Abstract
Because chronic Mycoplasma pneumoniae respiratory infection is hypothesized to play a role in asthma, the potential of M. pneumoniae to establish chronic respiratory infection with associated pulmonary disease was investigated in a murine model. BALB/c mice were intranasally inoculated once with M. pneumoniae and examined at 109, 150, 245, 368, and 530 days postinoculation. M. pneumoniae was detected in bronchoalveolar lavage fluid by culture or PCR in 70 and 22% of mice at 109 and 530 days postinoculation, respectively. Lung histopathology was normal up to 368 days postinoculation. At 530 days, however, 78% of the mice inoculated with M. pneumoniae demonstrated abnormal histopathology characterized by peribronchial and perivascular mononuclear infiltrates. A mean histopathologic score (HPS) at 530 days of 5.1 was significantly greater (P < 0.01) than that for controls (HPS score of 0). Serum anti-M. pneumoniae immunoglobulin G was detectable in all of the mice inoculated with M. pneumoniae and was inversely correlated with HPS (r = −0.95, P = 0.01) at 530 days postinoculation. Unrestrained whole-body plethysmography measurement of enhanced pause revealed significantly elevated airway methacholine reactivity in M. pneumoniae-inoculated mice compared with that in controls at 245 days (P = 0.03) and increased airway obstruction at 530 days (P = 0.01). Murine M. pneumoniae respiratory infection can lead to chronic pulmonary disease characterized by airway hyperreactivity, airway obstruction, and histologic inflammation.
While Mycoplasma pneumoniae is known to be a significant cause of acute respiratory illness in children and adults, the significance of chronic infection is undetermined. In humans, M. pneumoniae is reported to commonly be detectable by culture of the respiratory tract for up to several months after clinical recovery from acute pneumonia (5). Even after therapy with effective antibiotics, such as erythromycin or tetracycline, M. pneumoniae can still be cultured from respiratory secretions (6, 18). After receiving macrolides for 14 days, children have been found to have pulmonary structural abnormalities, by high-resolution computed tomography, suggestive of small airway obstruction 1 to 2 years after M. pneumoniae pneumonia with significantly increased frequency compared with that of controls. In these children, a greater antimycoplasma antibody titer was a significant risk factor for the development of abnormal pulmonary sequelae, suggesting that the host immune response may play a pathogenic role in this condition (10). The duration of M. pneumoniae infection in the human lower respiratory tract after acute pneumonia as determined by a method more sensitive than culture, such as PCR, has not been investigated in a controlled fashion.
Of potentially greater relevance, chronic M. pneumoniae respiratory infection has been hypothesized to play a role in asthma. By PCR, M. pneumoniae has been detected in the lower airways of chronic, stable asthmatics with significantly greater frequency than in nonasthmatic control subjects (11, 15). Although M. pneumoniae PCR was positive in these asthmatics, M. pneumoniae was not detected by culture, and serum antibodies to M. pneumoniae were not detected. The significance of a positive respiratory M. pneumoniae PCR was demonstrated by evidence that M. pneumoniae PCR-positive asthmatics had increased airway expression of tumor necrosis factor alpha (TNF-α) interleukin 4 (IL-4), and IL-5 compared with PCR-negative asthmatics and that the expression of these cytokines decreased with clarithromycin therapy in the PCR-positive subjects, but not in the PCR-negative subjects (M. Kraft, Q. Hamid, G. H. Cassell et al., Abstr. Am. J. Respir. Crit. Care Med. 159:A517, 1999). Clarithromycin therapy was also associated with a increase in forced expiratory volume (FEV1) (in seconds) in M. pneumoniae PCR-positive asthmatics, while it was not in PCR-negative subjects (Kraft et al., abstr. A517).
A mouse model of chronic respiratory infection with Mycoplasma pulmonis, a natural pathogen of rodents—but not humans—has been previously established (13), and it has been shown that rats chronically infected with M. pulmonis develop lung fibrosis accompanied by alterations in lung compliance (16). Therapy with dexamethasone was shown to reverse the chronic inflammatory airway disease induced by M. pulmonis infection and to reduce the CFU of M. pulmonis in the respiratory tract of rats (1). We previously described the microbiologic, histologic, cytokine or chemokine, antibody, and plethysmographic profile of acute murine M. pneumoniae pulmonary infection through 42 days of infection (9), but the characteristics of chronic murine M. pneumoniae infection have not been explored.
The aim of the present study was to determine whether chronic M. pneumoniae pulmonary infection could be established in mice and to characterize its course. Our investigations demonstrated that M. pneumoniae can establish a chronic pulmonary infection for up to approximately 18 months after inoculation and revealed evidence that M. pneumoniae infection in the respiratory tract can lead to chronic pulmonary inflammation and long-term functional sequelae.
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae ATCC 29342 was reconstituted in SP4 broth and subcultured after 24 to 48 h in a flask containing 20 ml of SP4 medium at 37°C. When the broth turned an orange hue (approximately 72 h), the supernatant was decanted, and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 108 to 109 CFU/ml (determined by plating dilutions on SP4 agar). Aliquots were stored at −80°C. All SP4 media contained nystatin (50 U/ml) and ampicillin (1.0 mg/ml) to inhibit growth of potential contaminants.
Animals and inoculation.
Mice were obtained from commercial vendors (Charles River and Harlan), who confirmed their mycoplasma- and murine virus-free status. They were housed in filter-top cages and allowed to acclimate to their new environment for 1 week. Methoxyflurane, an inhaled anesthetic, was used for inoculum sedation. Two-month-old female BALB/c mice were intranasally inoculated once (day 0) with 1.2 × 107 to 5.0 × 107 CFU of M. pneumoniae in 50 μl of SP4 broth. Control mice were inoculated with sterile SP4 broth. All mice were housed in the same animal room and received identical daily care. Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee.
Sample collection.
Samples were obtained from mice at 109, 150, 245, 368, and 530 days postinoculation. Mice were anesthetized (per kilogram of body weight) with an intraperitoneal injection of 75 mg of ketamine and 5 mg of acepromazine before cardiac puncture. Blood was centrifuged at 3,500 × g for 10 min, and the plasma was stored at −80°C. Bronchoalveolar lavage (BAL) specimens were obtained by infusing 0.5 ml of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Whole-lung specimens (including the trachea and both lungs) were collected and fixed with a 10% buffered formalin solution for histologic evaluation.
Culture.
Fifty microliters of neat BAL fluid was immediately cultured in SP4 broth and incubated at 37°C. Color change was utilized to determine positive cultures.
BAL PCR.
Mouse BAL samples were extracted for M. pneumoniae DNA with centrifuge columns according to the manufacturer's instructions (Qiagen GmbH, Germany). Single-step DNA amplification was performed with a programmable thermal cycler (GeneAmp PCR system 9600; Perkin-Elmer Cetus, Norwalk, Conn.) based on a modified method of Williamson et al. (20). The 100-μl reaction mixture consisted of 40 μl of DNA eluent and 60 μl of HotStar Taq DNA polymerase master mix (Qiagen GmbH) to maximize PCR sensitivity and specificity. The published primer sequences targeted the highly conserved region of the 16S ribosomal DNA (rDNA) gene of M. pneumoniae and amplified a unique 290-bp region (20). A 10-μl volume of amplified product was separated by electrophoresis in polyacrylamide gel and visualized under UV light after ethidium bromide staining. M. pneumoniae DNA extract from ATCC 29342 and sterile distilled water were amplified with each assay as positive and negative controls, respectively. In a preliminary in vitro serial dilution study, SP4 broth was spiked with culture-confirmed quantities of M. pneumoniae. This study demonstrated that the lower limit of detection of the PCR assay was 10 CFU/ml. The recommended procedures to avoid contamination were followed during all steps (12).
Histopathology.
The histopathologic score (HPS) was determined by a pathologist who was unaware of the infection status of the animals from which specimens were taken. HPS was based on grading of peribronchiolar or bronchial infiltrate, bronchiolar or bronchial luminal exudate, perivascular infiltrate, and parenchymal pneumonia (neutrophilic alveolar infiltrate). This HPS system assigned values from 0 to 26: the greater the score, the greater the inflammatory changes in the lung (3).
Plethysmography.
Whole-body, unrestrained plethysmography (Buxco, Troy, N.Y.) was utilized to monitor the respiratory dynamics of mice in a quantitative manner before and after methacholine exposure. Prior to methacholine exposure, previously inoculated mice were allowed to acclimate to the chamber, and then plethysmograph readings were recorded to establish baseline values. Next the mice were exposed to aerosolized methacholine (50 mg/ml); after exposure, plethysmograph readings were recorded again. Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh as measured by plethysmography has been previously validated in animal models of airway hyperresponsiveness (7, 8, 17, 19). Baseline Penh was evaluated at 109, 245, and 530 days postinoculation, while Penh with methacholine challenge was measured at 245 and 530 days.
Serology.
The presence of immunoglobulin M (IgM) and IgG antibodies to M. pneumoniae in the serum of infected mice was determined by enzyme-linked immunosorbent assay (ELISA). A sample was considered positive if its optical density (OD) reading was ≥2 standard deviations above the mean OD of the control serum samples (2). Five to six M. pneumoniae-inoculated mice, in addition to control mice, were randomly selected at each time point for serology determination by ELISA.
BAL cytokines or chemokines.
BAL specimens at 109, 245, and 530 days postinoculation were assessed for concentrations of TNF-α, IL-4, IL-6, MCP-1/JE, eotaxin, and transforming growth factor β1 (TGF-β1) by ELISA (R&D Systems, Minneapolis, Minn.).
Statistics.
The t test was used to compare indices of experimental versus control groups of animals at the same time point, if the data were normally distributed. In the instances in which the data were not normally distributed, the Mann-Whitney rank sum test was used for comparisons. The Pearson product moment test was used for correlations. A comparison was considered statistically significant if the P value was <0.05.
RESULTS
Clinical.
No visual differences could be detected between mice inoculated with M. pneumoniae and control mice after the first week of infection, as previously reported with our model of acute infection (9).
BAL culture.
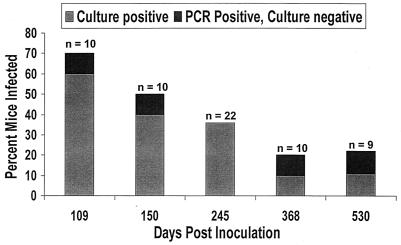
BAL cultures for M. pneumoniae were positive in 60, 36, and 11% of the mice at 109, 245, and 530 days postinoculation, respectively. The percentage of BAL culture-positive mice trended downward over the 530 days (Fig. 1). All control mice had negative BAL cultures.
FIG. 1.
Percentage of mice found to have M. pneumoniae in BAL fluid by culture or PCR after intranasal inoculation with M. pneumoniae.
BAL PCR.
M. pneumoniae PCR was performed with culture-negative BAL specimens. This increased the percentage of mice positive for M. pneumoniae infection by up to 11% above culture results, as shown in Fig. 1. PCR was negative in all control specimens.
Histopathology.
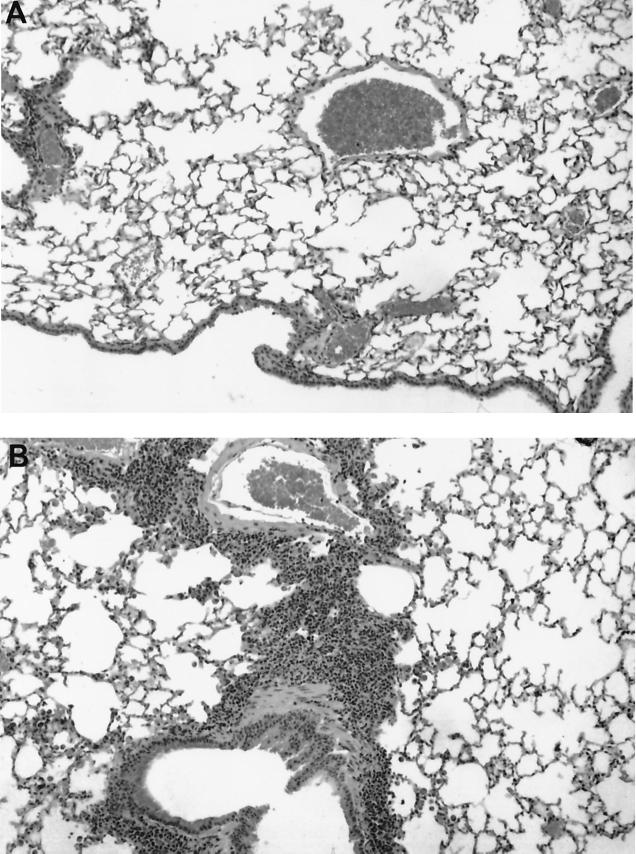
Histopathologic scores (HPS) for all M. pneumoniae-inoculated mice and control mice were 0 from 109 to 368 days postinoculation. At 530 days, HPS were elevated in 7 of 9 mice inoculated with M. pneumoniae. The mean HPS value of 5.1 at 530 days was significantly elevated (P < 0.01) compared with that in control mice, all of which had an HPS of 0. The abnormal histopathological findings in M. pneumoniae-inoculated mice at 530 days consisted of peribronchial and perivascular mononuclear infiltrates, but there was no intrabronchial exudate or parenchymal pneumonia (neutrophilic alveolar infiltrate). Of the two mice that had normal histopathology at 530 days postinoculation, one was culture and PCR positive for M. pneumoniae, and the other was culture and PCR negative. Of the seven mice that had abnormal histopathology at 530 days, all had negative BAL cultures, and one had a positive BAL PCR. Figure 2 demonstrates the histologic appearance of a control mouse lung at 530 days compared with that of a lung demonstrating peribronchial and perivascular infiltrates at 530 days postinoculation with M. pneumoniae.
FIG. 2.
Histopathologic appearance (magnification, ×100) of control mouse lung 530 days after inoculation with SP4 broth (A) compared with that of lung showing inflammation 530 days after inoculation with M. pneumoniae (B).
Plethysmography.
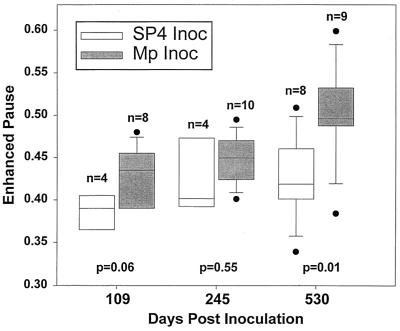
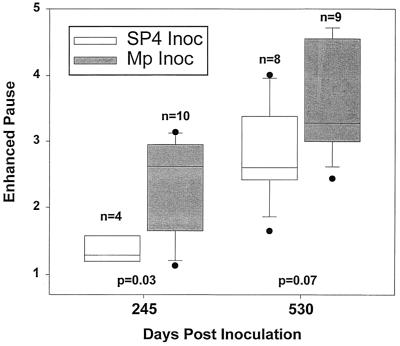
Median baseline Penh (before methacholine challenge) was increased in the mice inoculated with M. pneumoniae at 109, 245, and 530 days, but reached statistical significance (P = 0.01) only at 530 days compared with control mice at 530 days (Fig. 3). Penh after methacholine challenge was significantly elevated (P = 0.03) in the mice inoculated with M. pneumoniae compared with that in controls at 245 days (Fig. 4). At 530 days, the Penh after methacholine challenge was also elevated, but this was not statistically different (P = 0.07) from that in controls. Methacholine challenge only transiently elevated the Penh of the mice, demonstrating the reversibility of this functional abnormality.
FIG. 3.
Baseline Penh (prior to methacholine challenge) after inoculation (Inoc) with SP4 broth or M. pneumoniae (Mp). A box plot with outliers is shown: vertical boxes represent 25 to 75 percentiles; error bars represent 10 to 90 percentiles, and lines in vertical boxes represent median values.
FIG. 4.
Penh with aerosolized methacholine challenge after inoculation (Inoc) with SP4 broth or M. pneumoniae (Mp). A box plot with outliers is shown: vertical boxes represent 25 to 75 percentiles, error bars represent 10 to 90 percentiles, and lines in vertical boxes represent median values.
Serology.
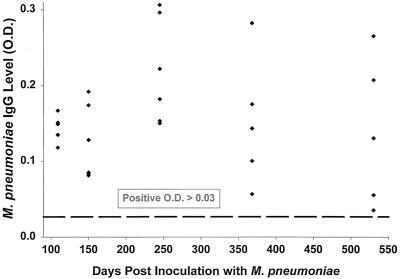
M. pneumoniae IgM was present in sera of 2 of 27 mice tested; 1 at day 109 and 1 at day 530 postinoculation. M. pneumoniae IgG was present in all 27 mice tested, as illustrated in Fig. 5.
FIG. 5.
M. pneumoniae IgG titers 109, 150, 245, 368, and 530 days after inoculation with M. pneumoniae. Each point represents one mouse.
BAL cytokines or chemokines.
TNF-α, IL-4, IL-6, and TGF-β1 were detected in low concentrations in BAL samples obtained from M. pneumoniae-inoculated mice at 109, 245, and 530 days postinoculation. However, no significant differences in cytokine concentrations were found between the M. pneumoniae-inoculated mice and controls. There was a trend (P = 0.08) for IL-4 to be elevated in the M. pneumoniae-inoculated mice compared with controls at 530 days. Concentrations of MCP-1/JE and eotaxin in BAL were below the level of detection of the assays at 109, 245, and 530 days postinoculation (lower limit of detection for these ELISAs was 16 pg/ml; R&D Systems).
Correlations.
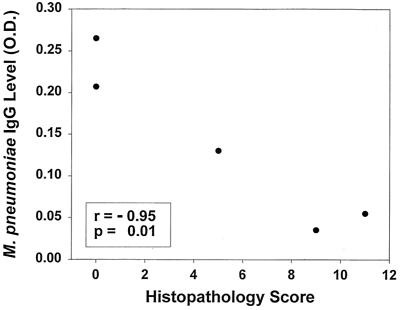
M. pneumoniae IgG titers inversely correlated with HPS at 530 days (r = −0.95, P = 0.01) (Fig. 6). An inverse trend was noted between Penh after methacholine challenge and HPS at 530 days (r = −0.62, P = 0.07). No other correlations were found among the culture, PCR, HPS, Penh, and antibody data.
FIG. 6.
Correlation between M. pneumoniae IgG titers and HPS 530 days after inoculation with M. pneumoniae.
DISCUSSION
The present studies indicate that experimental M. pneumoniae respiratory infection can lead to chronic infection, chronic pulmonary inflammation, and chronic pulmonary function abnormalities. In our model of acute murine M. pneumoniae pneumonia, M. pneumoniae in quantitative BAL cultures initially reached a high of 106 CFU/ml and then fell to a plateau of 101 to 102 CFU/ml during the first 21 days of infection, and histologic pulmonary inflammation resolved by 28 days postinoculation (9). Hence, the acute phase of infection in this model lasted approximately 1 month.
From our current investigation, it is apparent that a state of chronic infection can persist for at least 18 months. In this chronic phase, M. pneumoniae was persistently detected in the BAL fluid, although at a decreasing percentage (22% at approximately 18 months). Pulmonary histology was normal through 368 days, then at close to 18 months after infection, histology of the lungs revealed perivascular and peribronchial mononuclear infiltrates. These chronic histological findings lacked the intrabronchial neutrophilic exudate and parenchymal pneumonia (neutrophilic alveolar infiltrate) characteristic of acute infection, distinguishing the histopathology of chronic infection from acute infection. While M. pneumoniae was detected in the BAL fluid from two of nine mice at 530 days after inoculation, abnormal pulmonary histopathology was present in seven of the nine mice. It is possible that sampling of whole-lung specimens would have increased the detection rate of M. pneumoniae in the mice.
All mice tested at 530 days had positive IgG titers for M. pneumoniae (including the two mice with normal histology), and notably the concentrations of the IgG antibodies were strongly inversely correlated with the HPS. In fact, the animal with the highest IgG titer at 530 days had the only positive culture for M. pneumoniae and had a normal lung histology; the presumably elevated M. pneumoniae burden in this animal may have contributed to the greater IgG titer. These findings may indicate a protective role of high antibody titers against mechanisms responsible for pulmonary inflammation in this chronic model. Possibly, as the IgG titers fall, M. pneumoniae becomes able to exert a pathogenic affect that is inhibited by high M. pneumoniae antibody titers. It has been shown that M. pneumoniae is able to establish long-term intracellular infection in human cells (4). Speculatively, intracellular survival may be a mechanism by which M. pneumoniae is able to escape immune surveillance and then produce chronic lung disease as antibody titers decline. This would be consistent with the lack of M. pneumoniae antibody in chronic, stable asthmatics from whom M. pneumoniae can be isolated from the respiratory tract by PCR (11, 15). If these findings are supported by future studies, elucidation of the immunopathogenesis of chronic M. pneumoniae pulmonary infection could lead to the development of vaccines or to immune-based therapies specific for M. pneumoniae infection. Alternatively, it is possible that the IgG titers are merely a surrogate marker for another component of the immune response that is actually responsible for the inverse correlation between IgG titers and HPS.
In contrast to the findings in our model of acute murine M. pneumoniae pneumonia, we were unable to demonstrate significant elevations in BAL cytokines or chemokines by ELISA testing in this model of chronic pneumonia. The trend for IL-4 concentrations to be elevated in the BAL specimens obtained at 530 days after inoculation with M. pneumoniae deserves further investigation, given the association of asthma with IL-4. Sampling of whole-lung specimens or by a different method of detection might yield more significant results. The presence or absence of other Th2 cytokines is also of interest and will be investigated in future studies.
To assess the impact of chronic M. pneumoniae infection on murine pulmonary function, mice were evaluated by plethysmography under two different conditions: (i) at baseline without methacholine and (ii) after a standardized, aerosolized methacholine challenge. This revealed evidence of a chronic pulmonary process in mice inoculated with M. pneumoniae. Baseline Penh tended to be elevated at all days examined, but was significantly elevated above that of controls at 530 days postinoculation. In addition, exposure to methacholine revealed significant airway hyperreactivity at 245 days and a trend (P = 0.07) toward increased airway hyperreactivity at 530 days. (Testing more animals at this time point may have resulted in a significant difference.) These results reveal two abnormalities of pulmonary function in this chronic M. pneumoniae model: (i) obstructive airway disease and (ii) airway hyperreactivity. These observations are similar to pulmonary function tests in asthmatic patients and lend support to the contention that M. pneumoniae infection can result in chronic pulmonary disease with functional airway obstruction in some humans. We believe that this chronic M. pneumoniae murine model can be used to study infection-associated reactive airway disease.
M. pneumoniae respiratory infection is often regarded as a self-limited infection, and the resolution of acute infection within a few weeks without treatment, as shown in our model of acute infection, partly supports this concept. However, a subset of individuals may handle this infection in a less favorable manner and later develop chronic pulmonary abnormalities. Indeed, recent investigations have suggested that timely and effective antimicrobial treatment of acute M. pneumoniae respiratory infection in children can improve the course of reactive airway disease beyond the acute episode of wheezing and can prevent development of deleterious changes in pulmonary function tests (14; S. Esposito, F. Blasi, C. Arosio, et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2234, p. 700, 1999). With the findings of chronic infection, inflammation, and functional sequelae in this model, evaluation of effective therapeutic regimens to modulate inflammation and to eradicate M. pneumoniae has more relevance.
In conclusion, we have documented that M. pneumoniae is able to establish chronic respiratory infection, evoke chronic pulmonary inflammation, and elicit chronic pulmonary function abnormalities in mice. These findings provide strong evidence supporting the postulated association between M. pneumoniae infection and chronic pulmonary disease, possibly asthma, in humans. Future studies directed at characterizing the immunopathogenic mechanisms responsible for these abnormalities will provide the basis for novel therapeutic and preventative strategies aimed at reducing the potential impact of M. pneumoniae infections.
Acknowledgments
R.D.H. is the recipient of a Pediatric Infectious Diseases Society Fellowship award sponsored by Roche Laboratories. Additional support was provided by Abbott Laboratories and The American Lung Association. Lynn Duffy of Diagnostic Mycoplasma Laboratory, University of Alabama, Birmingham, provided resources for M. pneumoniae antibody titers.
Editor: E. I. Tuomanen
REFERENCES
- 1.Bowden, J. J., T. R. Schoeb, J. R. Lindsey, and D. M. McDonald. 1994. Dexamethasone and oxytetracycline reverse the potentiation of neurogenic inflammation in airways of rats with Mycoplasma pulmonis infection. Am. J. Respir. Crit. Care Med. 150:1391-1401. [DOI] [PubMed] [Google Scholar]
- 2.Cassell, G. H., G. Gambill, and L. Duffy. 1996. ELISA in respiratory infections of humans, p. 123-136. In J. G. Tully and S. Razin (ed.), Molecular and diagnostic procedures in mycoplasmology, vol. 2. Academic Press, San Diego, Calif. [Google Scholar]
- 3.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. [DOI] [PubMed] [Google Scholar]
- 4.Dallo, S. F., and J. B. Baseman. 2000. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb. Pathog. 29:301-309. [DOI] [PubMed] [Google Scholar]
- 5.Denny, F. W., W. A. Clyde, Jr., and W. P. Glezen. 1971. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J. Infect. Dis. 123:74-92. [DOI] [PubMed] [Google Scholar]
- 6.Foy, H. M., J. T. Grayston, G. E. Kenny, E. R. Alexander, and R. McMahan. 1966. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA 197:859-866. [PubMed] [Google Scholar]
- 7.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766-775. [DOI] [PubMed] [Google Scholar]
- 9.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, C. K., C. Y. Chung, J. S. Kim, W. S. Kim, Y. Park, and Y. Y. Koh. 2000. Late abnormal findings on high-resolution computed tomography after Mycoplasma pneumonia. Pediatrics 105:372-378. [DOI] [PubMed] [Google Scholar]
- 11.Kraft, M., G. H. Cassell, J. E. Henson, H. Watson, J. Williamson, B. P. Marmion, C. A. Gaydos, and R. J. Martin. 1998. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am. J. Respir. Crit. Care Med. 158:998-1001. (Erratum, 158:1692.) [DOI] [PubMed]
- 12.Kwok, S., and R. Higuchi. 1989. Avoiding false positives with PCR. Nature 339:237-238. [DOI] [PubMed] [Google Scholar]
- 13.Lindsey, J. R., and H. Cassell. 1973. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Am. J. Pathol. 72:63-90. [PMC free article] [PubMed] [Google Scholar]
- 14.Marc, E., M. Chaussain, F. Moulin, J. L. Iniguez, G. Kalifa, J. Raymond, and D. Gendrel. 2000. Reduced lung diffusion capacity after Mycoplasma pneumoniae pneumonia. Pediatr. Infect. Dis. J. 19:706-710. [DOI] [PubMed] [Google Scholar]
- 15.Martin, R. J., M. Kraft, H. W. Chu, E. A. Berns, and G. H. Cassell. 2001. A link between chronic asthma and chronic infection. J. Allergy Clin. Immunol. 107:595-601. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh, J. C., J. W. Simecka, S. E. Ross, J. K. Davis, E. J. Miller, and G. H. Cassell. 1992. Infection-induced airway fibrosis in two rat strains with differential susceptibility. Infect. Immun. 60:2936-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, C. B., W. T. Friedewald, and R. M. Chanock. 1967. Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy. N. Engl. J. Med. 276:1172-1175. [DOI] [PubMed] [Google Scholar]
- 19.van Schaik, S. M., G. Enhorning, I. Vargas, and R. C. Welliver. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 177:269-276. [DOI] [PubMed] [Google Scholar]
- 20.Williamson, J., B. P. Marmion, D. A. Worswick, T. W. Kok, G. Tannock, R. Herd, and R. J. Harris. 1992. Laboratory diagnosis of Mycoplasma pneumoniae infection. 4. Antigen capture and PCR-gene amplification for detection of the Mycoplasma: problems of clinical correlation. Epidemiol. Infect. 109:519-537. [DOI] [PMC free article] [PubMed] [Google Scholar]