Abstract
Objective
Because the role of thyroid autoimmunity in the development of lithium-induced thyroid dysfunction remains controversial, we compared the prevalence of thyroid autoantibodies in patients with affective disorders receiving long-term lithium maintenance therapy with that of age- and sex-matched controls.
Methods
We conducted a cross-sectional study with 100 adult patients with major affective disorders diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, revised (DSM-III-R), who were undergoing lithium therapy for 6 months or more at a specialized lithium university clinic and 100 age- and sex-matched controls with no history of an axis I psychiatric disorder. Serum autoantibodies against thyroid peroxidase (TPOAb), thyroglobulin (TgAb) and TSH receptors (TRAb) were measured.
Results
TPOAb were found in 7 patients and 11 controls, and TgAb were found in 8 patients and 15 controls. TRAb were not found in either group.
Conclusions
In this sample of patients with affective disorders, long-term lithium treatment did not increase the prevalence of thyroid autoimmunity.
Medical subject headings: hypothyroidism, lithium, mood disorders, thyroid antibodies
Abstract
Objectif
Comme le rôle de l'auto-immunité thyroïdienne dans l'apparition d'une dysfonction thyroïdienne causée par le lithium suscite toujours la controverse, nous avons comparé la prévalence d'autoanticorps thyroïdiens chez des patients qui ont des troubles de l'affectivité et qui suivent une thérapie d'entretien de longue durée au lithium à ce qui se passe chez des témoins jumelés selon l'âge et le sexe.
Méthodes
Nous avons effectué une étude transversale portant sur 100 patients adultes atteints de troubles majeurs de l'affectivité diagnostiqués conformément à la version révisée du Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R), qui suivaient une thérapie au lithium pendant six mois ou plus à une clinique universitaire spécialisée, et sur 100 témoins jumelés selon l'âge et le sexe qui n'avaient aucun antécédent de troubles psychiatriques de l'axe I. On a mesuré les autoanticorps sériques contre la thyroïde peroxydase (TPOAb), la thyroglobuline (TgAb) et les récepteurs de la TSH (TRAb).
Résultats
On a constaté la présence de TPOAb chez sept patients et 11 témoins et de TgAb chez huit patients et 15 témoins. On n'a pas trouvé de TRAb chez aucun sujet des deux groupes.
Conclusions
Dans cet échantillon de patients qui ont des troubles de l'affectivité, le traitement de longue durée au lithium n'a pas augmenté la prévalence de l'auto-immunité thyroïdienne.
Introduction
Lithium therapy for patients with affective disorders has long been acknowledged to induce thyroid dysfunction. Although it was noted early on that lithium-induced thyroid failure could occur without the presence of thyroid autoimmunity,1 the role of thyroid autoimmunity in the development of lithium-induced thyroid disorders remains unclear. Some studies have reported a high prevalence of antithyroid antibodies in patients with affective disorders receiving lithium therapy, suggesting that thyroid autoimmunity may mediate the antithyroid effects of lithium.2,3,4,5,6 Other studies, however, have not found an increased prevalence of antithyroid antibodies in patients with affective disorders receiving lithium when compared with the general population, healthy controls or controls with psychiatric disorders.7,8,9,10,11 Furthermore, patients who have thyroid autoimmunity before lithium exposure may show an increase in antibody titres12,13 and have an increased risk of developing hypothyroidism while receiving lithium therapy.9,13,14
For all thyroid antibodies, the prevalence and normal cutoff values vary with the assay method and manufacturer, and worldwide standardization has not yet been achieved.15 This, in addition to the evolution of assay techniques over the past several decades, may have contributed to the varying results reported in earlier studies of the effects of lithium on autoimmunity.15 Furthermore, because thyroid antibodies are associated with aging16 and female sex,17 the differing outcomes in earlier studies may have resulted from the lack of age- and sex-matched controls.7 Thus, in this cross-sectional study, we compared the prevalence of thyroid autoimmunity between 100 patients with affective disorders receiving lithium maintenance therapy and 100 age- and sex-matched healthy controls.
Methods
This study of thyroid autoimmunity was part of a series of studies investigating thyroid status in patients with affective disorders undergoing lithium treatment at the Berlin Lithium Clinic, a specialized outpatient clinic at the Department of Psychiatry, Benjamin Franklin University Hospital, which is an academic medical centre.18,19 Two hundred subjects participated in this study: 100 patients with affective disorders who were receiving lithium maintenance therapy at the clinic and 100 age- and sex-matched healthy controls. The patients were diagnosed with bipolar disorder (n = 64), major depressive disorder (n = 21) or schizoaffective disorder (n = 15). The approach to treatment used by this research clinic has been described in detail elsewhere.19 Before enrolment in the study, all the participants provided their written informed consent.
The inclusion criteria for patients were as follows: continuous maintenance treatment with lithium, with documented blood levels in the range of 0.6–1.2 mmol/L for at least 6 months; a diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, revised (DSM-III-R), of bipolar disorder, major depressive disorder or schizoaffective disorder;20 and age 18 years or more. Age- and sex-matched control subjects were recruited by the Berlin Lithium Clinic using word of mouth and study flyers. The inclusion criteria for controls were the following: no history of a psychiatric disorder as determined by a semistructured clinical interview using DSM-III-R criteria checklists; no history of lithium intake; and age 18 years or more.
The exclusion criteria for both patients and controls were the following: thyroidectomy (because other studies included thyroid volume measurements18); residence outside the county of Berlin (to ensure all participants were from the same geographic area); and a diet that interfered with iodine metabolism.
All participants underwent a detailed interview regarding their history of thyroid disorders. No reimbursement was offered. When thyroid abnormalities were detected, the participants were provided medical consultation.
Ten mL of cubital venous blood was drawn. A proportion of the serum samples were frozen, stored and later assessed in 1 assay for thyroid antibodies. The concentrations of thyroid-stimulating hormone ([TSH] normal range 0.27–4.20 mU/L) were routinely assessed in the General Clinical Laboratory of the Benjamin Franklin University Hospital, Berlin. TSH was evaluated by standardized immunoassays (ElecysR System, Boehringer Mannheim, Mannheim, Germany). Thyroid antibodies were determined in the thyroid hormone research laboratory of the Benjamin Franklin University Hospital. Thyroglobulin autoantibodies (TgAb) and thyroid peroxidase autoantibodies (TPOAb) were assayed by means of an enzyme-linked immunosorbent assay (ELISA) technique (Synelisa TG/TPOR, Pharmacia/Upjohn Diagnostics, Freiburg, Germany) using human thyroglobulin and recombinant thyroid peroxidase as antigens for antibody binding. TSH receptor autoantibodies (TRAb) were evaluated using the TRAK-AssayR (Brahms Diagnostika GmbH, Berlin, Germany), a radioreceptor assay using bovine iodine-125-labelled TSH as a tracer.
The distribution of positive and negative thyroid antibody levels among the groups were compared using the Pearson 2-sided asymptotic χ2 test. For all assays, the manufacturer's cutoff points were used to determine positive results. The differences in mean values in continuous variables between the 2 groups were tested using Student's t test. Test results were regarded as not significant when p > 0.05.
Results
The demographic characteristics of the patient and control groups are listed in Table 1. The mean duration of lithium maintenance treatment for the 100 patients was 11.2 (standard deviation [SD] 8.0) years. Sixty-seven patients were administered lithium carbonate, 24 received lithium sulfate and 9, lithium acetate. The mean lithium serum level for the 100 patients was 0.73 (SD 0.15) mmol/L.
Table 1
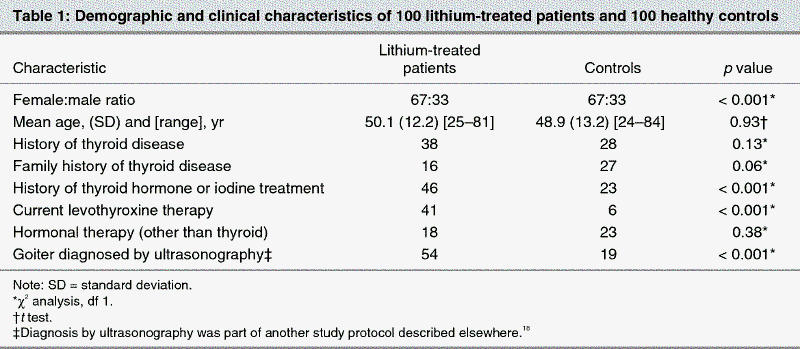
Forty-six of the 100 patients reported having taken thyroid hormones or iodine during their lifetime, 7 before lithium therapy was started, as compared with 23 in the control group. A positive history for any thyroid disorder was reported by 38 of the patients versus 28 in the control group. Sixteen patients and 27 individuals in the control group had a family history of any thyroid disorder.
Thyroid antibodies
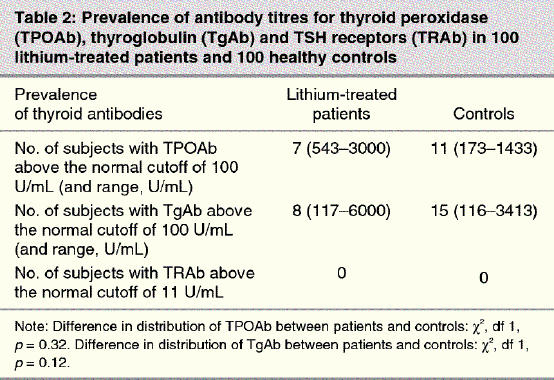
There was no significant difference in the distribution of positive TPOAb titres between the patient group (7%) and the control group (11%) by χ2 analysis (p = 0.32) (Table 2). For those with positive TPOAb titres, the range of the antibody levels was 543–3000 U/mL in the patient group and 173–1433 U/mL in the control group. The median value of TPOAb was 300 U/mL for the patients and 200 U/mL for the controls. There was also no significant difference between the distribution of positive TgAb titres between the patient group (8%) and control group (15%) by χ2 analysis (p = 0.12). For those with positive TgAb titres, the range of the antibody levels was again wider in the patient group (117–6000 U/mL) than in the control group (116–3413 U/mL). The median value of TgAb was 1100 U/mL for the patients and 500 U/mL for the controls. TRAb were not found in either group.
Table 2
Thyroid hormones
Twenty-four of the patients had TSH concentrations below normal; 22 of these were receiving therapy with levothyroxine as an augmentation agent for the treatment of an intractable mood disorder. Two controls had TSH concentrations below normal; 1 of these was receiving levothyroxine.
All the control subjects had normal levels of triiodothyronine. Two patients had decreased levels of triiodothyronine and 1 patient had increased levels, but this distribution was not significantly different (p = 0.24).
Two of the controls had decreased levels of levothyroxine. Among the 59 patients who were not currently receiving levothyroxine, 3 had decreased levels of levothyroxine; this distribution was not significantly different (p = 0.88). Only 1 of the 3 patients with a decreased level of levothyroxine had an increased level of TPOAb. None of the controls had an increase in levothyroxine, while 1 of the 59 patients who were not receiving levothyroxine had an increased level, but this distribution was not significantly different (p = 0.20).
Hypothyroidism
Although none of the study participants displayed clinical symptoms of hypothyroidism, 5 patients and 3 controls had TSH concentrations above the normal range. Considering only the 59 patients who were not receiving levothyroxine at the start of the study, latent hypothyroidism (defined as an increase in TSH along with normal levels of both levothyroxine and triiodothyronine) was detected in 5 or 8.5% of patients and in 3 or 3.2% of controls (p = 0.001).
Discussion
There was no significant difference in the prevalence of TPOAb, TgAb or TRAb between the patients with affective disorders undergoing long-term lithium maintenance therapy and the age- and sex-matched healthy controls. The prevalence rates for all thyroid antibodies for the healthy controls were within the reference limits for the general population.8 These results are also consistent with other studies, which, after adjustment for age and sex, also found no increase in the rate of thyroid autoimmunity in lithium-treated patients.7,8,9 Although we did not find an increased prevalence of thyroid autoimmunity in the lithium-treated patients, we did find significant evidence of thyroid dysfunction. First, 41% of patients currently used levothyroxine versus 6% of controls (p < 0.001), although some patients were taking levothyroxine for their mood disorder. Second, 46% of patients reported previous use of levothyroxine or iodine versus 23% of controls (p < 0.001). Third, subclinical hypothyroidism was found in 8.5% of patients who were not receiving levothyroxine versus 3.2% of controls (p = 0.001). Fourth, goiter was found by ultrasonographic diagnosis in 54% of patients versus 19% of controls as part of another study protocol.18 However, the hypothyroidism in the lithium-treated patients was not generally the result of thyroid autoimmunity, suggesting that the antithyroid action of lithium is independent of that caused by antithyroid antibodies.
When reviewing the control group, one might argue that the commonly reported family history of thyroid disorder suggests that those who participated in the study were unduly concerned about thyroid function. However, the high reported rates of thyroid disorder and family history of thyroid disorder in both the patients and controls are consistent with the high prevalence of goiter (30%–40%) in Germany, an iodine-deficient country.21,22 Furthermore, the exclusion of patients and controls with thyroidectomy may have decreased the prevalence of autoimmunity in both groups.
Assessment of thyroid function, including TPOAb, before starting lithium maintenance therapy is recommended for all patients with affective disorders. Close monitoring for thyroid dysfunction is especially needed for patients receiving lithium therapy who have additional risk factors for developing clinical hypothyroidism such as being female, advancing age, thyroid autoimmunity, residing in iodine-deficient areas23 and a family history of autoimmune thyroid disease. Furthermore, patients who have thyroid autoimmunity (TPOAb) at the start of lithium treatment should also be monitored for thyroid antibodies during long-term lithium therapy. Although the lithium-treated patients did not have a higher prevalence of thyroid autoimmunity, our finding that the highest thyroid antibody titres were present in lithium-treated patients is also consistent with the hypothesis that lithium can trigger thyroid autoimmunity in susceptible individuals12,13 and may increase the risk of developing thyroid disorders in patients with thyroid autoimmunity before lithium treatment.9,13,14 The appearance of TPOAb usually precedes the development of thyroid dysfunction15 and, in addition to lithium, may be a risk factor for development of autoimmune complications for other agents including amiodarone24 and interferon-α.25
Given the importance of lithium in the short-term and maintenance treatment of mood disorders,26,27,28 further prospective studies of the relation between lithium therapy, thyroid autoimmunity and thyroid function are warranted.
Acknowledgments
This study was supported, in part, by the Max Kade Foundation, New York City, NY (C.B.). We would like to thank Pharmacia/Upjohn Diagnostics, Freiburg, and Brahms Diagnostika GmbH, Berlin, for providing antibody kits for this study at no cost.
Footnotes
Contributors: Drs. Bschor and Bauer contributed to the conception and design of the study. Drs. Baethge, Blumentritt, Berghöfer, Adli and Finke contributed to the acquisition of data. Drs. Baethge, Glenn, Schlattmann and Finke contributed to the analysis and interpretation of the data. Drs. Baethge, Glenn and Schlattman contributed to drafting and reviewing the article. Drs. Blumentritt, Berghöfer, Bschor, Adli, Bauer and Finke contributed to reviewing the article critically. All authors gave final approval for the article to be published.
Competing interests: None declared for Drs. Baethge, Blumentritt, Bschor, Glenn, Adli and Schlattman. Dr. Berghöfer has received speaker fees from GlaxoSmithKline, Pfizer, Sanofi-Synthelabo (Aventis) and Eli Lilly. Dr. Bauer has received speaker fees from GlaxoSmithKline. Dr. Finke has received speaker fees from Sanofi-Aventis, Novartis and Pfizer and travel assistance from Novartis, Pfizer and Ipson.
Correspondence to: Dr. Michael Bauer, Department of Psychiatry and Psychotherapy, Charité-University Medicine Berlin, Campus Charité Mitte (CCM), Schumannstrasse 20/21, 10117 Berlin, Germany; fax 49 30 450 51 70 62; michael.bauer@charite.de
Submitted Dec. 21, 2004; Revised Aug. 16, 2005; Accepted Aug. 29, 2005
References
- 1.Crowe MJ, Lloyd GG, Bloch S, Rosser RM. Hypothyroidism in patients treated with lithium: a review and two case reports. Psychol Med 1973;3:337-42. [DOI] [PubMed]
- 2.Leroy MC, Villeneuve A, Lajeunesse C. Lithium, thyroid function and antithyroid antibodies. Prog Neuropsychopharmacol Biol Psychiatry 1988;12:483-90. [DOI] [PubMed]
- 3.Deniker P, Eyquem A, Bernheim R, Loo H, Delarue P. Thyroid autoantibody levels during lithium therapy. Neuropsychobiology 1978;4:270-5. [DOI] [PubMed]
- 4.Wilson R, McKillop JH, Crocket GT, Pearson C, Jenkins C, Burns F, et al. The effect of lithium therapy on parameters thought to be involved in the development of autoimmune thyroid disease. Clin Endocrinol (Oxf) 1991;34:357-61. [DOI] [PubMed]
- 5.Myers DH, Carter RA, Burns BH, Armond A, Hussain SB, Chengapa VK. A prospective study of the effects of lithium on thyroid function and on the prevalence of antithyroid antibodies. Psychol Med 1985;15:55-61. [DOI] [PubMed]
- 6.Lee S, Chow CC, Wing YK, Shek CC. Thyroid abnormalities during chronic lithium treatment in Hong Kong Chinese: a controlled study. J Affect Disord 1992;26:173-8. [DOI] [PubMed]
- 7.Haggerty JJ Jr, Silva SG, Marquard M, Mason GA, Chang HY, Evans DL, et al. Prevalence of antithyroid antibodies in mood disorders. Depress Anxiety 1997;5:91-6. [PubMed]
- 8.Kupka RW, Nolen WA, Post RM, McElroy SL, Altshuler LL, Denicoff KD, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biol Psychiatry 2002;51:305-11. [DOI] [PubMed]
- 9.Bocchetta A, Mossa P, Veluzzi F, Mariotti S, Zompo MD, Loviselli A. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8. [DOI] [PubMed]
- 10.Hornig M, Amsterdam JD, Kamoun M, Goodman DB. Autoantibody disturbances in affective disorders: A function of age and gender? J Affect Disord 1999;55:29-37. [DOI] [PubMed]
- 11.Spivak B, Radwan M, Bartur P, Brandon J, Tyano S, Weizman A. Haloperidol and lithium carbonate treatment did not influence serum immunoglobulin levels in schizophrenic and affective patients. Acta Psychiatr Scand 1991;84:225-8. [DOI] [PubMed]
- 12.Calabrese JR, Gulledge AD, Hahn K, Skwerer R, Kotz M, Schumacher OP, et al. Autoimmune thyroiditis in manic-depressive patients treated with lithium. Am J Psychiatry 1985;142:1318-21. [DOI] [PubMed]
- 13.Lazarus JH. The effects of lithium therapy on thyroid and thyropropin releasing hormone. Thyroid 1998;8:909-13. [DOI] [PubMed]
- 14.Johnston AM, Eagles JM. Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry 1999;175:336-9. [DOI] [PubMed]
- 15.Demers LM, Spencer CA, editors. Laboratory support for the diagnosis and monitoring of thyroid disease. National Academy of Clinical Biochemistry; 2002. Available: www.nacb.org/lmpg/thyroid_LMPG_PDF.stm (accessed 2005 Sep 1).
- 16.Mariotti S, Chiovato L, Franceschi C, Pinchera A. Thyroid autoimmunity and aging. Exp Gerontol 1998;33:535-41. [DOI] [PubMed]
- 17.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99. [DOI] [PubMed]
- 18.Bauer M, Blumentritt H, Schlattmann P, Müller-Oerlinghausen B. The prevalence of goiter in lithium-treated patients: a controlled thyroid ultrasonographic study [abstract]. J Bipolar Disord 1999; 1(Suppl 1):24.
- 19.Berghöfer A, Kossmann B, Müller-Oerlinghausen B. Course of illness and pattern of recurrences in patients with affective disorders during long-term lithium prophylaxis: a retrospective analysis over 15 years. Acta Psychiatr Scand 1996;93:349-54. [DOI] [PubMed]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, revised (DSM-III-R). 3rd ed. Washington: the Association; 1987.
- 21.Leutgeb U. Lithium und seine Wirkungen auf das Endokrinum, den Knochen und den peripheren Nerven – eine aktuelle Übersicht. Fortschr Neurol Psychiatr 1995;63:149-61. [DOI] [PubMed]
- 22.Kahaly GJ, Dietlein M. Cost estimation of thyroid disorders in Germany. Thyroid 2002;12:909-14. [DOI] [PubMed]
- 23.Leutgeb U. Ambient iodine and lithium-associated with clinical hypothyroidism. Br J Psychiatry 2000;176:495-6. [DOI] [PubMed]
- 24.Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001;22:240-54. [DOI] [PubMed]
- 25.Bell TM, Bansal AS, Shorthouse C, Sandford N, Powell EE. Low-titre auto-antibodies predict autoimmune disease during interferon-alpha treatment of chronic hepatitis C. J Gastroenterol Hepatol 1999;14:419-22. [DOI] [PubMed]
- 26.Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;359:241-7. [DOI] [PubMed]
- 27.Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA 2003;290:1467-73. [DOI] [PubMed]
- 28.Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 2004;161:217-22. [DOI] [PubMed]


