Abstract
The opportunistic fungal pathogen Candida albicans can cause superficial as well as systemic infections. Successful adaptation to the different host niches encountered during infection requires coordinated expression of various virulence traits, including the switch between yeast and hyphal growth forms and secretion of aspartic proteinases. Using an in vivo expression technology that is based on genetic recombination as a reporter of gene activation during experimental candidiasis in mice, we investigated whether two signal transduction pathways controlling hyphal growth, a mitogen-activated protein kinase cascade ending in the transcriptional activator Cph1p and a cyclic AMP-dependent regulatory pathway that involves the transcription factor Efg1p, also control expression of the SAP5 gene, which encodes one of the secreted aspartic proteinases and is induced by host signals soon after infection. Our results show that both transcriptional regulators are important for SAP5 activation in vivo. SAP5 expression was reduced in a cph1 mutant, although filamentous growth in infected tissue was not detectably impaired. SAP5 expression was also reduced, but not eliminated, in an efg1 null mutant, although this strain grew exclusively in the yeast form in infected tissue, demonstrating that in contrast to in vitro conditions, SAP5 activation during infection does not depend on growth of C. albicans in the hyphal form. In a cph1 efg1 double mutant, however, SAP5 expression in infected mice was almost completely eliminated, suggesting that the two signal transduction pathways are important for SAP5 expression in vivo. The avirulence of the cph1 efg1 mutant seemed to be caused not only by the inability to form hyphae but also by a loss of expression of additional virulence genes in the host.
The yeast Candida albicans is a member of the microflora on mucosal surfaces of healthy people, but it can also cause superficial and life-threatening systemic infections, especially in immunocompromised patients. The success of C. albicans both as a colonizer and as an infectious microorganism probably depends on many different characteristics of the fungus, including its ability to adhere to a variety of host tissues, its ability to switch between yeast and hyphal growth forms, and its secretion of hydrolytic enzymes, such as proteinases and phospholipases (3, 5, 24). Since C. albicans encounters different body niches during an infection, a flexible reaction to different environmental conditions is a prerequisite for optimal adaptation to the host at different infection stages. For example, the switch from yeast growth to hyphal growth may facilitate tissue invasion and evasion of macrophages, and the generation of yeasts budding off from the hyphae may allow more efficient spread through the bloodstream during disseminated infection and multiplication in infected tissues. Accordingly, mutants restricted to either the yeast form or the hyphal form are attenuated in terms of virulence (2, 18). The secreted aspartic proteinases (Saps) are also necessary for full virulence of C. albicans (6, 10, 14, 28). Different roles have been suggested for the Saps based on in vitro experiments; these roles include nitrogen supply (30), adherence (33), degradation of host barriers (4, 23), and evasion of host defense mechanisms (11, 25). C. albicans has a large gene family encoding Saps (21), and it is likely that the individual Sap isoenzymes evolved for optimal adaptation to specific functions or host niches. In fact, by using an in vivo expression technology (IVET) that is based on genetic recombination as a reporter of gene expression and allows detection of gene activation in single cells, we could demonstrate that individual SAP genes were differentially activated during infection depending on the host niche and the infection stage (31).
It is likely that expression of different virulence traits has to be coordinated, and common signal transduction pathways may be used to ensure induction of an appropriate set of virulence genes in response to environmental signals. A mitogen-activated protein kinase cascade ending in the transcription factor Cph1p and a cyclic AMP-dependent signaling pathway ending in the transcriptional regulator Efg1p are both necessary for induction of filamentous growth in C. albicans, depending on the conditions used (17, 18). Expression of some of the SAP genes, SAP4 to SAP6, in vitro has been linked to the hyphal form of C. albicans (9, 34). This linkage was confirmed by the finding that in contrast to expression in the wild-type parent strain, expression of SAP4 to SAP6 was not detectable in an efg1 mutant in vitro under conditions in which the strain failed to form hyphae (29). However, recent studies have demonstrated that the pattern of expression of virulence genes in pathogenic microorganisms in an infected host can be totally different from the expression pattern observed in vitro, including the dependence on regulatory factors (15, 16). Indeed, when IVET was used to study expression of the SAP genes during infection, we observed that the SAP5 gene, one of the putative hypha-specific genes, was induced very soon after contact with the host, at a time when no hyphae were detected in the infected animals (31). We suggested that expression of SAP5 during infection might be activated by signals that also induce hyphal growth, possibly involving the same signal transduction pathways, but might be independent of the hyphal morphology itself. Therefore, in the present study we investigated whether the transcriptional regulators CPH1 and EFG1, representing the two best-studied signaling pathways regulating hyphal formation in C. albicans (7, 35), were also necessary for SAP5 activation under experimental infection conditions. The SAP5 gene was chosen for this analysis because activation of this gene was observed in a large proportion of infecting cells and a negative influence of a regulatory mutation on SAP5 expression in vivo would be detected most easily (31). In addition, an efg1 mutation strongly attenuates virulence and prevents tissue invasion, so that certain host niches that induce activation of specific virulence genes are not accessible to an efg1 mutant (18). Since the SAP5 gene is activated very soon after infection, it was possible to study a possible effect of regulatory mutations on gene expression in a body location that is reached by both wild-type and mutant strains (i.e., under comparable in vivo conditions).
MATERIALS AND METHODS
Strains and growth media.
The C. albicans strains used in this study are listed in Table 1. The strains were maintained on minimal agar (6.7 g of yeast nitrogen base without amino acids [Bio 101, Vista, Calif.] per liter, 20 g of glucose per liter, 0.77 g of complete supplement medium [Bio 101] per liter, 15 g of agar per liter). For routine growth of the strains, YPD liquid medium (10 g of yeast extract per liter, 20 g of peptone per liter, 20 g of glucose per liter) was used. For induction of the SAP2 promoter, cells were grown overnight in YCB-BSA (23.4 g of yeast carbon base per liter, 4 g of bovine serum albumin per liter; pH 4.0). Screening for mycophenolic acid (MPA)-sensitive colonies was performed after 2 days of growth at 30°C on minimal agar containing 1 μg of MPA ml−1, which resulted in generation of large MPAr and small MPAr colonies (32). Uridine (100 μg ml−1) was added to the media to support growth of ura3 mutant strains.
TABLE 1.
C. albicans strains used in this study
| Strain(s) | Parent | Genotypea | Reference |
|---|---|---|---|
| CAI4 | Δura3::imm434/Δura3::imm434 | 8 | |
| CFI1 | CAI4 | ACT1/act1::FRT-MPAr-FRT | 32 |
| S2FI5B | CFI1 | sap2-1::PSAP2-1-ecaFLP-URA3/SAP2-2 | 31 |
| S5FI2A | CFI1 | sap5-1::PSAP5-ecaFLP-URA3/SAP5-2 | 31 |
| S5FI2B | CFI1 | SAP5-1/sap5-2::PSAP5-ecaFLP-URA3 | 31 |
| JKC18 | CAI4 | cph1::hisG/cph1::hisG | 17 |
| CFI2 | JKC18 | ACT1/act1::FRT-MPAr-FRT | This study |
| C2S2F1A and C2S2F1B | CFI2 | sap2-1::PSAP2-1-ecaFLP-URA3/SAP2-2 | This study |
| C2S5F1A and C2S5F1B | CFI2 | sap5-1::PSAP5-ecaFLP-URA3/SAP5-1/SAP5-2 | This study |
| HLC67 | CAI4 | efg1::hisG/efg1::hisG | 18 |
| CFI3 | HLC67 | ACT1/act1::FRT-MPAr-FRT | This study |
| C3S2F1D and C3S2F1E | CFI3 | sap2-1::PSAP2-1-ecaFLP-URA3/SAP2-1 | This study |
| C3S5F1A | CFI3 | SAP5-1/sap5-2::PSAP5-ecaFLP-URA3 | This study |
| C3S5F1B | CFI3 | sap5-1::PSAP5-ecaFLP-URA3/SAP5-2 | This study |
| HLC69 | CAI4 | cph1::hisG/cph1::hisG efg1::hisG/efg1::hisG | 18 |
| CFI4 | HLC69 | ACT1/act1::FRT-MPAr-FRT | This study |
| C4S2F1B and C4S2F1C | CFI4 | sap2-1::PSAP2-1-ecaFLP-URA3/SAP2-2 | This study |
| C4S5F1A | CFI4 | SAP5-1/sap5-2::PSAP5-ecaFLP-URA3 | This study |
| C4S5F1B | CFI4 | sap5-1::PSAP5-ecaFLP-URA3/SAP5-2 | This study |
Apart from the features indicated all strains are identical to their parents.
C. albicans transformation.
C. albicans strains were transformed by electroporation (13) with the following gel-purified linear DNA fragments: a SacI-SacI fragment from pAFI3 (32) containing the FRT-MPAr-FRT cassette between flanking ACT1 sequences (Fig. 1A), an XbaI-SacI fragment from pSFL53 (31) containing a PSAP5-ecaFLP reporter gene fusion (Fig. 2A), and an XbaI-SacI fragment from pSFL213 (31) containing a PSAP2-1-ecaFLP reporter gene fusion (Fig. 3A). MPA-resistant transformants were selected on minimal agar plates containing 100 μg of uridine ml−1 and 10 μg of MPA ml−1. Single colonies were picked after 5 to 7 days of growth at 30°C and restreaked on the same medium. Uridine-prototrophic transformants were selected on minimal agar plates without uridine.
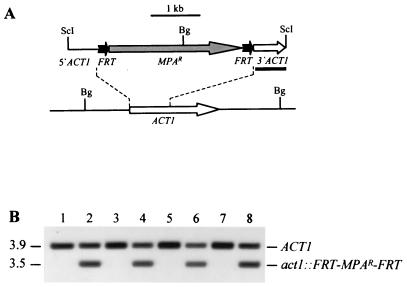
FIG. 1.
Integration of the deletable FRT-MPAr-FRT cassette into one of the ACT1 alleles of strains JKC18 (cph1/cph1), HLC67 (efg1/efg1), and HLC69 (cph1/cph1 efg1/efg1). (A) Integration scheme. The ACT1 coding region is represented by the open arrow, and the MPAr marker is represented by the grey arrow. The 34-bp FRT site is not drawn to scale. The probe used for verification of correct integration by Southern hybridization is represented by the black bar, and the diagnostic BglII sites are shown. Bg, BglII; ScI, SacI. (B) Southern hybridization of BglII-digested genomic DNA of parent strains and their transformants carrying the FRT-MPAr-FRT cassette with an ACT1-specific probe. The positions of the fragments are indicated on the right, and molecular sizes (in kilobases) are indicated on the left. Lane 1, CAI4; lane 2, CFI1; lane 3, JKC18; lane 4, CFI2; lane 5, HLC67; lane 6, CFI3; lane 7, HLC69; lane 8, CFI4.
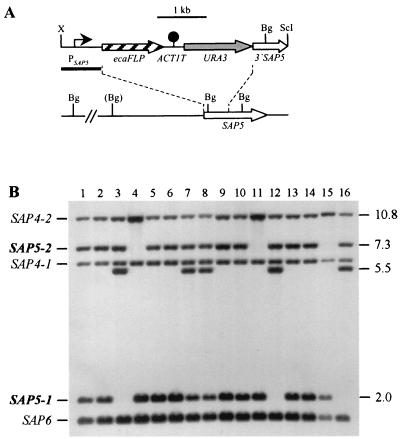
FIG. 2.
Integration of the PSAP5-ecaFLP fusion into one of the SAP5 alleles of strains CFI2 (cph1/cph1), CFI3 (efg1/efg1), and CFI4 (cph1/cph1 efg1/efg1). (A) Integration scheme. The coding regions of the SAP5 and ecaFLP genes are represented by the open and cross-hatched arrows, respectively. The SAP5 promoter (PSAP5) is indicated by the solid arrow, the ACT1 transcription termination sequence (ACT1T) is indicated by the solid circle, and the URA3 selection marker is indicated by the grey arrow. The probe used for verification of correct integration by Southern hybridization is represented by the black bar, and the diagnostic BglII sites are shown. The BglII site in parentheses is not present in the SAP5-2 allele of strain CAI4 and its derivatives. Bg, BglII; ScI, SacI; X, XbaI. (B) Southern hybridization of BglII-digested genomic DNA of parent strains and their transformants carrying the ecaFLP reporter gene with the SAP5 promoter fragment as the probe. The positions of the wild-type SAP5 alleles (boldface) and the cross-hybridizing SAP4 and SAP6 fragments are indicated on the left. The molecular sizes (in kilobases) of the wild-type SAP5 fragments and the fragments corresponding to the reporter gene fusions are indicated on the right. Lane 1, CAI4; lane 2, CFI1; lane 3, S5FI2A; lane 4, S5FI2B; lane 5, JKC18; lane 6, CFI2; lane 7, C2S5F1A; lane 8, C2S5F1B; lane 9, HLC67; lane 10, CFI3; lane 11, C3S5F1A; lane 12, C3S5F1B; lane 13, HLC69; lane 14, CFI4; lane 15, C4S5F1A; lane 16, C4S5F1B.
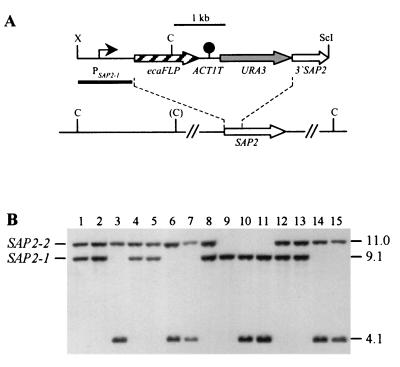
FIG. 3.
Integration of the PSAP2-ecaFLP fusion into one of the SAP2 alleles of strains CFI2 (cph1/cph1), CFI3 (efg1/efg1), and CFI4 (cph1/cph1 efg1/efg1). (A) Integration scheme. The coding regions of the SAP2 and ecaFLP genes are represented by the open and cross-hatched arrows, respectively. The SAP2 promoter (PSAP2-1) is indicated by the solid arrow, the ACT1 transcription termination sequence (ACT1T) is indicated by the solid circle, and the URA3 selection marker is indicated by the grey arrow. The probe used for verification of correct integration by Southern hybridization is represented by the black bar, and the diagnostic ClaI sites are shown. The ClaI site in parentheses is not present in the SAP2-2 allele of strain CAI4 and its derivatives. C, ClaI; ScI, SacI; X, XbaI. (B) Southern hybridization of ClaI-digested genomic DNA of parent strains and their transformants carrying the ecaFLP reporter gene with the SAP2 promoter fragment as the probe. The positions of the wild-type SAP2 alleles are indicated on the left, and the molecular sizes (in kilobases) of the wild-type SAP2 fragments and the fragment corresponding to the reporter gene fusion are indicated on the right. Lane 1, CAI4; lane 2, CFI1; lane 3, S2FI5B; lane 4, JKC18; lane 5, CFI2; lane 6, C2S2F1A; lane 7, C2S2F1B; lane 8, HLC67; lane 9, CFI3; lane 10, C3S2F1D; lane 11, C3S2F1E; lane 12, HLC69; lane 13, CFI4; lane 14, C4S2F1B; lane 15, C4S2F1C.
Isolation of chromosomal DNA and Southern hybridization.
Genomic DNA of C. albicans strains was isolated as described previously (20). DNA (10 μg) was digested with appropriate restriction enzymes, separated on a 1% (wt/vol) agarose gel, and, after ethidium bromide staining, transferred by vacuum blotting onto a nylon membrane and fixed by UV cross-linking. Southern hybridization with enhanced chemiluminescence-labeled probes was performed with an ECL labeling and detection kit obtained from Amersham (Braunschweig, Germany) according to the instructions of the manufacturer.
In vivo experiments.
For the in vivo experiments, 8- to 12-week-old female BALB/c mice (Harlan, Borchen, Germany) were used. To prepare the inoculum, C. albicans yeast cells grown overnight in YPD broth at 30°C were washed twice in phosphate-buffered saline (PBS) (Gibco, Karlsruhe, Germany) and resuspended in the same buffer. The phenotype of injected cells was controlled by spreading the inocula on MPA indicator plates. The percentage of MPA-sensitive cells was always less than 1%.
Mice were each infected intraperitoneally with 1 × 108 blastoconidia in 1.0 ml of PBS. At 30 min postinfection C. albicans cells were recovered by peritoneal lavage with 10 ml of PBS. Cells that adhered to the liver surface at 4 h but had not yet invaded (as determined by microscopic examination) were recovered after the peritoneal cavity was first washed to remove nonadherent cells. The organ was then cut out and homogenized with Tenbroeck tissue grinders (Wheaton Scientific) in 10 ml of sterile distilled water. After 24 and 48 h cells that adhered to or had invaded the liver were recovered in the same way. Aliquots of the lavages and the homogenates were spread on indicator plates to determine the percentage of MPA-sensitive cells.
Histology and alanine aminotransferase (ALT) activity determination.
For histological examination, organs were dissected, and blocks of tissue were fixed in a 10% formaldehyde solution in PBS. The tissue samples were further processed by using standard methods for paraffin embedding and cutting. Five-micrometer sections of the organs were cut. To stain the C. albicans cells, the periodic acid-Schiff reaction was used. The tissue sections were incubated in a 1% solution of periodic acid in distilled water for 5 min, and this was followed by washing with distilled water and incubation with Schiff reagent (Sigma), which consisted of 1% (wt/vol) pararosaniline HCl and 4% (wt/vol) sodium bisulfite in hydrochloric acid (0.25 mol/liter). The periodic acid-Schiff reagent reaction mixture was developed in tap water for 10 min. The tissue sections were counterstained with Mayer’s hemalum solution (Merck) for 10 s, dehydrated, and embedded in DePeX (Serva, Heidelberg, Germany).
ALT activity, an indicator of liver damage in infected mice, was determined as described previously (14).
RESULTS
Construction of C. albicans reporter strains in cph1 and efg1 mutant backgrounds.
So that we could use IVET to analyze the influence of CPH1 and EFG1 on virulence gene expression during infection, we integrated the deletable MPA resistance marker, flanked by direct repeats of the minimal FLP recombination target (FRT), into the genome of the cph1 mutant JKC18, the efg1 mutant HLC67, and the cph1 efg1 double mutant HLC69 (Fig. 1A). Southern hybridization analysis demonstrated that the resulting MPA-resistant transformants, CFI2 (Fig. 1B, lane 4), CFI3 (lane 6), and CFI4 (lane 8), had the deletable marker integrated into one of the ACT1 alleles in the same way as in previously described strain CFI1 (lane 2), as shown by the appearance of an expected 3.5-kb fragment in addition to the 3.9-kb wild-type fragment (lanes 1 to 8) hybridizing with an ACT1-specific probe.
A fusion of the ecaFLP reporter gene to the SAP5 promoter (PSAP5) was then integrated into the various signal transduction mutants carrying the deletable MPAr marker (Fig. 2A). The two SAP5 alleles in strain CAI4 and its derivatives can be distinguished by a BglII restriction site polymorphism. As previously shown for strains S5FI2A and S5FI2B (Fig. 2B, lanes 3 and 4) carrying the PSAP5-ecaFLP fusion in a wild-type background, derivatives of the efg1 mutant CFI3 and the cph1 efg1 double mutant CFI4 were isolated in which the reporter gene fusion was integrated into one of the two possible SAP5 alleles (strains C3S5F1A and C3S5F1B [lanes 11 and 12] and strains C4S5F1A and C4S5F1B [lanes 15 and 16]). All of the transformants of strain CFI2 tested had the reporter gene fusion integrated into the SAP5-1 allele; however, the original wild-type fragment did not disappear in these transformants, as was expected after allelic replacement (lanes 7 and 8). Close examination of the Southern blot revealed that the relative signal intensity of the band corresponding to the SAP5-1 allele was stronger in strain JKC18 (lane 5) and its derivative, CFI2 (lane 6), than in the other parent strains, CAI4 (lane 1), HLC67 (lane 9), and HLC69 (lane 13), and their derivatives, CFI1 (lane 2), CFI3 (lane 10), and CFI4 (lane 14). This suggests that duplication of the SAP5-1 allele had occurred in our copy of strain JKC18 and was maintained in all of its derivatives. Two independent transformants carrying the PSAP5-ecaFLP fusion integrated into one of the duplicated SAP5-1 alleles in the cph1 mutant background, strains C2S5F1A and C2S5F1B (lanes 7 and 8), were used for further analysis.
To control for a possible effect of the cph1 and efg1 mutations on FLP activity, a PSAP2-1-ecaFLP fusion was also integrated into strains CFI2, CFI3, and CFI4 (Fig. 3A), since the SAP2 promoter could be induced by growth of the cells in YCB-BSA. The two SAP2 alleles in strain CAI4 and its derivatives could be distinguished by ClaI restriction site polymorphism. Wild-type control strain S2FI5B contained the PSAP2-1-ecaFLP fusion integrated into the SAP2-1 allele (Fig. 3B, lane 3). To ensure optimal comparability, the reporter fusion was integrated into the same SAP2 allele in two independent transformants of strains CFI2 (strains C2S2F1A and C2S2F1B [lanes 6 and 7]), CFI3 (strains C3S2F1D and C352F1E [lanes 10 and 11]), and CFI4 (strains C4S2F1B and C4S2F1C [lanes 14 and 15]). We found in this analysis that heterozygosity was lost in strain CFI3, presumably due to a recombination event that may have been induced by the electroporation, so that this strain carried two copies of only the SAP2-1 allele based on the ClaI hybridization pattern (Fig. 3B, lane 9).
Induction of the SAP2 promoter resulted in excision of the MPAr marker from the genomes of all reporter strains with the same efficiency, irrespective of the genetic background (all of the cells were MPAs after overnight growth in YCB-BSA). However, in contrast to the wild-type strains and the cph1 mutants, the efg1 and cph1 efg1 mutants lost the MPAr marker after growth in SAP2-repressing minimal medium (more than 10% of the cells in each strain), suggesting that the SAP2 promoter is somewhat derepressed in the absence of a functional EFG1 gene. Therefore, SAP2 expression was not investigated further. However, these results demonstrated that the FLP recombinase works normally in all of the mutant backgrounds tested.
CPH1 and EFG1 contribute to SAP5 activation during infection.
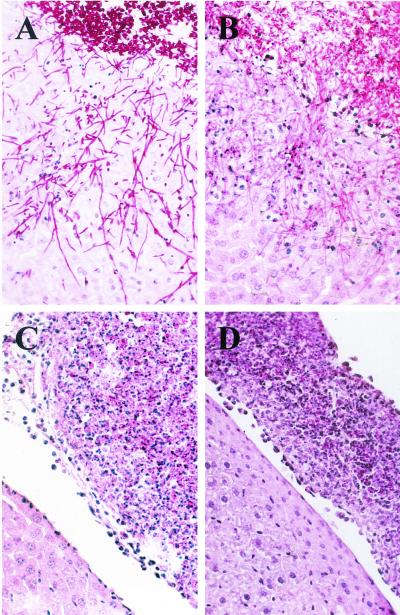
The various reporter strains carrying the PSAP5-ecaFLP fusion were then tested for SAP5 activation in a mouse model of Candida peritoneal infection. The wild-type reporter strains and the cph1 mutants formed germ tubes that had adhered to the surfaces of parenchymatous organs at 4 h and had invaded deeply 24 h after inoculation into the peritoneal cavity (Fig. 4A and B). In contrast, no germ tubes were observed with the efg1 single and cph1 efg1 double mutants (Fig. 4C and D), in agreement with previous studies performed by other researchers (18). The invasiveness of the strains correlated with organ damage, as measured by ALT activity 24 h after infection (14). The ALT activities of mice infected with the wild-type control strains (124 and 177 U in the two mice tested) and with the cph1 mutants (between 102 and 150 U in four mice) were similar. In contrast, the ALT activities were strongly reduced in both the efg1 single mutants (between 8 and 11 U in four mice) and the cph1 efg1 double mutants (between 7 and 10 U in four mice).
FIG. 4.
Microscopic appearance of reporter strains S5FI1A (wild type) (A), C2S5F1A (cph1) (B), C3S5F1A (efg1) (C), and C4S5F1A (cph1 efg1) (D) 24 h after infection. Peritoneal infection by the efg1 single mutant and the cph1 efg1 double mutant was characterized by inflammatory infiltrates composed of yeast cells surrounded by inflammatory cells which were attached to the liver surface (C and D), whereas the wild-type strain and the cph1 mutant invaded the liver (A and B).
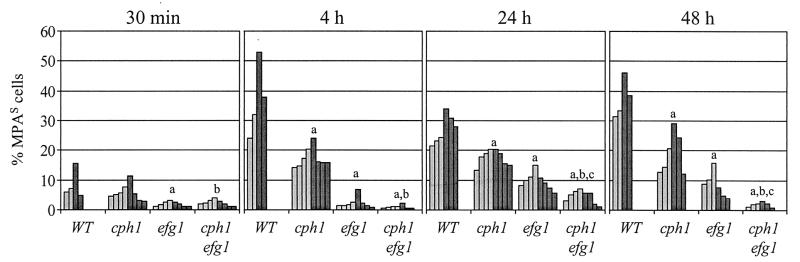
In agreement with our previous results, the SAP5 gene was detectably activated in a significant proportion of wild-type cells soon after infection, before invasion of internal organs had occurred (at 4 h), and a minor subpopulation of the cells was induced for SAP5 expression as soon as 30 min after inoculation into the peritoneal cavity (Fig. 5). Compared with the wild-type reporter strains, the proportion of cells in which the SAP5 gene was detectably activated was reduced in the cph1 and efg1 single mutants at all infection stages. The early activation of SAP5 was strongly dependent on the presence of a functional EFG1 gene, since FLP-mediated marker excision was detected only in a negligible percentage of the cells 4 h after infection (2.3% ± 1.9% MPAs cells [mean ± standard deviation], compared with 36.7% ± 12.1% MPAs cells for the wild type). Later, significant SAP5 activation was also observed in the efg1 mutants (9.6% ± 2.8% and 8.5% ± 4.3% MPAs cells at 24 and 48 h after infection, respectively, compared with 27% ± 4.9% and 37.4% ± 6.5% MPAs cells for the wild type), although the cells were not able to form hyphae and invade the liver. In contrast, the negative effect of cph1 inactivation on SAP5 expression was less pronounced than the effect of the efg1 mutation but was nevertheless clearly detectable at all infection stages (17.3% ± 3.3%, 17.5% ± 2.6%, and 18.9% ± 6.9% MPAs cells after 4, 24, and 48 h, respectively), although the invasiveness of the cph1 mutant was comparable to that of the wild type. In the absence of both CPH1 and EFG1, SAP5 expression was almost completely eliminated, since very few MPA-sensitive cells were recovered at all times investigated (1.1% ± 0.6%, 4.5% ± 2.1%, and 1.9% ± 0.8% MPAs cells after 4, 24, and 48 h, respectively). In each genetic background, the two independently constructed reporter strains gave comparable results. Together, these results demonstrated that SAP5 activation can occur in the absence of CPH1 or EFG1 and independent of hyphal growth, but both transcriptional regulators are necessary for full SAP5 activation in the host.
FIG. 5.
Expression of the SAP5 gene in wild-type (WT) and cph1, efg1, and cph1 efg1 mutant strains at various stages of an intraperitoneal infection. In vivo SAP5 induction was monitored by determining the percentage of MPA-sensitive cells recovered by peritoneal lavage after 30 min and recovered from the liver after 4, 24, and 48 h. The results are results from two independent experiments in which one or two mice were infected per strain and time point; each bar represents one animal. The light grey bars show the results obtained with strains S5FI2A, C2S5F1A, C3S5F1A, and C4S5F1A, and the dark grey bars show the results obtained with strains S5FI2B, C2S5F1B, C3S5F1B, and C4S5F1B. a, mutant strains were significantly different from the wild type (P < 0.005, as determined by Student’s t test); b, cph1 efg1 mutants were significantly different from the cph1 single mutant; c, cph1 efg1 mutants were significantly different from the efg1 single mutant.
DISCUSSION
The complex environments encountered by pathogens within their host during an infection can influence gene expression patterns in a way that is very different from the organism’s response to the simpler parameters that regulate gene activity in vitro (19). In vivo, the induction of virulence genes can depend on regulatory factors which are not required for expression in vitro, and conversely, the dependence of virulence gene expression on specific regulators in vitro may be bypassed in the host by the use of alternative signaling pathways, illustrating the importance of studying regulation of virulence genes in the context of bona fide host-pathogen interactions (15, 16).
The recombination-based IVET detects expression of a target gene whenever the promoter is sufficiently activated in a cell to result in recombinase-mediated marker excision. As in a previous study (31), expression of the SAP5 gene was observed only in a subpopulation of cells recovered from infected tissue. The heterogeneity in expression status can be explained in several ways. Both yeasts and hyphae were present in the infected host, and the different morphological forms probably differed with respect to gene expression. It is also likely that fungal cells reisolated from whole organs in fact inhabited many different microniches, only some of which may have induced SAP5 expression. Apart from these factors, it can be assumed that individual cells in a given population differ with respect to the activation status of genes even in the same environment, so that in some cells the promoter activity is below the threshold that is necessary to detect gene expression by IVET. As long as no differences are observed in the infection progress of two strains that are compared, a reduction in the number of cells in which a target gene is detectably activated can be assumed to be caused by reduced promoter activity in the population. This should have been the case for the cph1 mutants at all infection stages which we investigated, since we did not observe major differences in the capacities of these strains to infect internal organs in our experimental model system compared with the wild-type controls, in agreement with observations made by other workers (18). In contrast, the efg1 and cph1 efg1 mutants were unable to form hyphae and invade the internal organs. Especially at the late infection stages, when wild-type cells had invaded deep tissue, the different host niches in which wild-type and mutant cells were located, and not the defective signal transduction pathways alone, contributed to differences in gene expression. Nevertheless, at the early infection stages the mutants had access to the same host niches as the wild-type strains (i.e., the peritoneal cavity and the surfaces of the parenchymatous organs). Since in the wild-type strains SAP5 activation was clearly detectable in these host niches, the failure of the mutants to induce SAP5 at a significant level indicates the importance of EFG1 for SAP5 expression at this infection stage. At later times, even in the absence of hyphal formation and tissue invasion, reduced but significant SAP5 activation was observed also with the efg1 single mutants, which may have been induced by changes in the host environment caused by the infecting C. albicans cells. It seems that this activation was mediated by the Cph1p transcription factor, because almost no SAP5 induction was detected in the cph1 efg1 double mutants, indicating the importance of the two signal transduction pathways for SAP5 activation in an infected host. Interestingly, Efg1p also seems to negatively regulate expression of some genes directly or indirectly, since expression of the SAP2 gene was derepressed in all strains lacking a functional EFG1 gene.
Transcripts hybridizing with a probe specific for the SAP4 to SAP6 genes have also been detected during hyphal growth of C. albicans in vitro (9, 29). Because of the high levels of homology of the three genes, Northern hybridization did not reveal whether all three genes or only one or two of them were expressed under these conditions. In contrast, using antisera that recognize this subgroup of Saps, Borg-von Zepelin et al. observed expression of SAP4 to SAP6 only after phagocytosis by murine peritoneal macrophages and not in hyphae alone (1). We tried to detect SAP5 expression in vitro by incubating the strains containing the PSAP5-ecaFLP reporter fusion in a wild-type background in RPMI medium containing serum at 37°C, conditions that favor hyphal growth of C. albicans. Although germ tube formation and formation of hyphae were induced very efficiently, SAP5 expression was hardly detected, since very few cells became MPA sensitive (data not shown). This result was confirmed with strains carrying a different reporter gene, GFP, under the control of the SAP5 promoter. The fluorescence of the cells was hardly above the background level (unpublished observations), although like FLP, GFP has been demonstrated to be a useful reporter of SAP2 expression under SAP2-inducing conditions (22). Therefore, the transcripts detected by other workers during hyphal growth either may correspond to SAP4 and/or SAP6 but not SAP5 or SAP5 expression under hypha formation conditions in vitro is below the detection limit of our reporter systems. These observations, which are in agreement with those of Borg-von Zepelin et al. (1), suggest that SAP5 activation during infection is much stronger than the possible low-level expression under hypha-inducing conditions in vitro.
An issue to be considered when our IVET is used is that some of the colonies obtained after cells recovered from infected tissue are plated may be derived from more than one cell. If only one of the cells contains the MPAr marker, this gives rise to an MPAr colony, so the actual percentage of MPAs cells is underestimated. Microscopic analysis demonstrated that there was no clumping of cells that might be responsible for the reduced percentage of MPAs colonies of the efg1 and cph1 efg1 mutants. However, since the wild-type strains and the cph1 single mutants form hyphae in infected tissue, many colonies of these strains are derived from mycelial fragments, which can consist of several unseparated cells. Therefore, the percentages of MPAs cells of these strains may in fact have been somewhat higher than the observed percentages of MPAs colonies, and the difference between the wild type and the efg1 and cph1 efg1 mutants may have been even more pronounced. However, this would only emphasize our conclusion that full activation of the SAP5 gene during infection depends on CPH1 and EFG1.
We observed that our copy of cph1 mutant JKC18 contained a duplication of one of the SAP5 alleles. In addition, a change from heterozygosity to homozygosity for the SAP2 gene occurred during the construction of strain CFI3, which was derived from efg1 mutant HLC67. Such changes may be induced by genetic manipulation of strains (transformation, fluoroorotic acid selection) but may also occur due to natural genomic alterations (26, 27). These changes were detected only later when the reporter fusions were introduced into the loci and only because we made efforts to distinguish between the two alleles of the target locus. Nonspecific genomic alterations in derivatives of a parent strain may therefore be more common than suggested by the usually limited analyses of genetically engineered strains. However, we do not think that the additional copy of SAP5 or the homozygosity for SAP2 influenced our results for SAP5 regulation by CPH1 and EFG1, since no such alterations were observed in the reporter strains derived from the cph1 efg1 double mutant. The contribution of both CPH1 and EFG1 to SAP5 induction was clearly observable when the SAP5 expression in the double mutant strains was compared with the SAP5 expression in each of the single mutants.
Several important conclusions can be drawn from our results. SAP5 expression and hyphal morphology seem to be coincidentally linked in vitro, because both phenotypes are induced by the same or similar signals. Our results obtained with the efg1 mutants demonstrate that SAP5 can be expressed in the absence of hyphal formation in vivo, although SAP5 activation occurred at a reduced level in these strains due to the involvement of EFG1 in SAP5 induction. In the absence of both transcription factors, SAP5 expression was almost completely eliminated in vivo, at least in the infection model used in this study. Therefore, the avirulence of the cph1 efg1 mutant seems to be caused not only by the nonfilamentous phenotype but also by the loss of expression of additional virulence genes. Although the ability to grow in the hyphal form is certainly important for the virulence of C. albicans, it will be difficult to assess the contribution of morphology per se since many other cellular characteristics change upon the yeast-hypha transition (12). These observations illustrate the complex regulatory networks that control and coordinate virulence gene expression in C. albicans within its host.
Acknowledgments
This study was supported by the Bundesministerium für Bildung und Forschung (grant O1 K1 8906-0) and the Deutsche Forschungsgemeinschaft (grants MO 846/1 and KR 2002/1-1). Peter Staib was the recipient of a grant from the Studienstiftung des deutschen Volkes. Joachim Morschhäuser was the recipient of a Heisenberg fellowship from the Deutsche Forschungsgemeinschaft.
We thank Julia Köhler, Gerald Fink, and Steffen Rupp for providing C. albicans strains JKC18, HLC67, and HLC69.
Editor: T. R. Kozel
REFERENCES
- 1.Borg-von Zepelin, M., S. Beggah, K. Boggian, D. Sanglard, and M. Monod. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28:543–554. [DOI] [PubMed] [Google Scholar]
- 2.Braun, B. R., W. S. Head, M. X. Wang, and A. D. Johnson. 2000. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 156:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327–335. [DOI] [PubMed] [Google Scholar]
- 4.Colina, A. R., F. Aumont, N. Deslauriers, P. Belhumeur, and L. de Repentigny. 1996. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect. Immun. 64:4514–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187–218. [DOI] [PubMed] [Google Scholar]
- 6.De Bernardis, F., S. Arancia, L. Morelli, B. Hube, D. Sanglard, W. Schäfer, and A. Cassone. 1999. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J. Infect. Dis. 179:201–208. [DOI] [PubMed] [Google Scholar]
- 7.Ernst, J. F. 2000. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 146:1763–1774. [DOI] [PubMed] [Google Scholar]
- 8.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hube, B., M. Monod, D. A. Schofield, A. J. Brown, and N. A. Gow. 1994. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol. Microbiol. 14:87–99. [DOI] [PubMed] [Google Scholar]
- 10.Hube, B., D. Sanglard, F. C. Odds, D. Hess, M. Monod, W. Schäfer, A. J. Brown, and N. A. Gow. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaminishi, H., H. Miyaguchi, T. Tamaki, N. Suenaga, M. Hisamatsu, I. Mihashi, H. Matsumoto, H. Maeda, and Y. Hagihara. 1995. Degradation of humoral host defense by Candida albicans proteinase. Infect. Immun. 63:984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi, S. D., and J. E. Cutler. 1998. Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol. 6:92–94. [DOI] [PubMed] [Google Scholar]
- 13.Köhler, G. A., T. C. White, and N. Agabian. 1997. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J. Bacteriol. 179:2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretschmar, M., B. Hube, T. Bertsch, D. Sanglard, R. Merker, M. Schröder, H. Hof, and T. Nichterlein. 1999. Germ tubes and proteinase activity contribute to virulence of Candida albicans in murine peritonitis. Infect. Immun. 67:6637–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625–634. [DOI] [PubMed] [Google Scholar]
- 17.Liu, H., J. Köhler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726. [DOI] [PubMed] [Google Scholar]
- 18.Lo, H. J., J. R. Köhler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949. [DOI] [PubMed] [Google Scholar]
- 19.Mahan, M. J., D. M. Heithoff, R. L. Sinsheimer, and D. A. Low. 2000. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu. Rev. Genet. 34:139–164. [DOI] [PubMed] [Google Scholar]
- 20.Millon, L., A. Manteaux, G. Reboux, C. Drobacheff, M. Monod, T. Barale, and Y. Michel-Briand. 1994. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J. Clin. Microbiol. 32:1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monod, M., G. Togni, B. Hube, and D. Sanglard. 1994. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol. Microbiol. 13:357–368. [DOI] [PubMed] [Google Scholar]
- 22.Morschhäuser, J., S. Michel, and J. Hacker. 1998. Expression of a chromosomally integrated, single-copy GFP gene in Candida albicans, and its use as a reporter of gene regulation. Mol. Gen. Genet. 257:412–420. [DOI] [PubMed] [Google Scholar]
- 23.Morschhäuser, J., R. Virkola, T. K. Korhonen, and J. Hacker. 1997. Degradation of human subendothelial extracellular matrix by proteinase-secreting Candida albicans. FEMS Microbiol. Lett. 153:349–355. [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Garcia, F., M. Sanchez, C. Nombela, and J. Pla. 2001. Virulence genes in the pathogenic yeast Candida albicans. FEMS Microbiol. Rev. 25:245–268. [DOI] [PubMed] [Google Scholar]
- 25.Rüchel, R. 1986. Cleavage of immunoglobulins by pathogenic yeasts of the genus Candida. Microbiol. Sci. 3:316–319. [PubMed] [Google Scholar]
- 26.Rustchenko, E. P., D. H. Howard, and F. Sherman. 1994. Chromosomal alterations of Candida albicans are associated with the gain and loss of assimilating functions. J. Bacteriol. 176:3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rustchenko, E. P., D. H. Howard, and F. Sherman. 1997. Variation in assimilating functions occurs in spontaneous Candida albicans mutants having chromosomal alterations. Microbiology 143:1765–1778. [DOI] [PubMed] [Google Scholar]
- 28.Sanglard, D., B. Hube, M. Monod, F. C. Odds, and N. A. Gow. 1997. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schröppel, K., K. Sprößer, M. Whiteway, D. Y. Thomas, M. Röllinghoff, and C. Csank. 2000. Repression of hyphal proteinase expression by the mitogen-activated protein (MAP) kinase phosphatase Cpp1p of Candida albicans is independent of the MAP kinase Cek1p. Infect. Immun. 68:7159–7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staib, F. 1965. Serum-proteins as nitrogen source for yeastlike fungi. Sabouraudia 4:187–193. [DOI] [PubMed] [Google Scholar]
- 31.Staib, P., M. Kretschmar, T. Nichterlein, H. Hof, and J. Morschhäuser. 2000. Differential activation of a Candida albicans virulence gene family during infection. Proc. Natl. Acad. Sci. USA 97:6102–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staib, P., M. Kretschmar, T. Nichterlein, G. Köhler, S. Michel, H. Hof, J. Hacker, and J. Morschhäuser. 1999. Host-induced, stage-specific virulence gene activation in Candida albicans during infection. Mol. Microbiol. 32:533–546. [DOI] [PubMed] [Google Scholar]
- 33.Watts, H. J., F. S. Cheah, B. Hube, D. Sanglard, and N. A. Gow. 1998. Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinase genes. FEMS Microbiol. Lett. 159:129–135. [DOI] [PubMed] [Google Scholar]
- 34.White, T. C., and N. Agabian. 1995. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J. Bacteriol. 177:5215–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiteway, M. 2000. Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3:582–588. [DOI] [PubMed] [Google Scholar]