Abstract
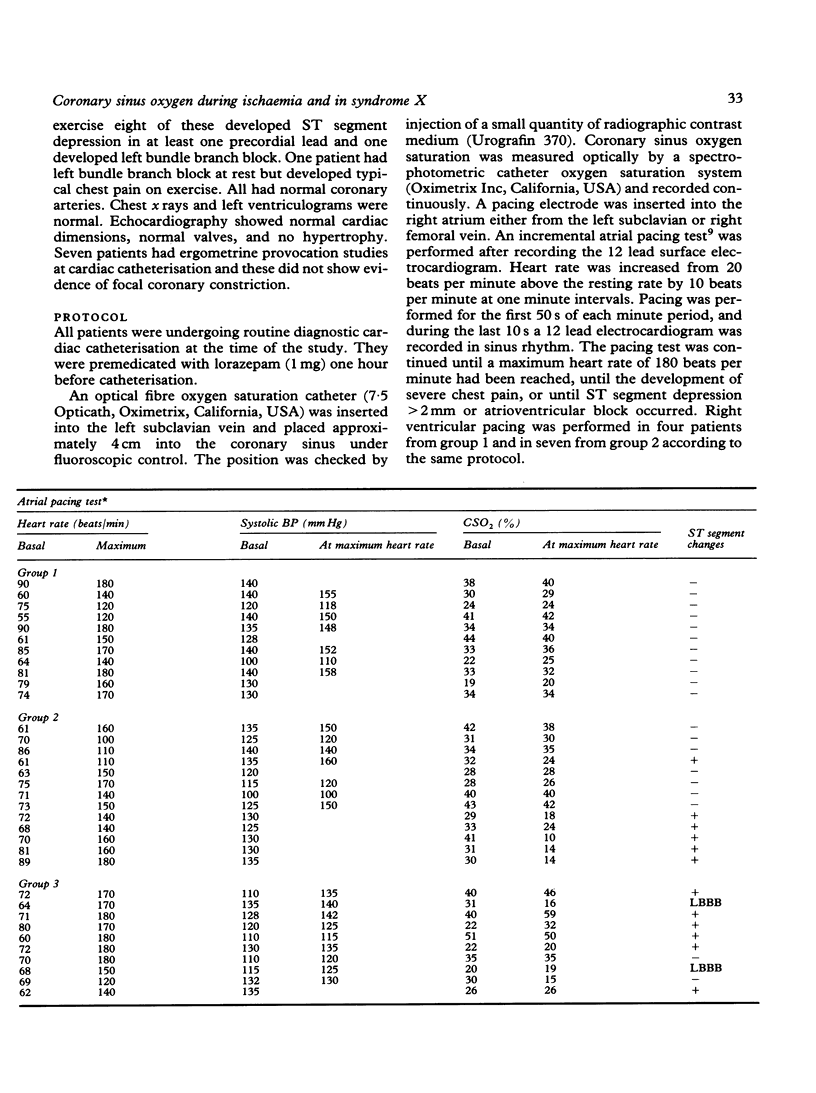
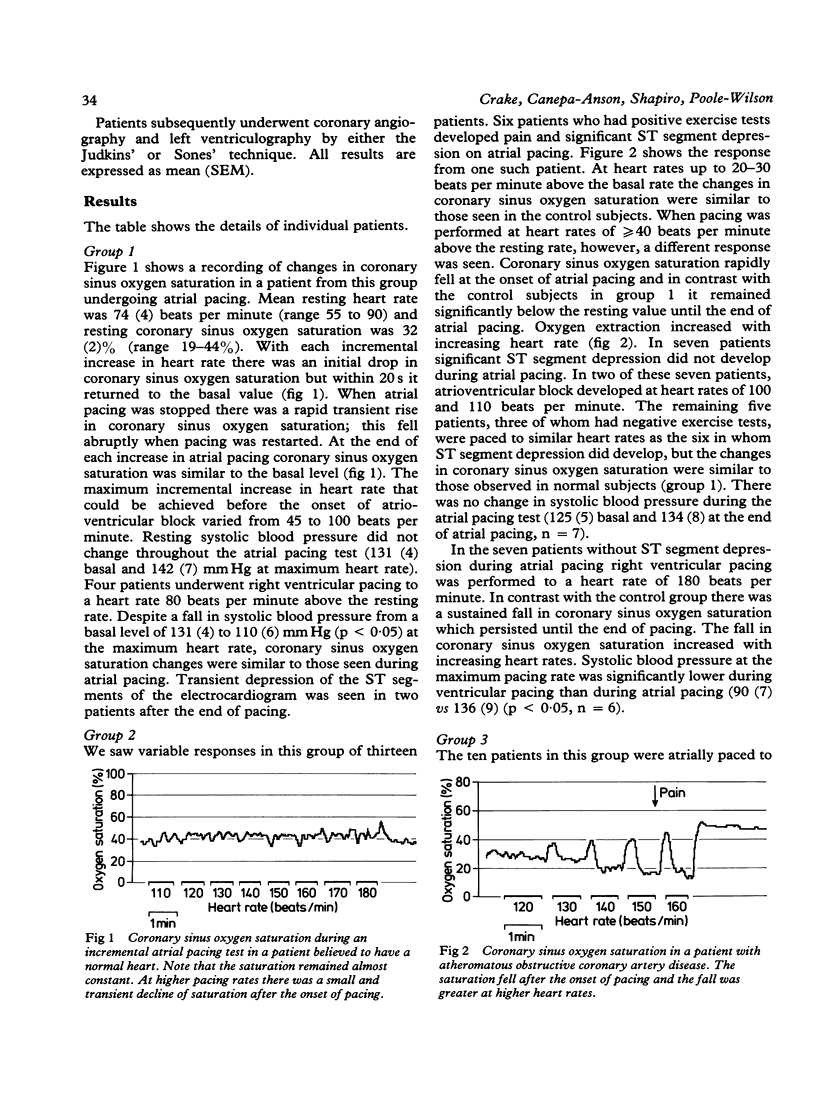
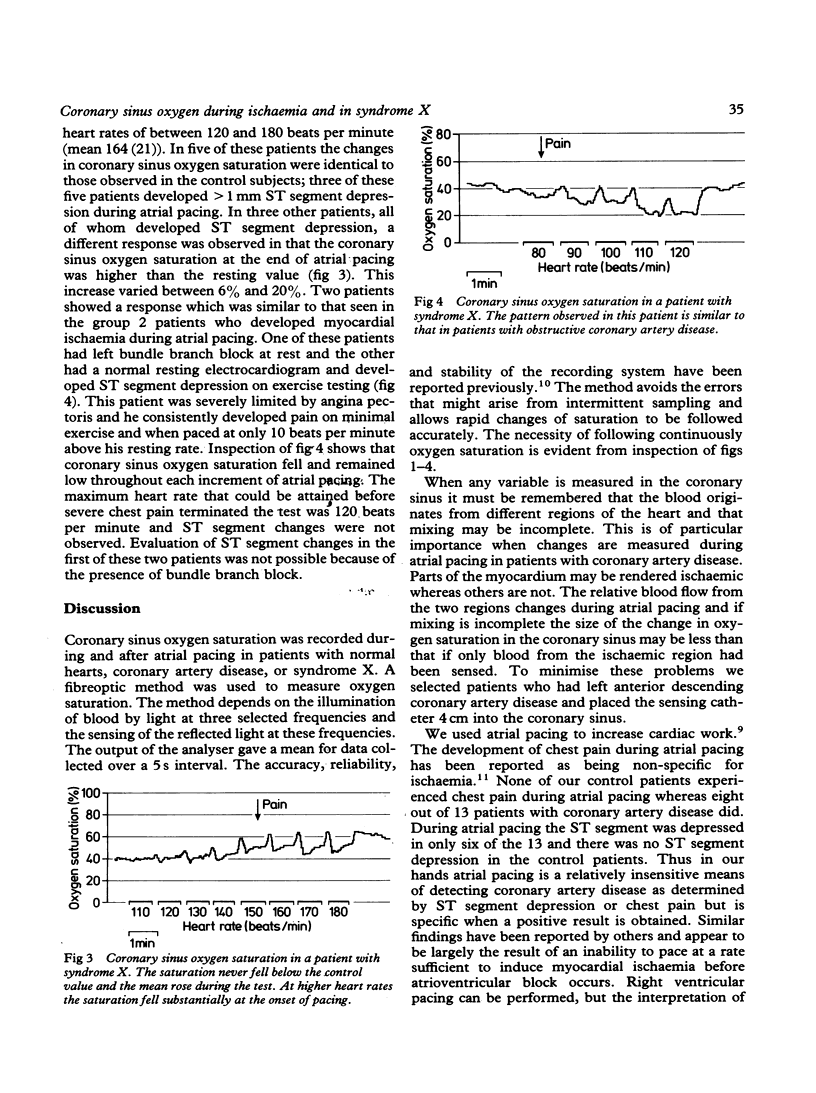
Coronary sinus oxygen saturation was measured continuously during incremental atrial pacing in 34 patients undergoing cardiac catheterisation. In eleven patients with normal coronary arteriograms, negative exercise tests, and no ST segment depression on the electrocardiogram, an increase in the rate of atrial pacing transiently decreased coronary sinus oxygen saturation but within 20 s oxygen saturation returned to the control value. In six patients with coronary artery disease ST segment depression developed during atrial pacing. The coronary sinus oxygen saturation fell and remained reduced until pacing was discontinued. The size of the fall of coronary sinus oxygen saturation increased with increasing heart rate. In seven patients with coronary artery disease the ST segments were unaltered during atrial pacing and coronary sinus oxygen saturation did not fall. Ten patients with syndrome X were studied. In six ST segment depression developed on atrial pacing. In five, three of whom developed ST segment depression, the changes in coronary sinus oxygen saturation during atrial pacing were similar to those observed in patients without any evidence of coronary artery disease. In three, all of whom developed ST segment depression, coronary sinus oxygen saturation gradually increased throughout the period of atrial pacing. In two patients coronary sinus oxygen saturation fell in a manner similar to that observed in patients with obstructive coronary artery disease who developed ST segment depression on pacing. Thus regulation of coronary blood flow in normal persons in response to an increase of heart rate is rapid. Oxygen extraction across the coronary bed can increase by up to 30% and a persistent increase in oxygen extraction is an indicator of myocardial ischaemia. The term "syndrome X" does not describe a homogeneous group of patients but in the majority coronary sinus oxygen saturation does not fall despite symptoms and changes on the electrocardiogram, indicating that inadequate coronary blood flow is not the dominant mechanism.
Full text
PDF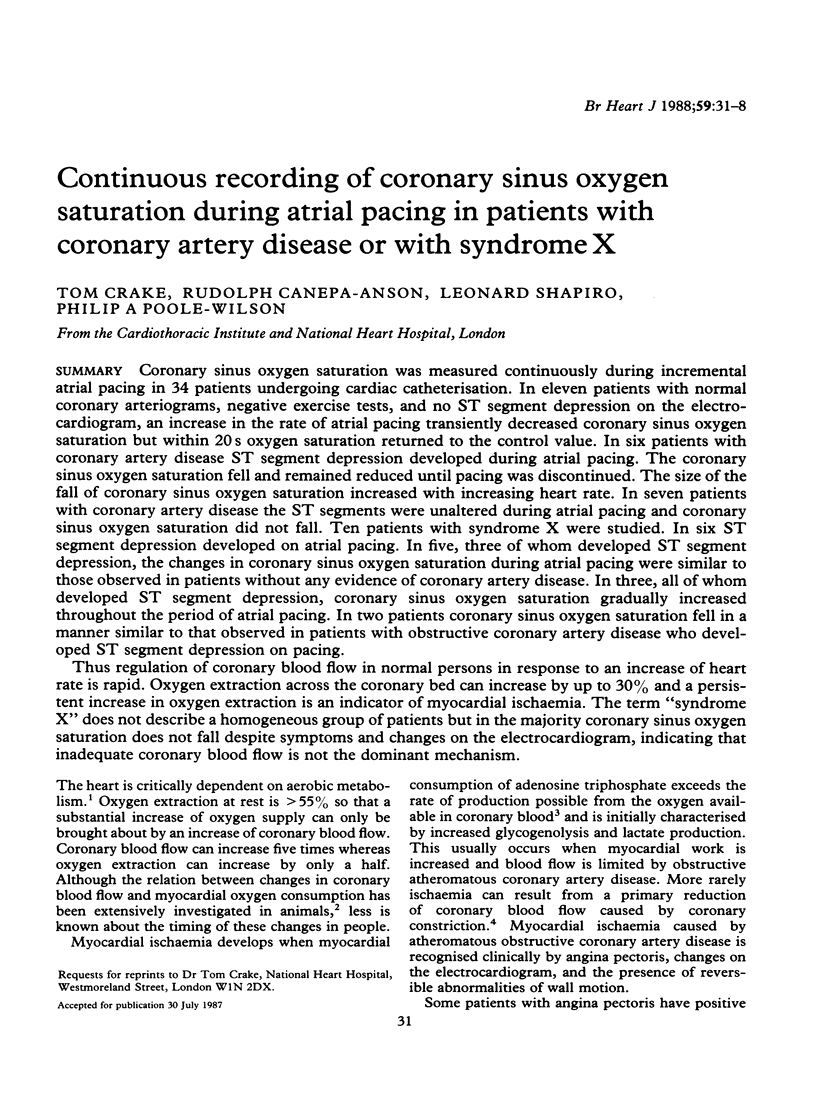




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbogast R., Bourassa M. G. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. Comparison with patients having significant coronary artery disease. Am J Cardiol. 1973 Sep 7;32(3):257–263. doi: 10.1016/s0002-9149(73)80130-4. [DOI] [PubMed] [Google Scholar]
- Cannon R. O., 3rd, Bonow R. O., Bacharach S. L., Green M. V., Rosing D. R., Leon M. B., Watson R. M., Epstein S. E. Left ventricular dysfunction in patients with angina pectoris, normal epicardial coronary arteries, and abnormal vasodilator reserve. Circulation. 1985 Feb;71(2):218–226. doi: 10.1161/01.cir.71.2.218. [DOI] [PubMed] [Google Scholar]
- Cannon R. O., 3rd, Watson R. M., Rosing D. R., Epstein S. E. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985 Aug 1;56(4):242–246. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- Chierchia S., Brunelli C., Simonetti I., Lazzari M., Maseri A. Sequence of events in angina at rest: primary reduction in coronary flow. Circulation. 1980 Apr;61(4):759–768. doi: 10.1161/01.cir.61.4.759. [DOI] [PubMed] [Google Scholar]
- Dart A. M., Davies H. A., Dalal J., Ruttley M., Henderson A. H. 'Angina' and normal coronary arteriograms: a follow-up study. Eur Heart J. 1980 Apr;1(2):97–100. doi: 10.1093/oxfordjournals.eurheartj.a061112. [DOI] [PubMed] [Google Scholar]
- Dart A. M., Davies H. A., Lowndes R. H., Dalal J., Ruttley M., Henderson A. H. Oesophageal spasm and angina: diagnostic value of ergometrine (ergonovine) provocation. Eur Heart J. 1980 Apr;1(2):91–95. doi: 10.1093/oxfordjournals.eurheartj.a061111. [DOI] [PubMed] [Google Scholar]
- Deanfield J. E., Shea M., Ribiero P., de Landsheere C. M., Wilson R. A., Horlock P., Selwyn A. P. Transient ST-segment depression as a marker of myocardial ischemia during daily life. Am J Cardiol. 1984 Dec 1;54(10):1195–1200. doi: 10.1016/s0002-9149(84)80066-1. [DOI] [PubMed] [Google Scholar]
- Dwyer E. M., Jr, Wiener L., Cox J. W. Angina pectoris in patients with normal and abnormal coronary arteriograms. Am J Cardiol. 1969 May;23(5):639–646. doi: 10.1016/0002-9149(69)90024-1. [DOI] [PubMed] [Google Scholar]
- Eliot R. S., Bratt G. The paradox of myocardial ischemia and necrosis in young women with normal coronary anteriograms. Relation to abnormal hemoglobin-oxygen dissociation. Am J Cardiol. 1969 May;23(5):633–638. doi: 10.1016/0002-9149(69)90023-x. [DOI] [PubMed] [Google Scholar]
- Epstein S. E., Cannon R. O., 3rd Site of increased resistance to coronary flow in patients with angina pectoris and normal epicardial coronary arteries. J Am Coll Cardiol. 1986 Aug;8(2):459–461. doi: 10.1016/s0735-1097(86)80067-5. [DOI] [PubMed] [Google Scholar]
- Gage J. E., Hess O. M., Murakami T., Ritter M., Grimm J., Krayenbuehl H. P. Vasoconstriction of stenotic coronary arteries during dynamic exercise in patients with classic angina pectoris: reversibility by nitroglycerin. Circulation. 1986 May;73(5):865–876. doi: 10.1161/01.cir.73.5.865. [DOI] [PubMed] [Google Scholar]
- James T. N. Pathology of small coronary arteries. Am J Cardiol. 1967 Nov;20(5):679–691. doi: 10.1016/0002-9149(67)90012-4. [DOI] [PubMed] [Google Scholar]
- Kaski J. C., Crea F., Nihoyannopoulos P., Hackett D., Maseri A. Transient myocardial ischemia during daily life in patients with syndrome X. Am J Cardiol. 1986 Dec 1;58(13):1242–1247. doi: 10.1016/0002-9149(86)90390-5. [DOI] [PubMed] [Google Scholar]
- Kemp H. G., Elliott W. C., Gorlin R. The anginal syndrome with normal coronary arteriography. Trans Assoc Am Physicians. 1967;80:59–70. [PubMed] [Google Scholar]
- Legrand V., Hodgson J. M., Bates E. R., Aueron F. M., Mancini G. B., Smith J. S., Gross M. D., Vogel R. A. Abnormal coronary flow reserve and abnormal radionuclide exercise test results in patients with normal coronary angiograms. J Am Coll Cardiol. 1985 Dec;6(6):1245–1253. doi: 10.1016/s0735-1097(85)80209-6. [DOI] [PubMed] [Google Scholar]
- Levy R. D., Shapiro L. M., Wright C., Mockus L., Fox K. M. Syndrome X: the haemodynamic significance of ST segment depression. Br Heart J. 1986 Oct;56(4):353–357. doi: 10.1136/hrt.56.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho P., Hintze T. H., Vatner S. F. Regulation of large coronary arteries by increases in myocardial metabolic demands in conscious dogs. Circ Res. 1981 Sep;49(3):594–599. doi: 10.1161/01.res.49.3.594. [DOI] [PubMed] [Google Scholar]
- Mammohansingh P., Parker J. O. Angina pectoris with normal coronary arteriograms: hemodynamic and metabolic response to atrial pacing. Am Heart J. 1975 Nov;90(5):555–561. doi: 10.1016/0002-8703(75)90217-3. [DOI] [PubMed] [Google Scholar]
- Markham R. V., Jr, Winniford M. D., Firth B. G., Nicod P., Dehmer G. J., Lewis S. E., Hillis L. D. Symptomatic, electrocardiographic, metabolic, and hemodynamic alterations during pacing-induced myocardial ischemia. Am J Cardiol. 1983 Jun;51(10):1589–1594. doi: 10.1016/0002-9149(83)90192-3. [DOI] [PubMed] [Google Scholar]
- Meller J., Goldsmith S. J., Rudin A., Pichard A. D., Gorlin R., Teichholz L. E., Herman M. V. Spectrum of exercise thallium-201 myocardial perfusion imaging in patients with chest pain and normal coronary angiograms. Am J Cardiol. 1979 Apr;43(4):717–723. doi: 10.1016/0002-9149(79)90069-9. [DOI] [PubMed] [Google Scholar]
- Mosseri M., Yarom R., Gotsman M. S., Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation. 1986 Nov;74(5):964–972. doi: 10.1161/01.cir.74.5.964. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Bünger R. Metabolic control of coronary blood flow. Prog Cardiovasc Dis. 1987 Mar-Apr;29(5):369–387. doi: 10.1016/0033-0620(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Opherk D., Zebe H., Weihe E., Mall G., Dürr C., Gravert B., Mehmel H. C., Schwarz F., Kübler W. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation. 1981 Apr;63(4):817–825. doi: 10.1161/01.cir.63.4.817. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A. Angina--pathological mechanisms, clinical expression and treatment. Postgrad Med J. 1983;59 (Suppl 3):11–21. [PubMed] [Google Scholar]
- Quyyumi A. A., Wright C., Fox K. Ambulatory electrocardiographic ST segment changes in healthy volunteers. Br Heart J. 1983 Nov;50(5):460–464. doi: 10.1136/hrt.50.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remme W. J., van den Berg R., Mantel M., Cox P. H., van Hoogenhuyze D. C., Krauss X. H., Storm C. J., Kruyssen D. A. Temporal relation of changes in regional coronary flow and myocardial lactate and nucleoside metabolism during pacing-induced ischemia. Am J Cardiol. 1986 Dec 1;58(13):1188–1194. doi: 10.1016/0002-9149(86)90379-6. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Livesley B., Oram S., Olsen E. G., Armstrong P. Angina pectoris with normal coronary arteries. Transvenous myocardial biopsy in diagnosis. Lancet. 1974 Sep 21;2(7882):677–680. doi: 10.1016/s0140-6736(74)93260-7. [DOI] [PubMed] [Google Scholar]
- Schofield P. M., Brooks N. H., Bennett D. H. Left ventricular dysfunction in patients with angina pectoris and normal coronary angiograms. Br Heart J. 1986 Oct;56(4):327–333. doi: 10.1136/hrt.56.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. G., McHale P. A., Greenfield J. C., Jr Coronary vasodilation after a single ventricular extra-activation in the conscious dog. Circ Res. 1982 Jan;50(1):38–46. doi: 10.1161/01.res.50.1.38. [DOI] [PubMed] [Google Scholar]
- Selwyn A. P., Allan R. M., L'Abbate A., Horlock P., Camici P., Clark J., O'Brien H. A., Grant P. M. Relation between regional myocardial uptake of rubidium-82 and perfusion: absolute reduction of cation uptake in ischemia. Am J Cardiol. 1982 Jul;50(1):112–121. doi: 10.1016/0002-9149(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Selwyn A. P., Forse G., Fox K., Jonathan A., Steiner R. Patterns of disturbed myocardial perfusion in patients with coronary artery disease. Regional myocardial perfusion in angina pectoris. Circulation. 1981 Jul;64(1):83–90. doi: 10.1161/01.cir.64.1.83. [DOI] [PubMed] [Google Scholar]
- Sowton G. E., Balcon R., Cross D., Frick M. H. Measurement of the angina threshold using atrial pacing. A new technique for the study of angina pectoris. Cardiovasc Res. 1967 Oct;1(4):301–307. doi: 10.1093/cvr/1.4.301. [DOI] [PubMed] [Google Scholar]
- Webb S. C., Canepa-Anson R., Rickards A. F., Poole-Wilson P. A. High potassium concentration in a parenteral preparation of glyceryl trinitrate. Need for caution if given by intracoronary injection. Br Heart J. 1983 Oct;50(4):395–396. doi: 10.1136/hrt.50.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. C., Poole-Wilson P. A. Potassium exchange in the human heart during atrial pacing and myocardial ischaemia. Br Heart J. 1986 Jun;55(6):554–559. doi: 10.1136/hrt.55.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. Extracellular potassium accumulation during myocardial ischemia: implications for arrhythmogenesis. J Mol Cell Cardiol. 1981 Jul;13(7):699–704. doi: 10.1016/0022-2828(81)90277-7. [DOI] [PubMed] [Google Scholar]


