm). The cells were allowed to adhere for 30 min and then were washed and fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer containing 7.5% sucrose. The bacteria were examined by backscatter electron imaging with an AUTRATA modified YAG detector with an Hitachi S-900 field emission scanning microscope at 5 keV as described previously (34).
RESULTS
AS on pCF10 increases vegetation size in a rabbit endocarditis model.
Recent results had shown that AS of the plasmid pCF10 (Asc10) is a virulence factor in rabbit endocarditis (38). This was established by utilizing a recombinant plasmid (pINY1801) constitutively expressing Asc10. To assess if Asc10 from wild-type plasmid pCF10 was expressed in vivo, the endocarditis model was employed. New Zealand White rabbits were catheterized through the left carotid artery and then intravenously challenged with 2 × 109 cells with the plasmid-free E. faecalis strain OG1SSp or with the isogenic strain containing wild-type pCF10. Virulence, as reflected by the weight of cardiac vegetations formed 3 days after infection, was significantly higher in rabbits challenged with the plasmid-containing strain (Table 2). Four of 13 rabbits injected with OG1SSp(pCF10) had vegetations with a weight of 100 mg or more, and vegetations occurred in all rabbits. In comparison, 3 of 10 rabbits injected with OG1SSp showed no vegetations, 3 had vegetation weights below 10 mg, and 2 showed weights over 40 mg. Bacterial culture of vegetations confirmed their infection by the bacterial strains. Cell numbers in the vegetations ranged between 108 and 1010 per g. No correlation between the strains used could be observed. Rabbits that were infected with OG1SSp(pCF10) were also more likely to show involvement of other organs, such as liver necrosis and up to 30% enlarged spleens: 9 of 13 with OG1SSp(pCF10) and 3 of 7 and 3 of 10 for OG1SSp(pCF121) and OG1SSp, respectively. Rabbits were also challenged with strain OG1SSp(pCF121), which does not express AS due to a Tn 917 insertion in the prgB gene. The transposon insertion in pCF121 was shown not to affect downstream genes, because transfer of this plasmid on solid media remained unaffected (7). The vegetation weights in these rabbits were not different from those obtained with the plasmid-free strain, strongly suggesting that Asc10 is responsible for the increased virulence of the wild-type strain. Since the cells were not exposed to cCF10 prior to injection, these results were consistent with induction of AS expression in vivo.
TABLE 2.
Wet weight of vegetations induced by challenge of New Zealand White rabbits with E. faecalis strains in experimental endocarditis
| E. faecalis strain | Median vegetation wt (mg) | No. of rabbits | Lowest/highest observed wt (mg) |
|---|---|---|---|
| OG1SSp | 13.4 | 10 | 0/55 |
| OG1SSp(pCF10) | 70.7a | 13 | 17/23 |
| OG1SSp(pCF121) | 17.2 | 6 | 6/24 |
Significance was determined according to the Mann-Whitney U test (P < 0.01).
AS is induced in E. faecalis in contact with human plasma.
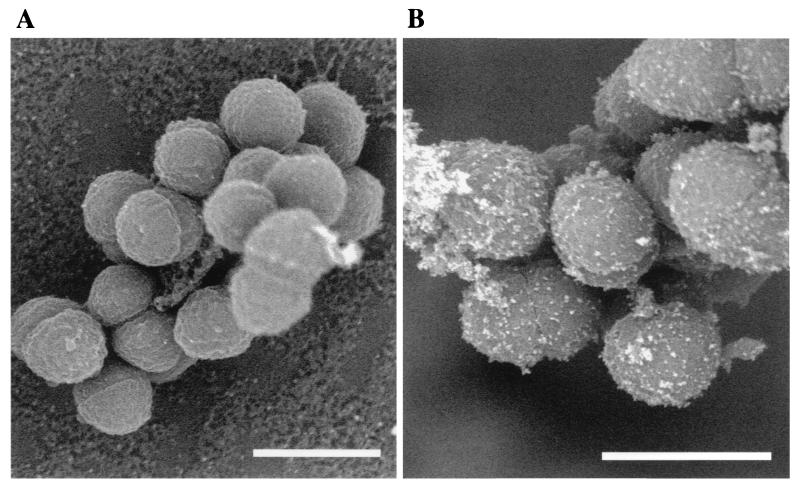
The results of the endocarditis model suggested that AS was expressed in an in vivo situation. To verify this hypothesis, OG1RF(pCF10) cells were grown in human plasma for several hours and then labeled with a monoclonal antibody directed against AS. Plasma-grown cells showed good labeling with antibody directed against AS (Fig. 2B), comparable to cCF10-induced cells as demonstrated previously (24). Plasmid-free OG1RF cells grown in plasma did not bind the antibody, demonstrating the specificity of the antibody for AS (Fig. 2A). No labeling was seen in control cells grown in THB without addition of cCF10 24; data not shown). This clearly showed that AS protein was induced and on the cell surface in pCF10 cells in contact with human plasma.
FIG. 2.
AS is expressed in contact with human plasma. Cells were grown in human plasma for 4 h, washed, and then labeled with a monoclonal antibody against AS. (A) OG1RF cells after growth in plasma. (B) OG1RF(pCF10). Only pCF10-containing cells showed labeling for AS in human plasma, indicating plasma induction of AS. AS was not expressed in cells that were not induced with cCF10 (data not shown).
Plasmid transfer is activated in plasma in vitro and in vivo.
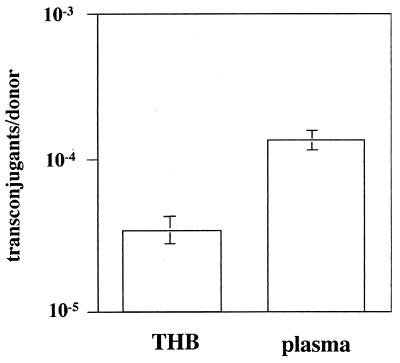
The expression of AS in human plasma raised the question of whether plasmid transfer also occurs in plasma. A series of mating assays were therefore conducted to assess the level of plasmid transfer in plasma. Transfer of plasmid pCF10 was significantly elevated in comparison to that in uninduced (no added cCF10 peptide) cells (Fig. 3), demonstrating that plasmid transfer coincided with the induction of AS expression. An identical experiment performed with rabbit plasma led to a comparable result (data not shown). A mixed infection with a donor and recipient strain was performed to verify that this observation also had relevance in vivo. Endocarditis was induced by challenging catheterized rabbits simultaneously with the plasmid donor strain OG1SSp(pCF10) and the potential recipient OG1RF. Their different chromosomally encoded antibiotic resistance markers (streptomycin and rifampin, respectively) could easily distinguish the strains. After enumeration of bacteria in the formed vegetations, the potential recipients were screened for the content of plasmid pCF10 (Table 3). Approximately 65 to 70% of OG1RF cells contained pCF10 after day 3, accounting for a remarkably high plasmid transfer rate of 10−2 transconjugants per donor. Similar transfer rates were observed in a subdermal chamber (Wiffle ball) in a rabbit model (unpublished results). Also evident was a significant shift in cell ratio in favor of OG1SSp(pCF10) compared to OG1RF, strongly suggesting an advantage for plasmid-containing cells.
FIG. 3.
Plasmid transfer is activated in human plasma. OG1SSp(pCF10) cells were grown for 2 h in THB and human plasma, respectively. Cells were then brought together for a 10-min mating with the recipient OG1RF. Matings were performed in triplicate; a representative experiment of several independent experiments is shown. Rabbit plasma showed a similar result (not shown). Significance was determined by Student's t test (P < 0.01).
TABLE 3.
Plasmid transfer in vivo occurs at a high frequencya
| Expt | OG1SSp(pCF10)/OG1RF ratio
|
OG1RF(pCF10)/ OG1RF ratio recovered from vegetations (%) | Transconjugant/ donor rate | |
|---|---|---|---|---|
| Prior to injection | End of experiment | |||
| I | 2:1 | 7.6:1 | 398:600 (66) | 8.4 × 10−2 |
| II | 1:4 | 8.8:1 | 373:536 (70) | 8.9 × 10−2 |
The donor and recipient strain were injected into the rabbits in a ratio as stated. Each experiment included a pair of rabbits. The induced endocarditis was allowed to persist for 3 days.
Plasma induction requires the pheromone-binding protein PrgZ.
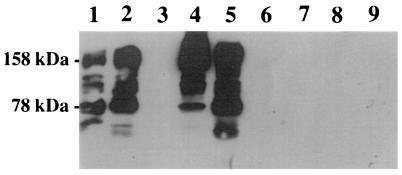
The specificity of the pCF10 plasmid transfer for cCF10 is remarkable. It has been demonstrated that the addition of pheromones cAD1 and cPD1, specific for the transfer of the plasmids pAD1 and pPD1, respectively, did not induce the transfer of pCF10 (13). Alterations in the cCF10 sequence, for example, replacing the second position valine with alanine, led to a drastic reduction in induction capacity (M. H. Antiporta and G. M. Dunny, personal communication). It was therefore of interest to determine if induction in human plasma was dependent on the pheromone-sensing system. The requirement for the import of cCF10 into the cell led us to investigate the potential role of PrgZ, the cCF10-specific OppA homologue. We used a two-plasmid system consisting of plasmid pINY1801, encoding both positive regulatory elements and prgB (encoding AS), and pMSP6043, which contains the negative regulation (prgY) as well as prgZ, the structural gene of the pheromone receptor. This two-plasmid system showed wild-type behavior, as demonstrated previously with lacZ replacing prgB. PrgZ was inactivated by insertion of a spectinomycin cassette in plasmid pMSP6043 (resultant plasmid, pMSP6049), leading to a significant decrease in sensitivity to cCF10 (29). A comparison of the two strains by Western blotting for AS confirmed the marked decrease in sensitivity for cCF10 (Fig. 4). Whereas the wild type (MSP4301) showed induction of AS at a cCF10 concentration as low as 1 ng/ml (Fig. 4, lane 2), the prgZ mutant (MSP4901) remained unresponsive at 1 and 10 ng/ml (lane 6, 7) and required further increases in the cCF10 concentration (lane 5). If the strains were grown in 100% human plasma, MSP4301 showed a pronounced induction of AS, as expected from the results obtained in vivo. No AS induction by human plasma was seen in MSP4901, indicating that the presence of PrgZ was required for expression of the virulence factor AS.
FIG. 4.
AS induction in human plasma is PrgZ dependent. Western blot of cell surface extracts with a polyclonal anti-AS antibody for comparison of AS expression in MSP4301 (prgZ+) and MSP4901 (prgZ mutant). Lanes: 1 to 4, MSP4301; 5 to 9, MSP4901; 1 and 6, 10 ng of cCF10 per ml; 2 and 7, 1 ng of cCF10 per ml; 3 and 8, no peptide; 5, 100 ng of cCF10 per ml; 4 and 9, 100% human plasma. Multiple bands are the result of AS degradation. Cell pellets were washed three times before extraction. An equivalent amount of protein was loaded in each lane.
Plasma influences the activity of cCF10 and iCF10.
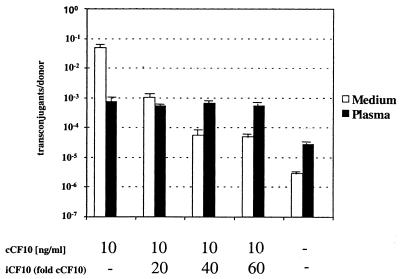
The involvement of PrgZ in induction by human plasma suggested that cCF10 could play a role in the observed AS expression. For this to occur, one of the components of the negative regulatory network would have to be overcome. The first contributing factor outside the cell is the secretion of iCF10, a competitive inhibitor of cCF10, creating an 80:1 molar ratio. A shift in this balance in favor of cCF10 could enable the cell to sense its own pheromone and lead to AS expression. To investigate if human plasma altered the peptide balance, a pCF10 plasmid transfer assay was performed in THB and plasma (Fig. 5). The addition of cCF10 to donor cells at 10 ng/ml led to a transfer rate of 10−2 in comparison to 10−6 in uninduced cells. Addition of a 20- to 60-fold excess of iCF10 reduced the plasmid transfer as expected. Plasmid transfer in plasma showed a 10-fold increase over the background level, in confirmation of the results seen in the Western blot analysis of AS expression. Addition of cCF10 to plasma increased plasmid transfer by another order of magnitude over the level observed with plasma alone, but this level was approximately 100-fold lower than the level observed with the identical concentration of cCF10 in THB. This indicated an interaction of plasma components with cCF10. However, the most striking difference between the experiment performed with human plasma and that performed with THB was the complete lack of effect by the addition of the inhibitor iCF10 in plasma. Neither a 20- nor a 60-fold excess of iCF10 relative to cCF10 could significantly decrease the plasmid transfer in human plasma, whereas 60-fold excess iCF10 reduced plasmid transfer in medium 1,000-fold. This suggested that plasma influenced the balance between the two-peptide activities produced by the donor cells and rendered iCF10 incapable of preventing induction by cCF10.
FIG. 5.
Plasma influences the peptide balance. Influence of addition of different ratios of exogenous cCF10 and iCF10 on the frequency of transconjugants per donor in THB (white bars) and human plasma (black bars). The concentration of cCF10 (1), where added, is 10 ng/ml. The concentration of iCF10 added was 20-, 40-, or 60-fold of that of cCF10, respectively. −, no peptides added. Cells were exposed to inducing conditions for 2 h, and mating between the donor and recipient was performed for 10 min (for details, see Materials and Methods).
DISCUSSION
AS has been established as a virulence factor of E. faecalis in several model systems. Recently, it was reported that AS promotes the adhesion of E. faecalis to fibrin (24) as well as to the extracellular matrix proteins fibronectin, vitronectin, collagen type I, and thrombospondin (36). In direct interaction with cultured mammalial cells, AS-mediated uptake was demonstrated with intestinal epithelial cells (34, 43) and a colon cell line (26). Increased adhesion to renal tubular cells was demonstrated (28). AS promoted increased association of E. faecalis with polymorphonuclear leukocytes (PMNs) (35, 42) and macrophages (39), whereby uptake of the bacteria failed to trigger the normal killing mechanisms of these cells, such as lowered phagosomal pH and the oxidative burst, presumably enabling cells expressing AS to evade host defenses. Fewer data are available for animal models that examined the ability of AS to act as a virulence factor in vivo. Increased vegetation weight in endocarditis was established in the pAD1 system (6) and with the pCF10 AS (38). Although in the work of Chow et al., wild-type pAD1 plasmid was used, this was not the case in the pCF10 system. In the pAD1 system, the wild-type plasmid—with inactivated cytolysin—alone was sufficient for an increase in vegetation weight, suggesting in vivo induction. In addition, Kreft et al. (28) reported clumping of pAD1-containing cells, suggesting AS induction caused by a component in the growth medium of the cultured renal tubular cells. However, no mechanism for this induction or the characterization of the inducing factor was provided. Although fairly similar in structure, the gene regulation of the AS of pAD1 and pCF10 shows some noticeable differences (9). We therefore set out to investigate if AS on the plasmid pCF10 (Asc10) played a role in virulence and was induced in vivo. Rabbits that were challenged with OG1SSp(pCF10) showed significant increases in vegetation weight in comparison to the plasmid-free and AS− strain OG1SSp. This effect was shown to be due to AS, since the strain OG1SSp(pCF121) with a transposon insertion in AS was reduced in its virulence to plasmid-free wild-type levels. The increase in vegetation size was consistent with earlier observations of AS in rabbit endocarditis (6, 38). These results also strongly suggested that Asc10 expression was induced in vivo. This notion was supported by immunogold labeling of Asc10 on the surface of OG1RF(pCF10) cells grown in plasma. No labeling was seen when no pheromone was added to OG1RF(pCF10) cells (24) and with plasmid-free OG1RF, emphasizing the tight control of AS expression and the specificity of the monoclonal antibody, respectively.
E. faecalis is often seen as potential source for mobile elements or plasmids encoding antibiotic resistances and as a threat for transmission of resistance (e.g., vancomycin) to more virulent bacterial species (e.g., Staphylococcus aureus). This possibility is suggested by the ability to mobilize drug resistance determinants into other species (8, 15). It was therefore of interest to evaluate if not only AS but also plasmid transfer of pCF10 was activated in plasma. An in vitro experiment showed increased plasmid transfer into recipient cells and suggested that, in addition to AS expression, plasmid transfer was activated by contact of the cells with human plasma. The relevance of this observation in vivo was obtained by combined infection of rabbits with the donor OG1SSp(pCF10) and the recipient OG1RF. The examination of bacteria in the vegetation revealed that 65 to 70% of OG1RF had received the plasmid. Therefore, efficient plasmid transfer occurred in the context of the rabbit endocarditis model. In fact, the plasmid transfer rate of 10−2 can only be reached in the laboratory if the pheromone cCF10 is added at a concentration of 10 ng/ml, which is 3 magnitudes higher than the natural amount secreted from a recipient (8 pg/ml) (33). Thus, pheromone produced by recipients in vivo may have contributed to in vivo induction, but we believe that it was not the major cause. The shift in ratio of donors to recipients was striking. With inoculum donor/recipient ratios of 2:1 and 1:4, respectively, the population was altered significantly in favor of OG1SSp(pCF10) during in vivo growth, indicating a strong positive selection for possession of plasmid pCF10 and providing a further indication that AS was induced in vivo. The shift in favor of OG1SSp(pCF10) especially in the latter experiment was surprising, since OG1RF transconjugants should gain the same advantage. The predominance of OG1SSp(pCF10) at the end of each experiment might be due to faster elimination of OG1RF in the bloodstream and reduced ability to adhere in the early stages of the experiment. Rakita and coworkers (35) as well as Süsmuth et al. (39) have recently reported that AS-expressing E. faecalis cells, despite the faster uptake into PMNs and macrophages, were able to survive inside these cells for a considerable amount of time. These observations could provide an explanation for the growth advantage of the pCF10 cells seen in our model. Although the phagocytosis of bacterial cells is difficult to achieve for phagocytic cells in a vegetation (18), one might speculate that cells can seed into the bloodstream and adhere again to the vegetation if they are capable of evading phagocytic cells during their time in the bloodstream. This could explain the increased size of the vegetations with AS+ cells. Evasion of the immune system by hiding in PMNs and macrophages could also play a role in the recent observation that antibodies against AS are not protective in rabbit endocarditis (30).
Transfer of the E. faecalis sex pheromone plasmids is exceptionally well controlled, because cells without external stimulation with their respective pheromone do not express AS. In addition, specificity towards the pheromones is also striking. Mating induction experiments involving pCF10, pAD1, and pPD1 have shown that when donor cells carrying two of these plasmids are induced by one of the pheromones, only the cognate plasmid shows elevated transfer (13, 19). The exclusivity and specificity of the sex pheromone system raised the question of how induction of AS can be achieved in vivo, especially in a complex system like plasma. Due to the high specificity, it was of interest to see what influence a lack of the cCF10-specific receptor protein PrgZ would have on the inducibility of AS in plasma. Previous results obtained with prgB::lacZ fusion strains (22) had demonstrated the requirement of PrgZ for response to natural levels of cCF10. Induction of AS expression could be detected on a Western blot at a cCF10 concentration of 1 ng/ml with intact PrgZ. No AS expression was seen when cCF10 was not present, therefore reflecting wild-type pCF10. The absence of PrgZ in strain MSP4901 confirmed the decreased sensitivity for cCF10. Intriguingly, the prgZ mutant was unable to show AS expression in human plasma, demonstrating the absolute requirement for the presence of PrgZ in the plasma induction process.
An obvious hypothesis accounting for the requirement of PrgZ is that plasma can change the activities of iCF10 and cCF10 peptides secreted by donor cells. Plasmid transfer was thus compared in human plasma and THB. At a constant cCF10 concentration, the addition of iCF10 led to a decrease in plasmid transfer as expected. With the use of the competitive inhibitor, human plasma had several interesting effects on the mating. The increase in plasmid transfer in plasma was mentioned above. A further increase of plasmid transfer could be accomplished by adding cCF10; however, the additional inducing effect, while significant, did not reach the level of induction of the same amount of cCF10 added to medium. This suggested that human plasma partially interfered with the action of cCF10. The most surprising result was that the addition of the inhibitor peptide iCF10 did not alter the induction of plasmid transfer induced by the cCF10, in contrast with its effect in laboratory medium. This strongly suggests that iCF10 may be selectively bound or degraded by a component in plasma.
We have demonstrated for the first time that the AS of the sex pheromone plasmid pCF10 was induced in vivo in a rabbit endocarditis model and that this led to an increase in virulence. This observation provides credence for the consideration of AS as a virulence factor of E. faecalis. In addition to the effect on virulence, plasmid transfer was also significantly activated, adding to the concerns that E. faecalis could act as a reservoir for resistance determinants that could be distributed to other bacterial species. Induction was dependent on the pheromone sensor PrgZ, and mechanisms of induction involved interaction of plasma components with the peptides cCF10 and iCF10, since neither can reach its full potential of either induction or inhibition, respectively. The action of the proposed plasma component was more pronounced on the inhibitor peptide, totally abolishing its activity. Our cumulative results to date suggest a possible mechanism for the induction of the virulence factor AS in E. faecalis, a component or components in plasma that interferes with the bacterial produced peptides. The nature of this interference remains to be elucidated. The data also demonstrate for the first time the influence of host factors on a sensing system that previously had been believed was used only for communication between donor and recipient bacterial mating partners.
Acknowledgments
We thank Martin Dinges, Pamela Gahr, Michael Kim, Brian Carlson, and Cécile Ottenwaelter for excellent technical help.
This work was supported by NIH grant HL51987.
Editor: J. D. Clements
REFERENCES
- 1.An, F. Y., M. C. Sulavik, and D. B. Clewell. 1999. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J. Bacteriol. 181:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour, L. M. 1994. Virulence factors among gram-positive bacteria in experimental endocarditis. Infect. Immun. 62:2143-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae, T., S. Clerc-Bardin, and G. M. Dunny. 2000. Analysis of expression of prgX, a key negative regulator of the transfer of the Enterococcus faecalis pheromone-inducible plasmid pCF10. J. Mol. Biol. 297:861-875. [DOI] [PubMed] [Google Scholar]
- 4.Bensing, B. A., D. A. Manias, and G. M. Dunny. 1997. Pheromone cCF10 and plasmid pCF10-encoded regulatory molecules act post-transcriptionally to activate expression of downstream conjugation functions. Mol. Microbiol. 24:285-294. [DOI] [PubMed] [Google Scholar]
- 5.Buttaro, B. A., M. H. Antiporta, and G. M. Dunny. 2000. Cell-associated pheromone peptide (cCF10) production and pheromone inhibition in Enterococcus faecalis. J. Bacteriol. 182:4926-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow, J. W., L. A. Thal, M. B. Perri, J. A. Vazquez, S. M. Donabedian, D. B. Clewell, and M. J. Zervos. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 37:2474-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie, P. J., S.-M. Kao, J. C. Adsit, and G. M. Dunny. 1988. Cloning and expression of genes encoding pheromone-inducible antigens of Enterococcus (Streptococcus) faecalis. J. Bacteriol. 170:5161-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie, P. J., R. Z. Korman, S. A. Zahler, J. C. Adsit, and G. M. Dunny. 1987. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J. Bacteriol. 169:2529-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clewell, D. B. 1999. Sex pheromone systems in enterococci, p. 47-65. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 10.Clewell, D. B., F. Y. An, S. E. Flannagan, M. Antiporta, and G. M. Dunny. 2000. Enterococcal sex pheromone precursors are part of signal sequences for surface lipoproteins. Mol. Microbiol. 35:246-247. [DOI] [PubMed] [Google Scholar]
- 11.Dunny, G., M. Yuhasz, and E. E. Ehrenfeld. 1982. Genetic and physiological analysis of conjugation in Streptococcus faecalis. J. Bacteriol. 151:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunny, G. M. 1991. Electroporation of enterococci, streptococci, and bacilli, p. 302. In G. M. Dunny, P. P. Cleary, and L. L. McKay (ed.), Genetics and molecular biology of streptococci, lactococci, and enterococci. ASM Press, Washington, D.C.
- 13.Dunny, G. M., M. H. Antiporta, and H. Hirt. 2001. Peptide pheromone-induced transfer of plasmid pCF10 in Enterococcus faecalis: probing the genetic and molecular basis for specificity of the pheromone response. Peptides 22:1529-1539. [DOI] [PubMed] [Google Scholar]
- 14.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 75:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunny, G. M., and D. B. Clewell. 1975. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J. Bacteriol. 124:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunny, G. M., R. A. Craig, R. L. Carron, and D. B. Clewell. 1979. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2:454-465. [DOI] [PubMed] [Google Scholar]
- 17.Dunny, G. M., C. Funk, and J. Adsit. 1981. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid 6:270-278. [DOI] [PubMed] [Google Scholar]
- 18.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrenfeld, E. E., R. E. Kessler, and D. B. Clewell. 1986. Identification of pheromone-induced surface proteins in Streptococcus faecalis and evidence of a role for lipoteichoic acid in formation of mating aggregates. J. Bacteriol. 168:6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli, D., A. Friesenegger, and R. Wirth. 1992. Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance. Mol. Microbiol. 6:1297-1308. [DOI] [PubMed] [Google Scholar]
- 21.Galli, D., F. Lottspeich, and R. Wirth. 1990. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol. Microbiol. 4:895-904. [DOI] [PubMed] [Google Scholar]
- 22.Hedberg, P. J., B. A. Leonard, R. E. Ruhfel, and G. M. Dunny. 1996. Identification and characterization of the genes of Enterococcus faecalis plasmid pCF10 involved in replication and in negative control of pheromone-inducible conjugation. Plasmid 35:46-57. [DOI] [PubMed] [Google Scholar]
- 23.Hirt, H., Y. Chen, P. M. Schlievert, and G. M. Dunny. 1998. Use of electroporation in genetic analysis of enterococcal virulence. Methods Cell Sci. 20:79-84. [Google Scholar]
- 24.Hirt, H., S. L. Erlandsen, and G. M. Dunny. 2000. Heterologous inducible expression of Enterococcus faecalis pCF10 aggregation substance Asc10 in Lactococcus lactis and Streptococcus gordonii contributes to cell hydrophobicity and adhesion to fibrin. J. Bacteriol. 182:2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirt, H., G. Wanner, D. Galli, and R. Wirth. 1993. Biochemical, immunological and ultrastructural characterization of aggregation substances encoded by Enterococcus faecalis sex-pheromone plasmids. Eur. J. Biochem. 211:711-716. [DOI] [PubMed] [Google Scholar]
- 26.Isenmann, R., M. Schwarz, E. Rozdzinski, R. Marre, and H. G. Beger. 2000. Aggregation substance promotes colonic mucosal invasion of Enterococcus faecalis in an ex vivo model. J. Surg. Res. 89: 132-138. [DOI] [PubMed] [Google Scholar]
- 27.Kao, S. M., S. B. Olmsted, A. S. Viksnins, J. C. Gallo, and G. M. Dunny. 1991. Molecular and genetic analysis of a region of plasmid pCF10 containing positive control genes and structural genes encoding surface proteins involved in pheromone-inducible conjugation in Enterococcus faecalis. J. Bacteriol. 173:7650-7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreft, B., R. Marre, U. Schramm, and R. Wirth. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect. Immun. 60:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard, B. A., A. Podbielski, P. J. Hedberg, and G. M. Dunny. 1996. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 93:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick, J. K., H. Hirt, C. M. Waters, T. J. Tripp, G. M. Dunny, and P. M. Schlievert. 2001. Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis. Infect. Immun. 69:3305-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megran, D. W. 1992. Enterococcal endocarditis. Clin. Infect. Dis. 15:63-71. [DOI] [PubMed] [Google Scholar]
- 32.Mori, M., Y. Sakagami, Y. Ishii, A. Isogai, C. Kitada, M. Fujino, J. C. Adsit, G. M. Dunny, and A. Suzuki. 1988. Structure of cCF10, a peptide sex pheromone which induces conjugative transfer of the Streptococcus faecalis tetracycline resistance plasmid, pCF10. J. Biol. Chem. 263:14574-14578. [PubMed] [Google Scholar]
- 33.Nakayama, J., G. M. Dunny, D. B. Clewell, and A. Suzuki. 1995. Quantitative analysis for pheromone inhibitor and pheromone shutdown in Enterococcus faecalis. Dev. Biol. Stand. 85:35-38. [PubMed] [Google Scholar]
- 34.Olmsted, S. B., G. M. Dunny, S. L. Erlandsen, and C. L. Wells. 1994. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J. Infect. Dis. 170:1549-1556. [DOI] [PubMed] [Google Scholar]
- 35.Rakita, R. M., N. N. Vanek, K. Jacques-Palaz, M. Mee, M. M. Mariscalco, G. M. Dunny, M. Snuggs, W. B. Van Winkle, and S. I. Simon. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect. Immun. 67:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozdzinski, E., R. Marre, M. Susa, R. Wirth, and A. Muscholl-Silberhorn. 2001. Aggregation substance-mediated adherence of Enterococcus faecalis to immobilized extracellular matrix proteins. Microb. Pathog. 30:211-220. [DOI] [PubMed] [Google Scholar]
- 37.Ruhfel, R. E., D. A. Manias, and G. M. Dunny. 1993. Cloning and characterization of a region of the Enterococcus faecalis conjugative plasmid, pCF10, encoding a sex pheromone-binding function. J. Bacteriol. 175:5253-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlievert, P. M., P. J. Gahr, A. P. Assimacopoulos, M. M. Dinges, J. A. Stoehr, J. W. Harmala, H. Hirt, and G. M. Dunny. 1998. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect. Immun. 66:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Süsmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tortorello, M. L., J. C. Adsit, D. Krug, D. Antczak, and G. M. Dunny. 1986. Monoclonal antibodies to cell surface antigens involved in sex pheromone induced mating in Streptococcus faecalis. J. Gen. Microbiol. 132:857-864. [DOI] [PubMed] [Google Scholar]
- 41.Tortorello, M. L., and G. M. Dunny. 1985. Identification of multiple cell surface antigens associated with the sex pheromone response of Streptococcus faecalis. J. Bacteriol. 162:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanek, N. N., S. I. Simon, K. Jacques-Palaz, M. M. Mariscalco, G. M. Dunny, and R. M. Rakita. 1999. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol. Med. Microbiol. 26:49-60. [DOI] [PubMed] [Google Scholar]
- 43.Wells, C. L., E. A. Moore, J. A. Hoag, H. Hirt, G. M. Dunny, and S. L. Erlandsen. 2000. Inducible expression of Enterococcus faecalis aggregation substance surface protein facilitates bacterial internalization by cultured enterocytes. Infect. Immun. 68:7190-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]