Abstract
Oropharyngeal candidiasis is associated with defects in cell-mediated immunity and is commonly seen in human immunodeficiency virus positive individuals and AIDS patients. A model for oral candidiasis in T-cell-deficient BALB/c and CBA/CaH nu/nu mice was established. After inoculation with 108 Candida albicans yeasts, these mice displayed increased levels of oral colonization compared to euthymic control mice and developed a chronic oropharyngeal infection. Histopathological examination of nu/nu oral tissues revealed extensive hyphae penetrating the epithelium, with polymorphonuclear leukocyte microabscess formation. Adoptive transfer of either naive or immune lymphocytes into immunodeficient mice resulted in the recovery of these animals from the oral infection. Reconstitution of immunodeficient mice with naive CD4+ but not CD8+ T cells significantly decreased oral colonization compared to controls. Interleukin-12 and gamma interferon were detected in the draining lymph nodes of immunodeficient mice following reconstitution with naive lymphocytes. This study demonstrates the direct requirement for T lymphocytes in recovery from oral candidiasis and suggests that this is associated with the production of cytokines by CD4+ T helper cells.
Oral candidiasis is a predominant feature in patients suffering from human immunodeficiency virus (HIV) and AIDS (28, 35), and these patients, as well as those with chronic mucocutaneous candidiasis (27), commonly exhibit defects in cell-mediated immunity.
The homozygous athymic nude mouse (nu/nu) is deficient in T lymphocyte number and function, analogous to DiGeorge syndrome in humans. In nu/nu mice, the thymus is almost totally absent due to the failure of the development of the thymic anlage, which arises from the ectoderm of the third pharyngeal pouch (36). This leads to many defects in the immune system, including depletion of lymphocytes from thymus-dependent areas of lymph nodes and spleen, a much reduced lymphocyte population composed mostly of B cells, relatively normal immunoglobulin M (IgM) response to thymus-independent antigens, very poor response to thymus-dependent antigens, and greatly increased susceptibility to infection (37).
T-cell-deficient nude mice have been widely used to evaluate the role of cell-mediated immune responses against Candida albicans infections in systemic (12, 19, 22, 30, 39), vaginal (10, 11), and mucosal (gastrointestinal) (13, 14) models of the disease. Work by our group (C. S. Farah, T. Gotjamanos, and R. B. Ashman, submitted for publication) has shown that an oral infection of mild to moderate severity and relatively short duration could be established in inbred mice of different genetic backgrounds. However, although oral C. albicans infection is known to elicit cell-mediated immune responses, there is no unequivocal evidence that T cells are essential for recovery from a primary infection with this yeast. A model of oral candidiasis in immunodeficient mice would allow immunological manipulation of the system and facilitate evaluation of the role of T cells in this infection.
As clinical observations support a role for T cells in oral candidiasis, T-cell-deficient mice provide an ideal model to study the role of T-cell-mediated immunity in oral candidiasis. The present paper reports the establishment of an oral C. albicans infection in T-cell-deficient mice and demonstrates the critical role of T cells in the clearance of the yeast from the oral tissues after primary infection.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free BALB/c and CBA/CaH female nude mice, their heterozygous littermates, and euthymic controls, 6 to 8 weeks of age, were purchased from the Animal Resources Centre, Perth, or from the Walter and Eliza Hall Institute, Melbourne, Australia. These mice undergo routine microbiological screening and do not harbor C. albicans in the gut. Animal experiments were approved by the Animal Experimentation Ethics Committee of the University of Queensland and were carried out in accordance with the National Health and Medical Research Council's Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 1997. Mice were housed in standard cages and provided with food and water ad libitum.
Yeast.
C. albicans isolate 3630, derived from a patient with cutaneous candidiasis, was obtained from the Mycology Reference Laboratory at the Royal North Shore Hospital, Sydney, Australia, and stored at −70°C in Sabouraud's broth with 15% (vol/vol) glycerol. For use, yeasts were grown in Sabouraud's broth for 48 h at room temperature with continuous agitation on a magnetic stirrer. Blastospores were washed in phosphate-buffered saline (PBS) and adjusted to the appropriate concentration for inoculation.
Oral infection.
Mice were inoculated orally with 108 live C. albicans yeasts in 20 μl of PBS. The infection was monitored by swabbing the oral cavity with sterile cotton swabs moistened with sterile PBS and plating on Sabouraud's agar plates. Agar plates were incubated for 48 h at 37°C. All inoculation and sampling procedures were conducted under halothane anesthesia using an inhalation apparatus and a scavenging system. CFU were counted on Sabouraud's agar plates. The counts were assigned into five groups correlating with the level of recoverable yeasts from the oral cavity. This provided a semiquantitative measure of the level of floridity of the infection. The scoring system used was as follows: 0, no detectable yeasts; 1, 1 to 10 CFU/plate; 2, 11 to 100 CFU/plate; 3, 101 to 1,000 CFU/plate; and 4, 1,000+ CFU/plate.
Histopathology.
Mice were sacrificed at various time points throughout the course of the experiment for histopathological examination of tissues. Thymus glands were fixed in 10% neutral buffered formalin (pH 7.0), sectioned, and stained with hematoxylin and eosin. Skulls were also fixed in buffered formalin and decalcified in a 5% formic acid-sodium formate mixture. Frontal sections of the skull were taken at approximately 3-mm intervals, with consecutive sections stained with hematoxylin and eosin and according to the periodic acid-Schiff technique. Sections were examined by light microscopy.
Footpad injection.
Mice were injected subcutaneously in the footpad with 107 heat-killed C. albicans yeasts in 20 μl of PBS. This dose has previously been shown to produce optimal delayed-type hypersensitivity responses in mice (3). The contralateral footpad was injected with an equal volume of sterile PBS. Footpad thickness was measured using dial calipers (Mitutoyo). Each measurement was repeated three times on a minimum of five mice.
Preparation of splenocytes.
Spleens were removed from donor mice, and spleen cells were obtained by pressing the spleens through a sterile metal sieve, followed by filtration through an 80-μm nylon mesh. The cells were resuspended in 6 ml of PBS, and lymphocytes were separated on a Ficoll gradient by underlaying the cell suspension with 4 ml of Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). The gradient was centrifuged at 700 × g for 25 to 30 min at room temperature. The buffy coat interface was carefully removed, washed twice in PBS, and resuspended in 1 ml of PBS. Viable lymphocytes were counted in a hemacytometer after staining with trypan blue dye and adjusted to the appropriate concentration for injection into recipient mice.
Adoptive lymphocyte transfer in immunodeficient mice.
At the height of oral infection (day 6 following oral challenge), 3 × 107 nonimmune lymphocytes from naive donor mice or 3 × 107 immune lymphocytes from previously immunized donor animals were injected intravenously into the respective immunodeficient recipient mice. Control animals received no cells and were used to monitor the normal course of infection. Donor mice were primed orally with 108 viable C. albicans yeasts and rested for 4 to 8 weeks before use.
Thymus transplant.
Immunodeficient mice were inoculated orally with 108 viable yeasts. At the height of infection (day 6), thymuses from naive inbred mice, 6 to 8 weeks of age, were surgically removed and transplanted into nude animals subcutaneously under the right axilla. Control animals had sham operations performed. The surgical sites were closed with wound clips. At the termination of the experiment, the transplant site was examined visually and histologically to determine whether grafting of the thymus was successful.
Mouse CD4+ and CD8+ T-cell enrichment.
T-cell enrichment was carried out using commercially available CD4+ and CD8+ mouse T-cell enrichment columns (R&D, Minneapolis, Minn.), according to the manufacturer's instructions. Spleen lymphocytes from naive inbred mice were obtained as described above and separated into CD4+ and CD8+ T cells on high-affinity negative-selection columns. Briefly, 1 ml of monoclonal antibody cocktail was mixed with 2 × 108 leukocytes in single-cell suspension and incubated at room temperature for 15 min. The cells were washed twice in 1× column buffer and resuspended in 2 ml of the same buffer. The antibody-treated cells were then applied to a subset column, incubated at room temperature for 10 min, and eluted with 10 ml of 1× column buffer. The isolated cells were centrifuged at 200 × g for 5 min and suspended in PBS at the appropriate concentration for injection.
Fluorescence-activated cell sorting (FACS) analysis (CellQuest version 3.1, FACSCalibur; Becton Dickinson, San Jose, Calif.) using fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4 (IgG2a) and R-phycoerythrin (PE)-conjugated rat anti-mouse CD8 (IgG2a) antibodies (Pharmingen, San Diego, Calif.) confirmed that the purity of cells isolated using these columns was 93.7% for CD4 and 89.8% for CD8.
CD4+ and CD8+ T-cell reconstitution.
BALB/c nude mice were infected with 108 C. albicans yeasts. At the height of oral infection (day 6 following oral challenge), nude recipient mice were reconstituted with naíve CD4+ or CD8+ T cells as follows: either 2.5 × 106 CD4+ cells, 2.5 × 106 CD8+ cells, or a combination of 1.5 × 106 CD4+ and 1.5 × 106 CD8+ cells. A positive control group received 3 × 107 naíve lymphocytes, while a negative control group received no cells.
Cell surface antigen staining.
Spleens or lymph nodes were isolated as described earlier and suspended in 0.1% PBS-NaN3. No Ficoll treatment was required for lymph node cells. A total of 106 cells were stained in 50 μl of 0.1% PBS-NaN3 for 30 min at 4°C in the dark, using an appropriate concentration of a fluorochrome-conjugated monoclonal antibody specific for a cell surface antigen or isotype control: 1 μl, 500 μg of FITC-rat anti-mouse CD4 (IgG2a); 1.5 μl, 200 μg of R-PE-rat anti-mouse CD8 (IgG2a); 1 μl, 500 μg of FITC-rat IgG2a isotype control immunoglobulin; and 1.5 μl, 200 μg of R-PE-rat IgG2a isotype control immunoglobulin (Pharmingen, San Diego, Calif.) per ml. The cells were washed twice in 0.1% PBS-NaN3 at 350 × g for 5 min and resuspended in 500 μl of 1% formalin. Cells were analyzed by FACS.
Cytokine ELISA.
Lymphocytes isolated from the submandibular and superficial cervical lymph nodes were cultured ex vivo for 3 days at 4 × 106 cells/ml in RPMI 1640 tissue culture medium without antigen stimulation. The supernatant was collected, filtered through a 0.8-μm filter, and stored at −20°C until analyzed. The culture supernatant was assayed for interleukin-4 (IL-4), IL-10, IL-12, and gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) using matched antibody pairs and recombinant cytokines as standards. Briefly, Immuno-Polysorb microtiter plates (Nunc, Rothskild, Denmark) were coated with capture rat monoclonal anti-IL-4 (IgG1), IL-10 (IgG1), IL-12 (IgG2a), or IFN-γ (IgG1) antibody (Pharmingen) at 1 μg/ml in sodium bicarbonate buffer overnight at 4°C. The wells were washed and then blocked with 1% bovine serum albumin-PBS before the culture supernatants and the appropriate standard were added to each well. Biotinylated rat monoclonal anti-IL-4, -IL-10, -IL-12, or -IFN-γ antibody (Pharmingen) at 2 μg/ml was added as the second antibody. Detection was carried out with streptavidin-peroxidase and tetramethylbenzidine. The results were expressed as net Candida-induced counts, from which the background was subtracted, and are shown as supernatant cytokine titer (picograms per milliliter) (mean ± standard error of the mean [SEM]) from lymphocytes pooled from a minimum of three mice per group per time point.
RT-PCR.
For reverse transcription-PCR (RT-PCR), total RNA was extracted from oral tissues using Ultraspec RNA reagent (Biotecx Laboratories, Houston, Tex.) according to the manufacturer's instructions. cDNA was prepared by reverse transcription of 1 μg of each RNA, using an oligo(dT)15 primer and avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.). Briefly, 4 μl of 25 mM MgCl2 solution, 2 μl of 10× PCR amplification buffer [670 mM Tris-HCl, 166 mM (NH4)2SO4, 4.5% Triton X-100, 2 mg of gelatin/ml] (BioTech International, Nedlands, Australia), 2 μl of a 10 mM deoxynucleoside triphosphate (dNTP) mix, 0.5 μl of RNasin, 0.75 μl (15 U) of avian myeloblastosis virus reverse transcriptase, 0.5 μg of oligo(dT)15 primer, and 1 μg of an mRNA sample were incubated in a 20-μl reaction mix at 42°C for 1 h, heated to 99°C for 5 min, then cooled on ice. cDNA was amplified by PCR in an amplification mix consisting of 2 μl of 25 mM MgCl2, 2.5 μl of 10× reaction buffer, 2 μl of 25 mM dNTP mix, 0.5 U of Taq DNA polymerase (Geneworks, Thebarton, Australia), the appropriate primer, and 1 μl of cDNA in a total volume of 25 μl. Negative controls (without cDNA) were included for all primers used in each run.
The mixture was amplified using a PTC-100 programmable thermal cycler (MJ Research Inc.). The amplification protocol was 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min for 35 to 40 cycles. Following amplification, 10 μl of product was analyzed by electrophoresis through 2.5% (wt/vol) agarose gels. The gels were stained with ethidium bromide and the bands were visualized with a UV transilluminator (GelDoc 2000; Bio-Rad, Regents Park, Australia) with appropriate software (MultiAnalyst version 1.1; Bio-Rad, Hercules, Calif.). Primer sequences for CD4 and CD8 were obtained from published data (34) and synthesized by Geneworks, Adelaide, South Australia. Data are representative of at least two separate experiments.
Statistics.
Quantitative data were analyzed using the statistical features of GraphPad Prism version 2.01 (GraphPad Inc., San Diego, Calif.). Student's t test and one-way analysis of variance were used, with a P value of <0.05 considered significant unless otherwise stated.
RESULTS
Colonization levels in nu/nu and nu/+ mice.
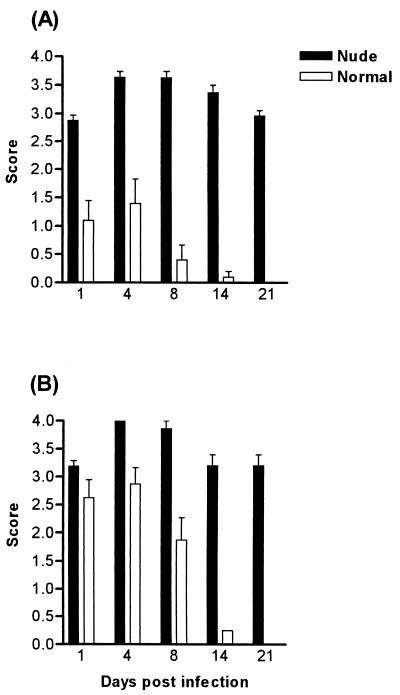
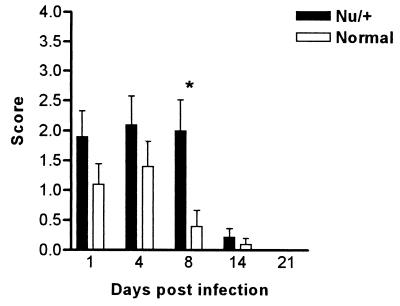
Athymic (nude) mice bred on the BALB/c and CBA/CaH backgrounds were infected orally with 108 C. albicans yeasts and monitored for 21 days by swabbing the oral cavity. In both strains, higher numbers of yeasts were recovered from the oral cavities of these animals compared to euthymic controls, and the severity and the duration of the infection were significantly greater (P < 0.001) (Fig. 1). The mice were chronically colonized, with the infection lasting more than 70 days (data not shown). In the BALB/c nu/+ heterozygous mice, the infection was more severe than that seen in the euthymic mice, but colonization levels were only significantly different on day 8 for nu/+ mice (Fig. 2). The nu/+ mice also cleared the infection within 21 days.
FIG. 1.
Time course of C. albicans clearance in BALB/c nu/nu (A) and CBA/CaH nu/nu (B) immunodeficient mice compared to wild-type controls following oral infection with 108 C. albicans yeasts. Bars represent scores (mean ± SEM) for a minimum of 30 mice (10 mice/group in three separate experiments). SEM is 0 if error bars are not shown. All groups were significantly different (P < 0.001) except CBA/CaH nu/nu and CBA/CaH on day 1.
FIG. 2.
Time course of C. albicans clearance in heterozygous BALB/c nu/+ and wild-type +/+ counterparts following oral infection with 108 C. albicans yeasts. Bars represent scores (mean ± SEM) for a minimum of 10 mice/group. Each experiment was repeated twice. ∗, significantly different at P < 0.05.
Histopathology of oral tissues.
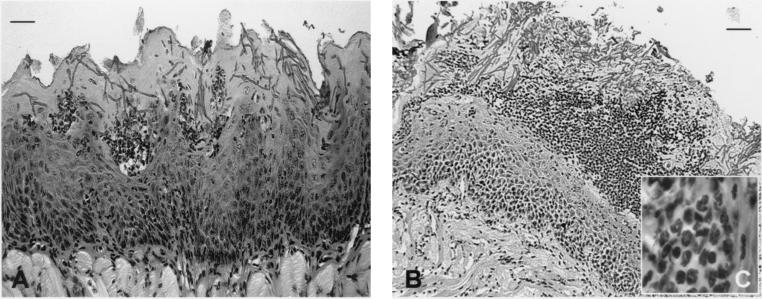
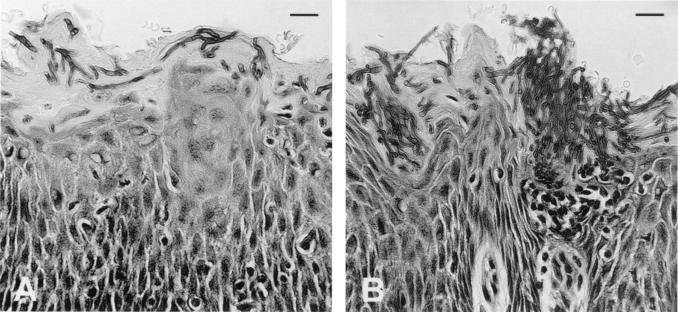
Histopathological examination of the oral tissues from infected BALB/c (Fig. 3A) and CBA/CaH nu/nu (Fig. 3B) mice showed hyphal elements penetrating the keratinized epithelium and microabscess formation comprising mostly polymorphonuclear phagocytes (Fig. 3C). The two strains differed in the severity of the infection, with an increased fungal burden seen in CBA/CaH nu/nu mice (Fig. 3B). Sections of the tongue showed a markedly hyperplastic irregular keratinized epithelium, with almost complete loss of surface papillae, acanthosis, and thickening and blunting of rete pegs. There was also a heavy surface hyperkeratosis. The dorsal surface of the tongue carried an extensive and heavy superficial infiltration of Candida cells. The tissues were acanthotic and showed a loss of distinct stratum granulosum and a consequent decrease in surface keratinization. The basal half was loosely textured, with predominant intercellular edema. There were multiple foci of transmigrating neutrophils and a lesser number of superficial intraepithelial neutrophil accumulations, consistent with a Munro's microabscess (Fig. 3C).
FIG. 3.
Histopathological sections of tongue from immunodeficient BALB/c nu/nu (A) and CBA/CaH (B) nu/nu mice on day 6 after oral C. albicans infection with 108 yeasts. There is heavy infiltration of Candida hyphae penetrating the superficial hyperkeratotic epithelium, and large numbers of PMNLs forming microabscesses (C). Sections were stained with periodic acid-Schiff. Bars, 50 μm (A) and 100 μm (B). Total magnification, ×600 (C).
The microabscesses were mostly confined to the superficial zone of the epithelium in what might be described as a pseudo-membrane (Fig. 3). The superficial lamina propria was fibrotic and contained a moderately dense inflammatory infiltrate that was predominantly lymphocytic/lymphohistiocytic, with scattered neutrophils and occasional eosinophils. In CBA/CaH nu/nu mice, the gingival tissues showed a heavily hyperplastic keratinized epithelium. The sulcular and crestal gingival tissues showed marked infection with candidal hyphae and associated intraepithelial abscess formation consisting of clusters of neutrophils, with some neutrophils present in the superficial lamina propria (data not shown).
Thymus transplantation.
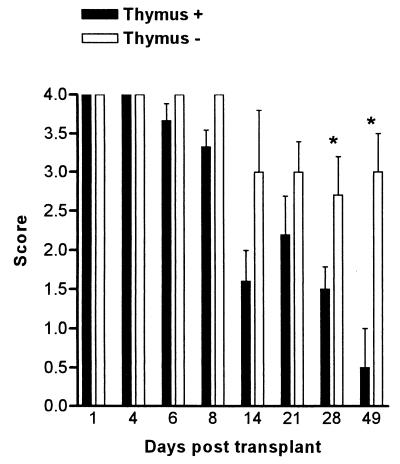
BALB/c nu/nu mice were transplanted with thymus glands from naive syngeneic mice 6 to 8 weeks of age. The thymus was transplanted into nude BALB/c mice 6 days after colonization with C. albicans. Mice that received the thymus transplant significantly reduced the infection by day 49, whereas control animals that only had sham surgery performed were still heavily colonized with the yeast (Fig. 4).
FIG. 4.
Time course of C. albicans clearance in BALB/c nu/nu mice after thymus transplantation. Bars represent scores (mean ± SEM) for a minimum of 10 mice/group. Each experiment was repeated twice. Comparison was made between animals that received a thymus transplant (Thymus +) and controls (Thymus −) that received no thymus. ∗, significantly different at P < 0.001. SEM is 0 if error bars are not shown.
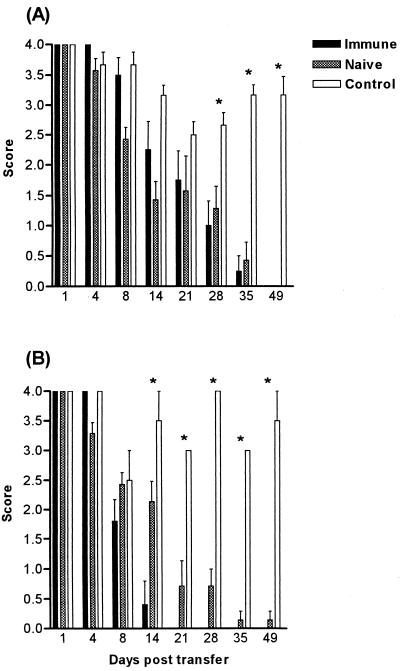
Adoptive transfer of naive and immune lymphocytes.
BALB/c and CBA/CaH nu/nu mice were adoptively transferred with 3 × 107 lymphocytes from animals of the same background. The lymphocytes were collected from either naive or immune (orally primed) animals. Control animals received no cells. BALB/c nude mice that received either naive or immune lymphocytes cleared the infection by day 49, whereas the control mice were still heavily colonized (P < 0.001) (Fig. 5A). CBA/CaH nude mice that received naive cells also significantly reduced the Candida infection by day 49, whereas animals that received immune cells were able to clear the infection by day 21 (Fig. 5B). A comparison of clearance rates following the transfer of either naive or orally primed lymphocytes revealed no significant difference in BALB/c nude mice, but showed accelerated clearance in CBA/CaH mice that received immune cells (Fig. 5).
FIG. 5.
Time course of C. albicans clearance in BALB/c nu/nu (A) and CBA/CaH nu/nu (B) mice reconstituted with 3 × 107 immune (orally primed) or naive lymphocytes. Bars represent scores (mean ± SEM) for a minimum of 10 mice/group. Each experiment was repeated twice. Comparison was made between animals that received lymphocytes and controls that received no cells. ∗, significantly different at P < 0.001. SEM is 0 if error bars are not shown.
Reconstitution with CD4+ and CD8+ T lymphocytes.
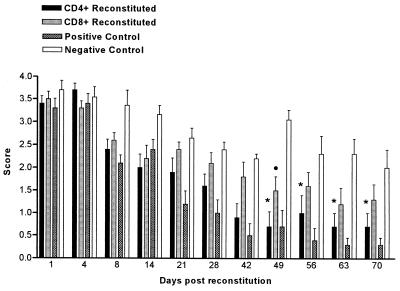
Reconstitution of infected BALB/c immunodeficient mice with naive CD4+ T cells resulted in a significant clearance of the yeast from the oral cavity of these animals and eventual recovery from chronic oral candidiasis (Fig. 6). The difference in colonization levels was significant (P < 0.001) from day 49 onwards. Animals reconstituted with CD4+ T cells alone followed a similar pattern of clearance to those reconstituted with a pool of lymphocytes. CD8+ T cells alone were unable to clear the infection in nu/nu mice, although there was a slight decrease in colonization levels compared to animals that received no cells. At day 70, these animals still showed significantly higher levels of colonization compared to positive control animals reconstituted with lymphocytes (Fig. 6). Animals reconstituted with a mixed population of CD4+ and CD8+ T cells were able to clear the infection in a fashion similar to that of mice administered CD4+ cells alone. Colonization levels were significantly less than in controls (P < 0.001) from day 49 onwards (data not shown).
FIG. 6.
Time course of C. albicans clearance in BALB/c nu/nu mice reconstituted with 2.5 × 106 naive CD4+ or CD8+ T cells. The positive control group received 3 × 107 naive lymphocytes, while the negative control group received no cells. Bars represent scores (mean ± SEM) for a minimum of 10 mice/group. Each experiment was repeated twice. Comparison was made between animals that received either CD4+ or CD8+ T cells and negative controls. ∗, significantly different at P < 0.001 for CD4 group; •, significantly different at P < 0.05 for CD8 group. Positive control group was significantly different from negative control group at P < 0.001 from days 21 to 70.
Footpad swelling in immunodeficient mice after lymphocyte reconstitution and thymus transplant.
BALB/c mice reconstituted with immune lymphocytes showed greater footpad swelling (P < 0.001) than control mice that received no lymphocytes and mice that received naive cells at 24, 48, and 96 h after footpad injection (Table 1). Measurement of footpad swelling in CBA/CaH mice also showed a difference between the immune and the naíve and control groups (Table 1). Footpad swelling was significantly greater (P < 0.001) in mice that received immune lymphocytes than in those that received naive cells. This situation existed for both BALB/c and CBA/CaH mice at all time points following footpad injections (Table 1). In thymus-transplanted mice, footpad swelling with heat-killed Candida yeasts was significantly greater (P < 0.05) than that with PBS (data not shown).
TABLE 1.
Footpad swelling following lymphocyte transfer in BALB/c and CBA/CaH nu/nu mice infected with C. albicansa
| Time (h) | Mean (SEM) increase in footpad thickness, mm
|
||||||
|---|---|---|---|---|---|---|---|
| BALB/c mice
|
CBA/CaH mice
|
||||||
| Immune | Naive | Control | Immune | Naive | Control | ||
| 24 | 2.06 (0.11) | 0.71 (0.05) | 0.43 (0.02) | 1.66 (0.08) | 0.75 (0.04) | 0.68 (0) | |
| 48 | 2.11 (0.22) | 0.70 (0.09) | 0.55 (0.08) | 2.023 (0.09) | 0.69 (0.059) | 0.58 (0.1) | |
| 96 | 1.85 (0.29) | 0.15 (0.02) | 0.05 (0.01) | 1.54 (0.11) | 0.29 (0.04) | 0.45 (0.02) | |
Results are presented as mean (SEM) increase in footpad thickness following injection with 107 heat-killed C. albicans yeasts in a minimum of five mice that were reconstituted with either immune or naive lymphocytes. Each experiment was repeated twice. Comparison was made between animals that received lymphocytes and controls that received no cells, and results were significantly different at P < 0.001 only for animals that received immune cells compared to controls at all time points.
There was a positive footpad response in mice reconstituted with naive CD4+ cells alone or a combination of CD4+ and CD8+ T cells (data not shown). The footpad response seen in mice reconstituted with naive CD8+ cells alone was similar to that in control animals (no lymphocyte transfer) and significantly less (P < 0.01) than that of CD4+-reconstituted mice (data not shown).
Histopathology of oral tissues in nu/nu mice following lymphocyte reconstitution.
Following lymphocyte reconstitution in nude BALB/c mice, histopathological sections of the tongue showed markedly fewer Candida hyphae penetrating the superficial epithelium (Fig. 7A). The neutrophilic inflammatory infiltrate was less obvious, but intraepithelial polymorphonuclear lymphocytes (PMNLs) were still found, and the lamina propria was mildly lymphocytic (Fig. 7).
FIG. 7.
Histopathological section of tongue from immunodeficient BALB/c nu/nu mouse following lymphocyte reconstitution (A) (day 35 posttransfer), and infected control (B) that received no cells. Mice were infected with 108 C. albicans yeasts and reconstituted with 3 × 107 naive lymphocytes on day 6 postinfection. Sections show fewer hyphae penetrating the epithelium in the reconstituted mouse, with some intraepithelial PMNLs (A), compared to the heavy hyphal penetration and PMNL microabscess formation in the untreated control (B). Sections were stained with periodic acid-Schiff. Bars, 45 μm.
CD4+ and CD8+ T-cell expression in lymph nodes and spleen after reconstitution.
The majority of T cells detected in lymph node and spleen of naive nude BALB/c mice were phenotypically CD8+. Upon reconstitution of immunodeficient mice with naive lymphocytes obtained from spleens of syngeneic mice, the percentage of CD4+ T cells rose to approximately 25% on day 4 and remained generally the same up to day 21 after lymphocyte transfer (data not shown). The CD8+ subpopulation increased from about 4% on day 0 to 15% on day 4 and remained between 15 and 20% till day 21. A CD4-to-CD8 ratio of 2:1 was achieved by day 4 after lymphocyte transfer in both spleen and draining lymph nodes. This was sustained in the spleen, but decreased to approximately 1:1 in the submandibular and superficial cervical lymph nodes on days 8 and 14 (data not shown).
CD4 and CD8 gene expression in oral tissues.
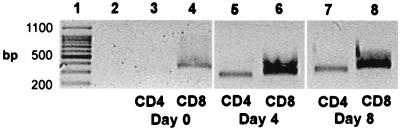
Since CD4+ and CD8+ T cells were capable of repopulating the draining lymph nodes and spleen in nude mice after lymphocyte reconstitution, it was desirable to demonstrate the presence of message for CD4 and CD8 in the oral tissues of these mice by RT-PCR. Agarose gels stained with ethidium bromide revealed no CD4 in oral tissues of nude mice infected with C. albicans without lymphocyte reconstitution (Fig. 8, lane 3). CD4 gene expression was detected in oral mucosa on days 4 and 8 after lymphocyte reconstitution (Fig. 8, lanes 5 and 7, respectively). CD4 was not detected in the tongue until day 21 following lymphocyte transfer (data not shown). CD8 was detected in infected controls (Fig. 8, lane 4) and reconstituted animals at all time points examined (Fig. 8, lanes 6 and 8).
FIG. 8.
Representative agarose gel stained with ethidium bromide showing CD4 and CD8 gene products in BALB/c nu/nu mice following lymphocyte reconstitution. RT-PCR was performed on BALB/c tongue and oral mucosa. Lane 1 shows size markers, while lane 2 shows the cDNA negative control for each gel. CD4 is not present in control nude mice (lane 3), whereas CD8 is present at all times (lanes 4, 6, and 8). CD4 is present in oral mucosa on day 4 (lane 5) and day 8 (lane 7) following lymphocyte transfer, but is not seen in the tongue until day 21 (data not shown).
Cytokine production by lymph node cells by ELISA.
ELISAs conducted on culture supernatant of lymphocytes from the draining lymph nodes of nude mice following lymphocyte reconstitution showed that the overwhelming cytokine produced was IL-12. This was apparent on days 8 (1,625 ± 262.1 pg/ml), 14 (1,059 ± 93.84 pg/ml), and 21 (1,405 ± 150 pg/ml) following lymphocyte transfer. There was some IFN-γ produced on day 8 (442.2 ± 68.93 pg/ml), but this soon returned to baseline levels on days 14 (46.38 ± 4.37) and 21 (46.23 pg/ml). Only very small amounts of IL-10 (38.9 to 67.7 pg/ml) were produced, with no IL-4 detected in the cell supernatant at any time point.
DISCUSSION
The current study demonstrates that BALB/c and CBA/CaH nu/nu mice are naturally susceptible to oral candidiasis and provide an excellent model to study various aspects of susceptibility and resistance to this disease. BALB/c nu/+ mice were slightly more susceptible to oral infection than their euthymic congenic counterparts, but less susceptible than the immunodeficient nu/nu mice. A prolonged chronic oral infection could be established in nu/nu mice which did not resolve for at least 70 days, confirming a requirement for T cells in recovery from this infection.
Previous work from our laboratory (21; Farah et al., submitted), along with other studies of experimental C. albicans infections of the mucosa (9, 17, 29, 33), has shown small numbers of yeasts and hyphae colonizing the palate and tongue of normal (euthymic) mice. Histopathological examination of the oral tissues from BALB/c and CBA/CaH nu/nu mice showed abundant hyphal elements penetrating the epithelium and microabscess formation, consisting mostly of polymorphonuclear phagocytes. The pseudomembrane-like appearance of C. albicans lining the oral cavity in nu/nu mice, especially CBA/CaH, is very similar to that in clinical thrush observed in patients with HIV and AIDS (28, 35). The increased fungal burden in the oral cavity of the CBA/CaH nu/nu strain might be related to the genetic susceptibility of the CBA/CaH strain to both oral (Farah et al., submitted) and systemic colonization (1-3, 5, 6).
The chronic oral infection established in nude mice provided a system in which immunological manipulation was exploited to confirm the importance of T cells in this infection. Nude mice that received a thymus transplant cleared the infection, as did mice that received syngeneic lymphocytes, confirming a role for T cells and thymus-dependent immunity in this model of oral infection. However, despite the difference in the severity of oral colonization, there was no difference in the rate of clearance between BALB/c nu/nu and CBA/CaH nu/nu mice after the transfer of naive lymphocytes into these mice. This is consistent with previous observations that tissue susceptibility to C. albicans is determined by innate and not by adaptive immune responses (7).
Surprisingly, lymphocytes transferred from orally primed animals markedly accelerated clearance in the CBA/CaH nude mice, but were no more effective than naive cells in promoting clearance of the yeast from the oral cavity of BALB/c nu/nu mice. This result is consistent with the increased antibody-mediated resistance against systemic challenge displayed by immune CBA/CaH compared to BALB/c mice (4, 8). The basis for this difference is unknown at present; however, BALB/c mice appear to have stronger innate immune defenses than CBA/CaH mice, so that the window for immunological enhancement of resistance is smaller in this strain. Since the host response is most probably mediated by cytokines produced by CD4+ T cells, it is possible that after activation, cells from naive mice can deliver levels of cytokines that are sufficient for optimal or near optimal recruitment and/or activation of the phagocytic effector cells. If this is the case, the increased number of Candida-specific cells present in the immune cell population may only be able to make a small difference to the magnitude or rapidity of the host response, particularly in the BALB/c strain.
There was a strong expression of delayed-type hypersensitivity in both BALB/c and CBA/CaH nude mice following the adoptive transfer of lymphocytes from primed but not naive mice. This is in sharp contrast to the patterns of clearance of oral infection after adoptive transfer of immune and naive cells discussed above, but is consistent with the reported lack of correlation between the magnitude of delayed-type hypersensitivity responses and clearance of systemic candidiasis (23). B-cell epitopes are known to differ between mouse strains (18), and it is likewise possible that subsets of CD4+ T cells also recognize different antigenic peptides of C. albicans presented in the context of major histocompatibility complex class II. Thus, delayed-type hypersensitivity cells may represent a memory subpopulation of CD4+ cells whose functions are irrelevant to the recruitment and/or activation of the effector cells responsible for clearance of the yeast from the oral cavity.
Naive CD4+ and CD8+ T-cell population subsets separated by a negative selection protocol were introduced intravenously into BALB/c nu/nu mice to examine the contribution of each to recovery from oral infection. The results show that CD4+ but not CD8+ T cells are capable of reducing the fungal burden in the oral tissues and eventually lead to the recovery of these mice from chronic oral candidiasis. Animals reconstituted with either CD4+ cells alone or a mixture of CD4+ and CD8+ T cells exhibit the same pattern of clearance as animals reconstituted with a pool of naive mixed lymphocytes. A significant delayed-type hypersensitivity response was also observed following reconstitution with CD4+ but not CD8+ T cells. It was clear that CD4+ T cells were able to localize in the infected oral mucosa and exert their activity there, as evidenced by the CD4 gene expression in the oral tissues following reconstitution.
Although animals that received only naive CD8+ T cells did not clear the infection, there was a slight decrease in colonization levels, which might have been due to the carryover of CD4+ T cells in the transferred cells. CD4+ cells constituted only a small number of the T-cell population in the lymph nodes of mice reconstituted with CD8+ T cells; and control mice that received no cells displayed comparable numbers of CD4+ T cells to that of the CD8+ group (data not shown). T-cell numbers in nude mice tend to increase with age (32), and this might account for the slight decline in yeast CFU recovered from these control mice late in the course of infection.
While α/β T cells often predominate in oral mucosal epithelium, the percentages of γ/δ T cells are always considerably higher than in the periphery (24, 31, 38). γ/δ T cells show a sharp increase in number following either oral (17, 20) or gastrointestinal (25) infection with C. albicans, and in a recent study, mice made deficient in α/β or γ/δ T cells (α- and δ-chain T-cell receptor knockout mice, respectively) were shown to be more susceptible to orogastric candidiasis than immunocompetent controls (26).
The most abundant cytokine detected in the submandibular and superficial cervical lymph nodes of infected BALB/c nude mice following reconstitution with naive lymphocytes was IL-12. IL-12 is produced by phagocytic cells, B cells, and dendritic cells and acts on T lymphocytes and NK cells by inducing proliferation and production of cytokines, especially IFN-γ. In vivo, IL-12 has been shown to be required for the induction of Th1 responses in systemic candidiasis (41), although its action may also be regulated by IFN-γ (15). In a model of gastrointestinal candidiasis, both Th1 cytokines, such as IFN-γ, and the Th2 cytokines IL-4 and IL-5 were produced by CD4+ cells from Peyer's patches and mesenteric lymph nodes at a time when the fungus was cleared from the stomach and intestine (16). In addition, treatment with soluble IL-4 receptor, which increased Th1 cells, was associated with enhanced yeast clearance. It was concluded that activation of Th1- but not Th2-like responses may be responsible for controlling gastrointestinal candidiasis and generating protective immunity. In contrast to these results, others have reported that neither IL-2 nor IFN-γ played a role in protecting the oral cavity from candidal infection (14), even though CD4+ T lymphocytes were essential for host recovery.
From the data presented in the current study and those reported by others (14-16), there is little evidence that IL-2, IL-4, and IL-10 play a significant role in mucosal candidiasis. IL-12 and IFN-γ were found in recovering reconstituted mice and may play some role in the clearance of C. albicans yeasts from the oral tissues of these animals. They are known to be involved in natural and acquired aspects of the immune response, and it is perhaps some aspect of this interaction that underlies the effector function of CD4+ T cells in the host response against oral candidiasis.
In conclusion, the present study demonstrates the essential role of CD4+ T cells in the clearance and recovery of immunodeficient mice from chronic oral candidiasis. Although these data are consistent with the body of evidence that implicates Th1 cytokines in recovery from candidiasis (40), the precise role of these cytokines in recovery from oral candidiasis remains to be determined.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the Australian Dental Research Foundation. C.S.F. was supported by an NH&MRC Dental Postgraduate Research Scholarship.
We thank Slavica Pervan for preparation of the histological samples.
Editor: T. R. Kozel
REFERENCES
- 1.Ashman, R. B. 1998. A gene (Carg1) that regulates tissue resistance to Candida albicans maps to chromosome 14 of the mouse. Microb. Pathog. 25:333-335. [DOI] [PubMed] [Google Scholar]
- 2.Ashman, R. B. 1997. Genetic determination of susceptibility and resistance in the pathogenesis of Candida albicans infection. FEMS Immunol. Med. Microbiol. 19:183-189. [DOI] [PubMed] [Google Scholar]
- 3.Ashman, R. B. 1987. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics 25:200-203. [DOI] [PubMed] [Google Scholar]
- 4.Ashman, R. B., E. M. Bolitho, and J. M. Papadimitriou. 1993. Patterns of resistance to Candida albicans in inbred mouse strains. Immunol. Cell. Biol. 71:221-225. [DOI] [PubMed] [Google Scholar]
- 5.Ashman, R. B., A. Fulurija, and J. M. Papadimitriou. 1997. Evidence that two independent host genes influence the severity of tissue damage and susceptibility to acute pyelonephritis in murine systemic candidiasis. Microb. Pathog. 22:187-192. [DOI] [PubMed] [Google Scholar]
- 6.Ashman, R. B., A. Fulurija, and J. M. Papadimitriou. 1998. A second Candida albicans resistance gene (Carg2) regulates tissue damage, but not fungal clearance, in sublethal murine systemic infection. Microb. Pathog. 25:349-352. [DOI] [PubMed] [Google Scholar]
- 7.Ashman, R. B., and J. M. Papadimitriou. 1987. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol. Cell. Biol. 65:163-171. [DOI] [PubMed] [Google Scholar]
- 8.Ashman, R. B., and J. M. Papadimitriou. 1993. Strain dependence of antibody-mediated protection in murine systemic candidiasis. J. Infect. Dis. 168:511-513. [DOI] [PubMed] [Google Scholar]
- 9.Balish, E., M. J. Balish, C. A. Salkowski, K. W. Lee, and K. F. Bartizal. 1984. Colonization of congenitally athymic, gnotobiotic mice by Candida albicans. Appl. Environ. Microbiol. 47:647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black, C. A., F. M. Eyers, A. Russell, M. L. Dunkley, R. L. Clancy, and K. W. Beagley. 1999. Increased severity of Candida vaginitis in BALB/c nu/nu mice versus the parent strain is not abrogated by adoptive transfer of T cell enriched lymphocytes. J. Reprod. Immunol. 45:1-18. [DOI] [PubMed] [Google Scholar]
- 11.Cantorna, M., D. Mook, and E. Balish. 1990. Resistance of congenitally immunodeficient gnotobiotic mice to vaginal candidiasis. Infect. Immun. 58:3813-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantorna, M. T., and E. Balish. 1991. Acquired immunity to systemic candidiasis in immunodeficient mice. J. Infect. Dis. 164:936-943. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna, M. T., and E. Balish. 1990. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect. Immun. 58:1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantorna, M. T., and E. Balish. 1991. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect. Immun. 59:2447-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cenci, E., A. Mencacci, G. Delsero, C. F. Dostiani, P. Mosci, A. Bacci, C. Montagnoli, M. Kopf, and L. Romani. 1998. IFN-gamma is required for IL-12 responsiveness in mice with Candida albicans infection. J. Immunol. 161:3543-3550. [PubMed] [Google Scholar]
- 16.Cenci, E., A. Mencacci, R. Spaccapelo, L. Tonnetti, P. Mosci, K. H. Enssle, P. Puccetti, L. Romani, and F. Bistoni. 1995. T helper cell type-1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J. Infect. Dis. 171:1279-1288. [DOI] [PubMed] [Google Scholar]
- 17.Chakir, J., L. Cote, C. Coulombe, and N. Deslauriers. 1994. Differential pattern of infection and immune response during experimental oral candidiasis in BALB/c and DBA/2 (H-2d) mice. Oral Microbiol. Immunol. 9:88-94. [DOI] [PubMed] [Google Scholar]
- 18.Costantino, P. J., N. F. Gare, and J. R. Warmington. 1995. Humoral immune responses to systemic Candida albicans infection in inbred mouse strains. Immunol. Cell. Biol. 73:125-133. [DOI] [PubMed] [Google Scholar]
- 19.Cutler, J. E. 1976. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J. Reticuloendothel. Soc. 19:121-124. [PubMed] [Google Scholar]
- 20.Elahi, S., G. Pang, R. Clancy, and R. B. Ashman. 2000. Cellular and cytokine correlates of mucosal protection in murine model of oral candidiasis. Infect. Immun. 68:5771-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farah, C. S., S. Hong, S. Wanasaengsakul, S. Elahi, G. Pang, T. Gotjamanos, G. J. Seymour, R. L. Clancy, and R. B. Ashman. 2001. Irradiation-induced oral candidiasis in an experimental murine model. Oral Microbiol. Immunol. 16:358-363. [DOI] [PubMed]
- 22.Fulurija, A., R. B. Ashman, and J. M. Papadimitriou. 1997. Increased tissue resistance in the nude mouse against Candida albicans without altering strain-dependent differences in susceptibility. J. Med. Vet. Mycol. 35:197-203. [PubMed] [Google Scholar]
- 23.Hurtrel, B., P. H. Langrange, and J. C. Michel. 1981. Absence of correlation between delayed-type hypersensitivity and protection in experimental systemic candidiasis in immunized mice. Infect. Immun. 31:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itohara, S., A. G. Farr, J. J. Lafaille, M. Bonneville, Y. Takagaki, W. Haas, and S. Tonegawa. 1990. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature 343:754-757. [DOI] [PubMed] [Google Scholar]
- 25.Jones-Carson, J., A. Vazquez-Torres, H. C. van der Heyde, T. Warner, R. D. Wagner, and E. Balish. 1995. γ/δ T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat. Med. 1:552-557. [DOI] [PubMed] [Google Scholar]
- 26.Jones-Carson, J., A. Vazquez-Torres, T. Warner, and E. Balish. 2000. Disparate requirement for T cells in resistance to mucosal and acute systemic candidiasis. Infect. Immun. 68:2363-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkpatrick, C. H., R. R. Rich, and J. E. Bennett. 1971. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann. Intern. Med. 74:955-978. [DOI] [PubMed] [Google Scholar]
- 28.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-358. [DOI] [PubMed] [Google Scholar]
- 29.Lacasse, M., C. Fortier, J. Chakir, L. Cote, and N. Deslauriers. 1993. Acquired resistance and persistence of Candida albicans following oral candidiasis in the mouse: a model of the carrier state in humans. Oral Microbiol. Immunol. 8:313-318. [DOI] [PubMed] [Google Scholar]
- 30.Lee, K. W., and E. Balish. 1981. Systemic candidosis in germfree, flora-defined and conventional nude and thymus-bearing mice. J. Reticuloendothel. Soc. 29:71-77. [PubMed] [Google Scholar]
- 31.Lundqvist, C., S. Baranov, S. Teglund, S. Hammarstrom, and M. L. Hammarstrom. 1994. Cytokine profile and ultrastructure of intraepithelial gamma-delta T cells in chronically inflamed human gingiva suggest a cytotoxic effector factor. J. Immunol. 153:2302-2312. [PubMed] [Google Scholar]
- 32.MacDonald, H. R., R. K. Lees, B. Sordat, P. Zaech, J. L. Maryanski, and C. Bron. 1981. Age-associated increase in expression of the T cell surface markers Thy-1, Lyt-1, and Lyt-2 in congenitally athymic (nu/nu) mice: analysis by flow microfluorometry. J. Immunol. 126:865-870. [PubMed] [Google Scholar]
- 33.Martin, M. V. 1989. A comparison of fluconazole and ketoconazole in the treatment of rat palatal candidosis. J. Med. Vet. Mycol. 27:63-70. [DOI] [PubMed] [Google Scholar]
- 34.Murray, L. J., R. Lee, and C. Martens. 1990. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur. J. Immunol. 20:163-170. [DOI] [PubMed] [Google Scholar]
- 35.Pankhurst, C., and M. Peakman. 1989. Reduced CD4+ T cells and severe oral candidiasis in absence of HIV infection. Lancet i:672. [DOI] [PubMed]
- 36.Pantelouris, E. 1968. Absence of a thymus in a mouse mutant. Nature 217:370-371. [DOI] [PubMed] [Google Scholar]
- 37.Pantelouris, E. 1973. Athymic development in the mouse. Differentiation 1:437-450. [DOI] [PubMed] [Google Scholar]
- 38.Poussier, P., and M. Julius. 1994. Intestinal intraepithelial lymphocytes: the plot thickens. J. Exp. Med. 180:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers, T. J., E. Balish, and D. D. Manning. 1976. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J. Reticuloendothel. Soc. 20:291-298. [PubMed] [Google Scholar]
- 40.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 41.Romani, L., A. Mencacci, L. Tonnetti, R. Spaccapelo, E. Cenci, S. Wolf, P. Puccetti, and F. Bistoni. 1994. Interleukin-12 but not interferon-gamma production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur. J. Immunol. 24:909-915. [DOI] [PubMed] [Google Scholar]