Abstract
Oropharyngeal and vaginal candidiases are the most common forms of mucosal fungal infections and are primarily caused by Candida albicans, a dimorphic fungal commensal organism of the gastrointestinal and lower female reproductive tracts. Clinical and experimental observations suggest that local immunity is important in host defense against candidiasis. Accordingly, cytokines and chemokines are present at the oral and vaginal mucosa during C. albicans infections. Since mucosal epithelial cells produce a variety of cytokines and chemokines in response to microorganisms and since C. albicans is closely associated with mucosal epithelial cells as a commensal, we sought to identify cytokines and/or chemokines produced by primary oral and vaginal epithelial cells and cell lines in response to C. albicans. The results showed that proinflammatory cytokines were produced by oral and/or vaginal epithelial cells at various levels constitutively with considerable interleukin-1α (IL-1α) and tumor necrosis factor alpha, but not IL-6, produced in response to C. albicans. In contrast, Th1-type (IL-12 and gamma interferon) and Th2-type-immunoregulatory (IL-10 and transforming growth factor β) cytokines and the chemokines monocyte chemoattractant protein 1 and IL-8 were produced in low to undetectable concentrations with little additional production in response to C. albicans. Taken together, these results indicate that cytokines and chemokines are variably produced by oral and vaginal epithelial cells constitutively, as well as in response to C. albicans, and are predominated by proinflammatory cytokines.
Mucosal candidiasis is a significant problem in both immunocompetent and immunocompromised individuals (23, 29, 34). Most episodes of oropharyngeal and vulvovaginal candidiases (OPC and VVC, respectively), the most common forms of mucosal candidiasis, are caused by Candida albicans, a commensal dimorphic fungal organism of the gastrointestinal and lower female reproductive tracts. Clinical observations show that hormonal influences and antibiotic usage appear to predispose to acute VVC, whereas recurrent VVC (RVVC), which does not share these patterns, is presumed to be due to an as-yet-undefined local immune deficiency. In contrast to RVVC, OPC is common when peripheral T cells are reduced (i.e., patients with chronic mucocutaneous candidiasis, with a history of corticosteroid usage, having undergone chemotherapy or allogeneic transplantation, or infected with human immunodeficiency virus [HIV]) (6, 23).
Host defense mechanisms against mucosal candidiasis are not well understood. Although cell-mediated immunity (CMI) is considered important for protection against mucosal and/or systemic candidiasis (4, 5), the role of systemic CMI against vaginal (12–15, 38) and more recently oral (27) candidiasis has been challenged to various degrees. Thus, investigations have shifted to the role of local oral and vaginal CMI. At the oral mucosa, some role for CMI has been suggested from clinical studies showing that a Th0-type (mixed) salivary cytokine profile is present in HIV-negative persons, while a dominant Th2-type salivary cytokine profile (associated with susceptibility to mucosal Candida infection) was observed in HIV-positive persons and was more pronounced in those with OPC (28). At the vaginal mucosa, however, a role for CMI has yet to be observed. Studies investigating local vaginal CMI in healthy women have shown a predominant Th1-type cytokine profile (interleukin-2 [IL-2], IL-12, and gamma interferon [IFN-γ] > IL-4, IL-5, and IL-10) in vaginal lavages (11) but without significant deviation in lavage fluid from women with RVVC.
Based on a questionable role for CMI against Candida at the oral and vaginal mucosa, recent attention has focused on innate immunity. Accordingly, we recently reported that primary epithelial cells from murine, nonhuman primate, and human vaginal mucosa (10, 36, 37), as well as human oral mucosa (35), significantly inhibited the growth of C. albicans in vitro. In addition to our observations, epithelial cells have recently been recognized as playing active roles in mucosal immune responses, including antigen presentation (16, 17, 40), antimicrobial activity (3, 26, 39, 41), and cytokine and chemokine production in response to microorganisms (20, 21).
Based on the fact that epithelial cells are the cell type most often associated with C. albicans at mucosal surfaces and produce a variety of cytokines and chemokines in response to microorganisms (20, 21), we sought to determine whether human oral and vaginal epithelial cells could potentially contribute to the local immune response by the production of cytokines and chemokines in response to C. albicans.
MATERIALS AND METHODS
Participants.
Informed consent was obtained from each participant, and all procedures in the conduct of clinical research were done in accordance with the Institutional Review Board at Louisiana State University Health Sciences Center, New Orleans. Specimens were collected exclusively from healthy volunteers.
Human vaginal and oral epithelial cell collection and isolation.
Human vaginal cells were collected from vaginal lavages from healthy volunteers. Briefly, a sterile speculum was inserted into the vaginal lumen, followed by administration of 5 ml of sterile phosphate-buffered saline from a sterile transfer pipette connected to a sterile 10-ml syringe. The lavage was performed with constant aspiration for 30 s, and the fluid was collected in a sterile test tube. Human oral cells were collected from whole unstimulated saliva. The lavage fluid or saliva was centrifuged for 10 min at 800 × g, and the supernatant was discarded. The resulting cell pellet was resuspended and used fresh or alternatively placed in cryosolution consisting of 50% fetal bovine serum (FBS; Gibco, Grand Island, N.Y.), 35% RPMI 1640 (Gibco), and 15% dimethyl sulfoxide (Sigma, St. Louis, Mo.) and frozen at −70°C until use. Results did not differ whether fresh or stored cells were used. For the isolation of epithelial cells, vaginal and oral cells were resuspended in 7 ml of Hanks balanced salt solution (HBSS; Gibco) and layered over a sterile 20-μm-pore-size nylon membrane. Epithelial cells retained on the membrane were collected with 50 ml of HBSS into a sterile glass petri dish. Thereafter, the cells were transferred to a 50-ml centrifuge tube and pelleted at 800 × g. The supernatant was discarded, and the epithelial cell-enriched pellet was resuspended in Phytone-peptone medium (Becton Dickinson) supplemented with 10% FBS and 1% penicillin (100 U/ml)-streptomycin (100 μg/ml; Gibco) (tissue culture medium). The epithelial cells were enumerated on a hemocytometer by using trypan blue dye exclusion. The purity of the epithelial cell-enriched pellet (>95%) was determined by enumerating five separate fields of 100 cells and is expressed as the mean percentage of cells with an epithelial morphology.
Human epithelial cell lines.
Two human epithelial cell lines were used. VK2/E6E7 (kind gift from R. Fichorova, Harvard Medical School, Cambridge, Mass.) (9) is an epithelial cell line derived from the vaginal mucosa of a healthy premenopausal female undergoing vaginal repair surgery. VK2/E6E7 is immortalized with human papillomavirus 16E6E7 and was maintained in keratinocyte serum-free medium (Gibco) supplemented with 50 μg of bovine pituitary extract, 0.1 ng of epidermal growth factor, 100 U of penicillin, and 100 μg of streptomycin/ml. KB (ATCC CCL-17) is an epithelial cell line originally derived from an epidermoid carcinoma in the oral mucosa of an adult male but has recently been reported to be contaminated with HeLa cells, a human cervical epithelial cell line (American Type Culture Collection, Manassas, Va.). KB cells were maintained in 90% Eagle minimal essential medium with nonessential amino acids and Earle’s balanced salt solution (Sigma) supplemented with 10% FBS and 1% penicillin-streptomycin. Both cell lines were maintained at 37°C and 7.5% CO2 and were passaged every 3 to 4 days.
Stimulator cells.
C. albicans 3153A was from the National Collection of Pathogenic Fungi (London, United Kingdom). The isolate was grown on Sabouraud-dextrose agar (Becton Dickinson, Cockeysville, Md.) at 30°C, and one colony was used to inoculate 10 ml of Phytone-peptone broth (Becton Dickinson) supplemented with 0.1% glucose. Broth cultures were grown to stationary phase for 18 h at 25°C in a shaking water bath. The blastoconidia were collected, washed with phosphate-buffered saline, and enumerated on a hemacytometer by using trypan blue dye exclusion.
Coculture supernatant collection for examination of cytokines and chemokines.
For examination of cytokines and chemokines, epithelial cells (1 × 105 to 5 × 105 cells/ml) were cocultured with Candida (1 × 107 to 5 × 107/ml) at a ratio of 1:100 in separate wells for 0, 24, 48, 72, and 96 h for primary epithelial cells and for 0, 24, and 96 h for epithelial cell line cells in a total volume of 2 ml of tissue culture medium in 24-well tissue culture plates (Costar, Corning, N.Y.). Preliminary studies showed that results with epithelial cell/Candida cell ratios of 1:10 and 1:1,000 were similar to that obtained with a ratio of 1:100. Controls included epithelial cells (constitutive production) cultured alone in separate wells, C. albicans cultured alone (negative control), and epithelial cells cultured with tumor necrosis factor alpha (TNF-α; 10 to 20 ng/ml [quality control]; Pharmingen, San Diego, Calif.) independently in tissue culture medium alone over the same 96-h period. At each time point, the epithelial cell-Candida coculture and the respective control cultures were aspirated from a new set of individual wells and centrifuged for 5 min at 800 × g. Thereafter, the supernatants were collected and stored at −70°C until assayed. Epithelial cells were also evaluated for viability at each time point and observed to be similar to those in the preculture conditions. Cocultures consisted of five volunteers examined individually and four separate experiments employing each epithelial cell line.
Cytokine and chemokine analysis of coculture supernatants.
Supernatants were assayed for proinflammatory (TNF-α, IL-1α, and IL-6), Th1 (IFN-γ and IL-12), Th2-immunoregulatory (IL-10 and transforming growth factor β [TGF-β]), and chemotactic (monocyte chemoattractant protein 1 [MCP-1] and IL-8) cytokines by enzyme-linked immunosorbent assay (ELISA) by using commercially available capture and biotinylated detection antibodies (Pharmingen). Standard curves were generated by using the respective recombinant human cytokines as previously described (28, 33, 38). The sensitivity of each set of antibodies ranged from 3 to 16 pg/ml. The absorbance values and concentrations of each cytokine were determined by using a Ceres 900 automated microplate reader (Bio-Tek Corp., Wisnooski, Vt.) and Kineticalc software (Bio-Tek). Data were expressed as picograms of cytokine per milliliter.
Statistical analysis.
The unpaired Student’s t test was used to analyze constitutive and Candida-specific cytokine production between primary oral and vaginal epithelial cells. Significant differences were defined as having a P value of <0.05.
RESULTS
To determine whether oral and vaginal epithelial cells produced cytokines and chemokines in response to C. albicans, epithelial cell-enriched populations collected from human whole unstimulated saliva and vaginal lavages, as well as human epithelial cell lines, were cocultured with C. albicans or medium alone. Candida was also cultured alone as a negative control for cytokine production. Supernatants collected at 0, 24, 48, 72, and 96 h were assayed for proinflammatory (TNF-α, IL-1α, and IL-6), Th1 (IFN-γ and IL-12), Th2-immunoregulatory (IL-10 and TGF-β), and chemotactic (MCP-1 and IL-8) cytokines. Results from the negative control culture (C. albicans alone) showed no significant presence of any cytokine or chemokine (data not shown). Candida-specific cytokine production by epithelial cells was defined as any amount consistently (over time) greater than that detected without Candida (constitutive). The mean constitutive and Candida-specific production of each cytokine and chemokine at 24 and 96 h of culture is summarized in Table 1.
TABLE 1.
Constitutive and Candida-specific cytokine and chemokine production by oral and vaginal epithelial cells
| Cytokine | Stimulus | Cytokine concn (mean pg/ml ± SEM) by:
|
|||
|---|---|---|---|---|---|
| Oral cells at:
|
Vaginal cells at:
|
||||
| 24 haI | 96 h | 24 h | 96 h | ||
| TNF-α | Medium alone | 6.3 ± 4.5 | 30 ± 5.6 | 3.4 ± 2.0 | 29 ± 8.6 |
| Medium + Candida | 29 ± 14 | 67 ± 23 | 25 ± 6.1 | 64 ± 16 | |
| IL-1α | Medium alone | 68 ± 19 | 250 ± 28 | 37 ± 21 | 50 ± 9.6 |
| Medium + Candida | 120 ± 39 | 640 ± 220 | 59 ± 27 | 68 ± 10 | |
| IL-6 | Medium alone | 2.6 ± 0.5 | 12 ± 6.4 | 0 | 5.4 ± 4.2 |
| Medium + Candida | 3.9 ± 1.6 | 14 ± 5.7 | 3.8 ± 1.2 | 12 ± 3.6 | |
| IL-12 | Medium alone | 0 | 20 ± 9.9 | 1.8 ± 0.8 | 5.7 ± 2.3 |
| Medium + Candida | 0 | 22 ± 7.8 | 3.2 ± 1.3 | 11 ± 3.1 | |
| IL-10 | Medium alone | 1.4 ± 0.8 | 9.4 ± 1.0 | 2.6 ± 1.2 | 21 ± 11 |
| Medium + Candida | 8.3 ± 6.2 | 31 ± 22 | 4.3 ± 1.8 | 21 ± 11 | |
| MCP-1 | Medium alone | 3.7 ± 3.2 | 32 ± 16 | 0 | 63 ± 27 |
| Medium + Candida | 10 ± 4.8 | 44 ± 17 | 5.0 ± 3.3 | 69 ± 29 | |
| IL-8 | Medium alone | 35 ± 21 | 38 ± 24 | 20 ± 7.9 | 27 ± 6.3 |
| Medium + Candida | 38 ± 23 | 39 ± 24 | 21 ± 7.2 | 32 ± 6.6 | |
Time spent in culture.
Proinflammatory cytokines.
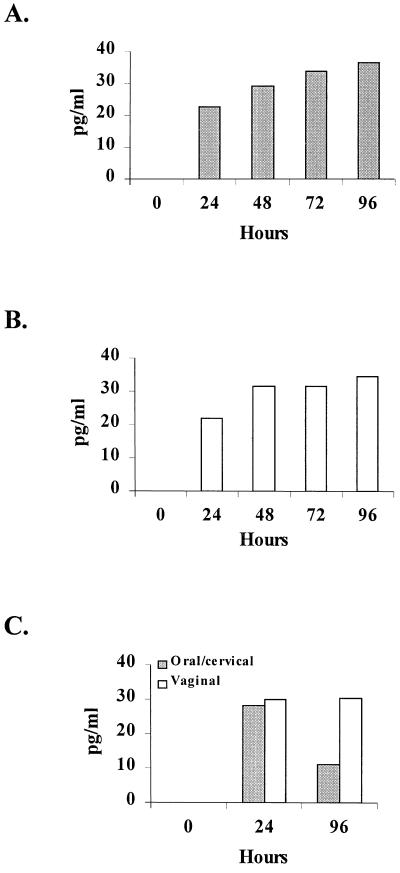
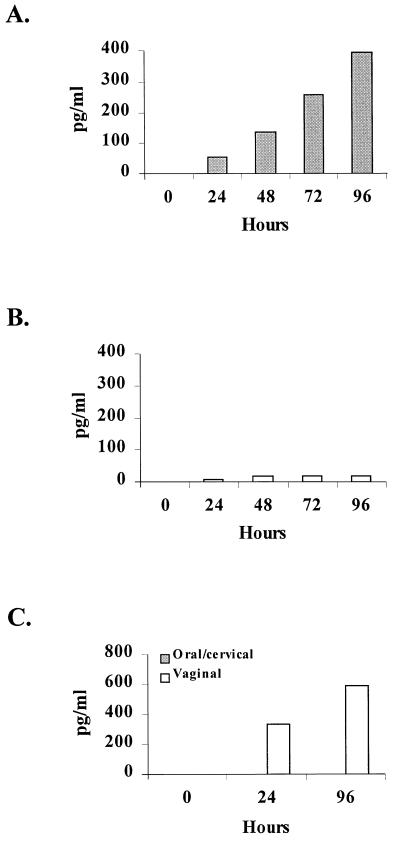
Figure 1 shows considerable Candida-specific TNF-α production for primary oral (Fig. 1A) and vaginal (Fig. 1B) epithelial cells and for oral/cervical and vaginal epithelial cell lines (Fig. 1C) that began at 24 h postculture and continued through 96 h. Figure 2 shows little to no appreciable amounts of IL-1α in response to C. albicans by primary vaginal epithelial cells throughout the 96-h culture (Fig. 2B), whereas primary oral epithelial cells produced increasing concentrations of IL-1α for 96 h (Fig. 2A) that were significantly higher than for primary vaginal epithelial cells (P < 0.05 for 72 and 96 h). The oral/cervical cell line showed no Candida-specific IL-1α, whereas the vaginal cell line produced considerable amounts in response to C. albicans (Fig. 2C). Both primary epithelial cell populations produced little to no IL-6 over the 96-h culture period in response to C. albicans (Table 1). Similarly, both epithelial cell lines produced little Candida-specific IL-6 but had considerable constitutive production (100 to 1,100 pg/ml by 96 h; data not shown). Culture of each epithelial cell population with TNF-α (control for epithelial cell lines and culture integrity) showed three- to fourfold increases in both IL-1α and IL-6 concentrations by both epithelial cell lines but not by either primary epithelial cell population (data not shown).
FIG. 1.
TNF-α production by oral and vaginal epithelial cells in response to C. albicans. Whole unstimulated saliva and vaginal lavage fluid were collected from healthy human volunteers, and epithelial cell-enriched populations were isolated through nylon membrane retention. Primary oral (A) and vaginal (B) epithelial cells and oral/cervical and vaginal epithelial line cells (C) were cocultured with C. albicans at an epithelial cell/Candida ratio of 1:100 for up to 96 h. Supernatants of the Candida-epithelial cell coculture were collected at specified time points, and TNF-α levels were quantified by ELISA. The figure shows the mean concentrations of Candida-specific (minus the concentrations in medium alone) production of TNF-α from five volunteers examined individually and four separate experiments with each epithelial cell line.
FIG. 2.
IL-1α production by oral and vaginal epithelial cells in response to C. albicans. Whole unstimulated saliva and vaginal lavage fluid were collected from healthy human volunteers, and epithelial cell-enriched populations were isolated through nylon membrane retention. Primary oral (A) and vaginal (B) epithelial cells and oral/cervical and vaginal epithelial line cells (C) were cocultured with C. albicans at an epithelial cell/Candida ratio of 1:100 for up to 96 h. Supernatants of the Candida-epithelial cell coculture were collected at specific time points, and IL-1α levels were quantified by ELISA. The figure shows the mean concentrations of Candida-specific (minus the concentrations in medium alone) production of IL-1α from five volunteers examined individually and four separate experiments with each epithelial cell line.
Th1/Th2-type cytokines.
Primary oral and vaginal epithelial cells produced little IL-12 and IL-10 throughout the 96-h culture period in response to C. albicans (Table 1). Similar results were observed for both cell lines (data not shown). IFN-γ and the immunoregulatory cytokine, TGF-β, were not present in any primary oral or vaginal epithelial cell or cell line culture (data not shown). IFN-γ, IL-10, TGF-β, and IL-12 were not produced by any epithelial cell population in response to TNF-α (data not shown).
Chemokines.
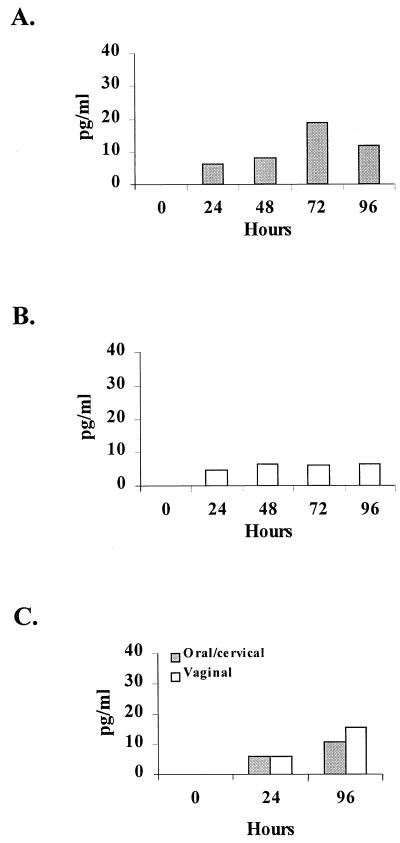
Candida-specific MCP-1 chemokine production is illustrated in Fig. 3. As an example of a relatively negative cytokine response, each epithelial population produced low concentrations of MCP-1 in response to C. albicans through the 96-h culture. Similar results were observed for IL-8 (Table 1). The epithelial cell lines also showed little to no Candida-specific IL-8 but had high constitutive levels (500 to 1,000 pg/ml by 96 h; data not shown). Stimulation of each epithelial cell population with TNF-α showed two- to threefold increases in IL-8 concentrations by both epithelial cell lines but not by either primary epithelial cell population (data not shown).
FIG. 3.
MCP-1 production by oral and vaginal epithelial cells in response to C. albicans. Whole unstimulated saliva and vaginal lavage fluid were collected from healthy human volunteers, and epithelial cell-enriched populations were isolated through nylon membrane retention. Primary oral (A) and vaginal (B) epithelial cells and oral/cervical and vaginal epithelial line cells (C) were cocultured with C. albicans at an epithelial cell/Candida ratio of 1:100 for up to 96 h. Supernatants of the Candida-epithelial cell coculture were collected at specific time points, and MCP-1 levels were quantified by ELISA. The figure shows the mean concentrations of Candida-specific (minus the concentrations in medium alone) production of MCP-1 from five volunteers examined individually and four separate experiments with each epithelial cell line.
DISCUSSION
This study investigated cytokine and chemokine production in response to C. albicans by enriched populations of epithelial cells isolated from the oral and vaginal mucosa and by human epithelial cell lines. Since Candida is in contact with epithelial cells more than any other cell type at mucosal sites and since epithelial cells secrete cytokines (20–22), the potential is high for epithelial cells to contribute to the local cytokine milieu present during asymptomatic or symptomatic mucosal Candida colonization. Although C. albicans exists as an asymptomatic commensal at both the oral and the vaginal mucosa, we investigated cytokine and chemokine production under conditions that simulated a C. albicans mucosal infection (i.e., predominantly hyphae from the natural transformation of C. albicans during the 96-h culture) to ascertain the potential role of epithelial cells in in vivo mucosal immune responses against the pathogenic form of the organism. In addition, we confirmed that C. albicans did not contribute to or interfere with the detection of the cytokines.
The cytokines examined in this study were chosen based on the fact that they represent important members of proinflammatory, regulatory, T-helper, and chemotactic cytokine classes. The coculture design in this study employed a variety of controls in order to best determine the ability of epithelial cells to produce cytokines constitutively and in response to Candida. Constitutive cytokine production by the cells may represent a homeostatic mechanism of innate immunity at mucosal tissues or consequences of the in vitro culture conditions.
Results showed that primary oral and vaginal epithelial cells produced considerable levels of TNF-α and IL-1α both constitutively and in response to C. albicans as early as 24 h postculture. The exception was primary vaginal epithelial cells that produced little to no Candida-specific IL-1α. IL-6, IFN-γ, IL-12, IL-10, MCP-1, and IL-8 were also variably produced in undetectable to low concentrations constitutively with little to no additional production in response to C. albicans through the 96-h culture. For the epithelial cell lines, high constitutive levels of IL-1α, IL-6, and IL-8 were observed as early as 24 h postculture, but there was little to no Candida-specific production of IL-6, IL-12, IL-10, MCP-1, and IL-8 and only moderate production of TNF-α. On the other hand, IL-1α was produced in relatively high concentrations by the vaginal cell line, but not by the oral/cervical cell line, in response to C. albicans, a result similar to that seen with primary oral epithelial cells. Interestingly, IL-1α was the one cytokine that was not consistently produced by either primary cells or the cell lines. A potential explanation is that IL-1α can be released from intracellular stores when the cell expires (32). Although we did not observe vast changes in cell viability between pre- and postculture, it is possible that IL-1α was released from dying cells that were variable in each culture. Finally, TGF-β was not produced at detectable levels by either primary or epithelial cell lines alone or in response to C. albicans.
The use of epithelial cell lines enabled us to predict the source of the cytokines produced in the enriched primary populations. Accordingly, since the cytokines in most cases were produced by both types of primary epithelial cells and the epithelial cell lines, and since the primary populations were >95% pure, our data suggest that the cytokines were indeed epithelial cell derived. We recognize, however, that the immortalized characteristics of the epithelial cell lines employed in these studies may not accurately predict the outcome of nonimmortalized epithelial cells (i.e., primary epithelial cells). Our results are also consistent with the high levels of IL-1α, IL-6, and IL-8 produced by the cell lines in response to TNF-α as an integrity control for the cells and the culture conditions (1, 7, 8). It is unclear why the primary epithelial cells did not similarly produce cytokines in response to TNF-α, although the state of differentiation may not be permissive for cytokine stimulation without the presence of the appropriate receptors. In contrast, the high concentrations of IL-6 and IL-8 produced by the cell lines, but not by the primary cells, may be due to the transformed characteristics of the epithelial cell lines. In any event, our results are consistent with results previously reported for these cell lines (7, 8).
It has been known for some time that epithelial cells of the gastrointestinal and urinary tracts produce cytokines in response to microorganisms such as Neisseria gonorrhoeae, Shigella dysenteriae, and Chlamydia trachomatis (18, 22, 30, 32). C. albicans can now be added to this list as the first fungal organism with similar properties. In fact, the concentrations of IL-1α and TNF-α produced in response to C. albicans were similar to those induced in these other epithelial cell populations. The only other attempt to stimulate cytokine production by epithelial cells in response to C. albicans was through the confluent culture of gingival epithelial cells or with the oral/cervical epithelial cell line employed in this study. Under these conditions, both IL-6 and IL-8 were produced in high concentrations by both types of epithelial cells (7, 8). Although it is unclear why the epithelial cell line in our study produced lower levels of IL-6 and IL-8 in response to C. albicans than did the previous study, culture conditions (i.e., tissue culture media, cell preparations, and effector/target ratios, etc.), the state of the epithelial cell line (i.e., prior to contamination with HeLa cells), and a different C. albicans strain may have contributed to the differences despite the similar levels produced in the presence of TNF-α.
Based on the documented role for T-helper responses in protection (Th1) or susceptibility (Th2) to Candida infection (4), Th1 and Th2 cytokines have been detected in mucosal secretions from both the oral and the vaginal mucosa (11, 28). However, data from our study showed low concentrations of both Th1 (IFN-γ and IL-12) and Th2 (IL-10) cytokines produced by oral and vaginal epithelial cells, with little to no modulation in response to C. albicans. Therefore, our data suggest that cells other than epithelial cells most likely contribute to these T-helper cytokines present during mucosal C. albicans infections.
Also interesting was the lack of TGF-β production by oral and vaginal epithelial cells. Clinical (P. L. Fidel, M. Barousse, T. Espinosa, R. R. Chesson, and K. Dunlap, submitted) and experimental (38) studies from our laboratory show the presence of TGF-β in vaginal secretions. As such, TGF-β has been postulated to play a downregulatory or tolerance role in vaginal immunity (38). However, data from this study suggest that, as with the Th1 and Th2 cytokines, cells other than epithelial cells are responsible for the presence of TGF-β in vaginal secretions. In contrast, the level of TGF-β is low in the saliva of healthy individuals as well as in those with OPC (P. L. Fidel, unpublished results) and thus would not be expected to be produced by oral epithelial cells.
Although the presence of proinflammatory cytokines in mucosal secretions has not been investigated during OPC or VVC, our study clearly shows the production of IL-1α and TNF-α by oral and vaginal epithelial cells in response to C. albicans. If similar proinflammatory cytokines are produced in vivo, an inflammatory condition would be expected. Indeed, edema and erythema are common symptoms of both OPC and VVC (19, 34). Interestingly, TNF-α and IL-1α have been reported to play an active role in the immune response to C. albicans infections (2, 25, 31) and have been used therapeutically during experimental infection (24).
Chemokines are considered critical for migration of cells to sites of pathogenic insults. Accordingly, some chemokines (MCP-1 in particular) have been constitutively present at the vaginal mucosa in mice and are elevated during infection (33). If a similar scenario occurs in human tissue, the moderate amounts of IL-8 and MCP-1 produced by oral and vaginal epithelial cells, together with little to no additional production in response to C. albicans, suggests that epithelial cells are not a primary source of these chemokines.
Additional considerations are the similarities in cytokine production between oral and vaginal epithelial cells with few exceptions. These results suggest that these epithelial cells, although anatomically distinct, are similar in their capacity to produce cytokines and respond to Candida. We recognize, however, that the studies and interpretations discussed here are from in vitro culture conditions and may not reflect fully the activity of the cells in the intact mucosal microenvironment.
In summary, this is the first comprehensive evaluation of cytokine production, albeit in vitro, by primary oral (buccal) and vaginal epithelial cells both constitutively and in response to C. albicans. Accordingly, our findings suggest that oral and vaginal epithelial cells are an important source of proinflammatory cytokines (TNF-α and/or IL-1α) in response to C. albicans that may contribute to the local immune response at either mucosal site. This, together with future studies examining additional cytokines and chemokines present in mucosal secretions and in epithelial cell-Candida cocultures from other patient populations (e.g., HIV-positive individuals with or without OPC or women with RVVC), will undoubtedly provide a more complete picture of the local host response to mucosal C. albicans infections.
Acknowledgments
This work was supported by National Institutes of Health Public Health Service grant DE-12178 from the National Institute of Dental and Craniofacial Research and grant AI-41693 from the National Institute of Allergy and Infectious Diseases.
Editor: T. R. Kozel
REFERENCES
- 1.Anderson, D. J., J. A. Politch, L. D. Tucker, R. Fichorova, F. Haimovici, R. E. Tuomala, and K. H. Mayer. 1998. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res. Hum. Retrovir. 14:S43–S49. [PubMed] [Google Scholar]
- 2.Blasi, E., L. Pitzurra, A. Bartoli, M. Puliti, and F. Bistoni. 1994. Tumor necrosis factor as an autocrine and paracrine signal controlling the macrophage secretory response to Candida albicans. Infect. Immun. 62:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P., T. O. Gabrielsen, I. Dale, F. Muller, M. Steinbakk, and M. K. Fagerhol. 1995. The leucocyte protein L1 (calprotectin): a putative nonspecific defense factor at epithelial surfaces. Adv. Exp. Med. Biol. 371:201–206. [DOI] [PubMed] [Google Scholar]
- 4.Cenci, E., A. Mencacci, R. Spaccapelo, L. Tonnetti, P. Mosci, K. H. Enssle, P. Puccetti, L. Romani, and F. Bistoni. 1995. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J. Infect. Dis. 171:1279–1288. [DOI] [PubMed] [Google Scholar]
- 5.Cenci, E., L. Romani, A. Vecchiarelli, P. Puccetti, and F. Bistoni. 1990. T cell subsets and IFN-gamma production in resistance to systemic candidosis in immunized mice. J. Immunol. 144:4333–4339. [PubMed] [Google Scholar]
- 6.Clift, R. A. 1984. Candidiasis in the transplant patient. Am. J. Med. 77:34–38. [PubMed] [Google Scholar]
- 7.Dongari-Bagtzoglou, A., and J. L. Ebersole. 1998. Increased presence of IL-6 and IL-8 secreting fibroblast subpopulations in adult periodontitis. J. Peridontol. 69:899–910. [DOI] [PubMed] [Google Scholar]
- 8.Dongari-Bagtzoglou, A., K. Wen, and I. B. Lamster. 1999. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol. Immunol. 14:364–370. [DOI] [PubMed] [Google Scholar]
- 9.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847–855. [DOI] [PubMed] [Google Scholar]
- 10.Fidel, P. L., Jr., J. L. Cutright, and C. Steele. 2000. Effects of reproductive hormones on experimental vaginal candidiasis. Infect. Immun. 68:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel, P. L., Jr., K. A. Ginsburg, J. L. Cutright, N. A. Wolf, D. Leaman, K. Dunlap, and J. D. Sobel. 1997. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J. Infect. Dis. 176:728–739. [DOI] [PubMed] [Google Scholar]
- 12.Fidel, P. L., Jr., W. Luo, C. Steele, J. Chabain, M. Baker, and F. L. Wormley. 1999. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect. Immun. 67:3135–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidel, P. L., Jr., M. E. Lynch, V. Redondo-Lopez, J. D. Sobel, and R. Robinson. 1993. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis (RVVC). J. Infect. Dis. 168:1458–1465. [DOI] [PubMed] [Google Scholar]
- 14.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1994. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect. Immun. 62:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1995. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect. Immun. 63:2403–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonella, P. A., and M. R. Neutra. 1984. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J. Cell Biol. 99:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonella, P. A., and D. W. Wilmore. 1993. Co-localization of class II antigen and endogenous antigen in the rat enterocyte. J. Cell Sci. 106:937–940. [DOI] [PubMed] [Google Scholar]
- 18.Hang, L., B. Wullt, Z. Shen, D. Karpman, and C. Svanborg. 1998. Cytokine repertoire of epithelial cells lining the human urinary tract. J. Urol. 159:2185–2192. [DOI] [PubMed] [Google Scholar]
- 19.Hauman, C. H., I. O. Thompson, F. Theunissen, and P. Wolfaart. 1993. Oral carriage of Candida in healthy and HIV-seropositive persons. Oral Surg. Oral Med. Oral Pathol. 76:570–572. [DOI] [PubMed] [Google Scholar]
- 20.Hedges, S. R., W. W. Agace, and C. Svanborg. 1995. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 3:266–270. [DOI] [PubMed] [Google Scholar]
- 21.Hedges, S. R., W. W. Agace, M. Svensson, A. Sjogren, M. Ceska, and C. Svanborg. 1994. Uroepithelial cells are part of a mucosal cytokine network. Infect. Immun. 62:2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung, H. C., L. Eckmann, S. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354–357. [DOI] [PubMed] [Google Scholar]
- 24.Kullberg, B. J. 1997. Trends in immunotherapy of fungal infections. Eur. J. Clin. Microbiol. Infect. Dis. 16:51–55. [DOI] [PubMed] [Google Scholar]
- 25.Kullberg, B. J., J. W. van’t Wout, and R. van Furth. 1990. Role of granulocytes in enhanced host resistance to Candida albicans induced by recombinant interleukin-1. Infect. Immun. 58:3319–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehrer, R., T. Ganz, D. Szklarek, and M. Seisted. 1988. Modulation of the in vitro candidiacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J. Clin. Investig. 81:1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leigh, J. E., M. Barousse, R. K. Swoboda, T. Myers, S. Hager, N. A. Wolf, J. L. Cutright, J. Thompson, J. D. Sobel, and P. L. Fidel, Jr. 2001. Candida-specific systemic cell-mediated immune reactivities in HIV-infected persons with and without mucosal candidiaisis. J. Infect. Dis. 183:277–285. [DOI] [PubMed] [Google Scholar]
- 28.Leigh, J. E., C. Steele, F. L. Wormley, Jr., W. Luo, R. A. Clark, W. R. Gallaher, and P. L. Fidel, Jr. 1998. Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:373–380. [DOI] [PubMed] [Google Scholar]
- 29.Macher, A. M. 1988. The pathology of AIDS. Public Health Rep. 103:246–254. [PMC free article] [PubMed] [Google Scholar]
- 30.Naumann, M., S. Webler, C. Bartsch, B. Wieland, and T. F. Meyer. 1997. Neisseria gonorrhoeae epithelial cell interactions leads to the activation of the transcription factors nuclear factor κB and activator protein-1 and the induction of inflammatory cytokines. J. Exp. Med. 186:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netea, M. G., L. J. H. van Tits, J. H. A. J. Curfs, F. Amiot, J. F. G. M. Meis, J. W. M. van der Meer, and B. J. Kullberg. 1999. Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J. Immunol. 163:1498–1505. [PubMed] [Google Scholar]
- 32.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saavedra, M., B. Taylor, N. W. Lukacs, and P. L. Fidel, Jr. 1999. Local production of chemokines during experimental vaginal candidiasis. Infect. Immun. 67:5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobel, J. D. 1997. Vaginitis. N. Engl. J. Med. 337:1896–1903. [DOI] [PubMed] [Google Scholar]
- 35.Steele, C., J. E. Leigh, R. K. Swoboda, and P. L. Fidel, Jr. 2000. Growth inhibition of Candida by human oral epithelial cells. J. Infect. Dis. 182:1479–1485. [DOI] [PubMed] [Google Scholar]
- 36.Steele, C., H. Ozenci, W. Luo, M. Scott, and P. L. Fidel, Jr. 1999. Growth inhibition of Candida albicans by vaginal cells from naive mice. J. Med. Mycol. 37:251–260. [PubMed] [Google Scholar]
- 37.Steele, C., M. Ratterree, and P. L. Fidel, Jr. 1999. Differential susceptibility to experimental vaginal candidiasis in macaques. J. Infect. Dis. 180:802–810. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, B. N., M. Saavedra, and P. L. Fidel, Jr. 2000. Local Th1/Th2 cytokine production during experimental vaginal candidiasis. J. Med. Mycol. 38:419–431. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg, A., S. Krisanaprakornkit, and B. A. Dale. 1998. Epithelial antimicrobial peptides: review and significance for oral applications. Crit. Rev. Oral Biol. Med. 9:399–414. [DOI] [PubMed] [Google Scholar]
- 40.Wira, C. R., and R. M. Rossoll. 1995. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology 136:4526–4534. [DOI] [PubMed] [Google Scholar]
- 41.Xu, T., S. M. Levitz, R. D. Diamond, and F. G. Oppenheim. 1991. Anticandidal activity of major human salivary histatins. Infect. Immun. 59:2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]