Abstract
Fractionation of commercial preparations of lipoteichoic acids (LTA) by hydrophobic interaction chromatography (HIC) and nuclear magnetic resonance spectroscopy revealed very inhomogeneous compositions and decomposition of the LTA structure: LTA content of the preparations averaged 61% for Streptococcus pyogenes, 16% for Bacillus subtilis, and 75% for Staphylococcus aureus. The decomposition was characterized by a loss of glycerophosphate units as well as alanine and N-acetylglucosamine substituents. All preparations contained—to varying degrees—non-LTA, non-lipopolysaccharide (LPS) immunostimulatory components as indicated by their elution profile in HIC, lack of phosphate, and negative Limulus amoebocyte lysate (LAL) test results. After purification, the commercial LTA from Bacillus subtilis and S. pyogenes but not LTA from S. aureus induced the release of tumor necrosis factor alpha, interleukin 1 beta (IL-1β), IL-6, and IL-10 in human blood. While pure LTA are negative in the LAL assay, endotoxin equivalents of more than 10 ng of LPS/mg of LTA were found in the commercial preparations. Taken together, these data indicate that these crude preparations with relatively high endotoxin contamination are not suitable for characterizing the activation of immune cells by LTA.
Gram-negative bacteria are recognized by the innate immune system via their endotoxins, i.e., lipopolysaccharides (LPS), which are components of the outer membrane (28). No structural equivalent to LPS has been clearly identified in gram-positive bacteria, although the inflammatory immune response to gram-negative and gram-positive bacteria can hardly be distinguished by the symptoms. Recently lipoteichoic acids (LTA) from the cytoplasmic membrane of gram-positive bacteria (7) have become increasingly recognized as an immunostimulatory principle (1, 5, 10, 18, 25, 26, 32). For example, commercial preparations of LTA from Staphylococcus aureus were shown to exhibit potent immunostimulation in vitro (3, 11) and in vivo (4).
It was demonstrated, however, that a potent immunostimulatory nonhydrophobic phosphate-containing fraction can be separated from commercially available S. aureus LTA by reverse-phase chromatography (20, 21); the resulting purified LTA had no immunostimulatory potential but instead inhibited CD14-mediated activation of monocytes by LPS. Also, separation of immunostimulatory glycerophosphate-containing glycolipid fractions from otherwise inactive LTA was reported for Enterococcus hirae (13, 14, 31, 34), employing, in sequence, hydrophobic interaction chromatography (HIC) and anion exchange chromatography on DEAE. The latter findings raised doubts as to the immunostimulatory potential of LTA in general. However, we have shown recently that decomposition of LTA from S. aureus during phenol extraction results in the loss of its immunostimulatory potential. We developed a gentler extraction procedure using butanol, which yielded bioactive, pure LTA (24). A recent report (9) again raised the problem of endotoxin contamination of commercial LTA preparations (17, 33).
This study addresses the immunostimulatory potential of commercial LTA from three bacterial species which we purified by HIC and analyzed by nuclear magnetic resonance (NMR). It aims to resolve some discrepancies reported between experiments with crude commercial extracts and those with highly purified material.
MATERIALS AND METHODS
Chromatography of commercial LTA from S. aureus, S. pyogenes, and B. subtilis on octyl-Sepharose.
Twenty-five milligrams of LTA from S. aureus, Streptococcus pyogenes, or Bacillus subtilis (Sigma, Deisenhofen, Germany), dissolved in equilibration buffer, was subjected to chromatography on an octyl-Sepharose column (6, 8) (CL-4B, 1.5 by 29 cm; Amersham Pharmacia Biotech) equilibrated with 0.1 M sodium acetate buffer (pH 4.7, 15% propanol). The column was washed with 40 ml of equilibration buffer at 15 ml/h. Elution was performed using a linear gradient (15 to 60% propanol, 150 ml each) in 0.1 M sodium acetate buffer at 15 ml/h. Aliquots of 4 ml each were collected, and their phosphate content was determined (29). All fractions were negative for endotoxins in the chromogenic Limulus amoebocyte lysate (LAL) assay (Hämochrom, Essen, Germany), i.e., the LAL reactivity was lower than that for 1 pg of LPS/ml. The purified LTA were analyzed for chain length, chain substitution, and lipid anchor structure by NMR.
Improved preparation of LTA from S. aureus and B. subtilis.
A more gentle structure-preserving isolation procedure was reported recently (24). Briefly, the classical phenol-water extraction at 68°C and subsequent dialysis were replaced by butanol extraction at room temperature and lyophilization. B. subtilis (DSMZ 1087) and S. aureus (DSM 20233) were grown in 4-liter shakers and 42-liter fermenters, respectively. After harvest by centrifugation at 4°C, the pelleted bacteria were sonicated (Branson, Danbury, Conn.) on ice or alternatively disrupted with a cell mill (Büchi, Uster, Switzerland).
NMR analysis of LTA.
NMR of LTA was undertaken at 600.13 MHz (1H) and 300 K. The NMR spectra were referred to 3-(trimethylsilyl) 3,3,2,2-tetradeuteropropionic acid Na salt. Homonuclear assignments were taken from double-quantum filtered correlation spectroscopy, total correlation spectroscopy, rotating frame Overhauser enhancement spectroscopy, and nuclear Overhauser effect spectroscopy (DQF = COSY, TOCSY, ROESY, and NOESY, respectively) spectra. 13C assignments were based on heteronuclear multiple-quantum correlation.
The average chain length of the phosphoglycerol backbone and the degree of substitution were quantified directly from the 1H NMR integrals of LTA. The integral ratio of δH 5.4 and δH 5.08 as well as the integral ratio of δH 1.62 and 2.1 yielded the ratio of d-alanine to α-d-N-acetylglucosamine (GN). The total amount of glycerol was determined from the integral δH 3.7 to 4.2 (corrected for the underlying five GN resonances) plus the glycerol methine resonance at δH 5.4.
Whole-blood cytokine response.
Incubations of human whole blood in the presence of the bacterial stimuli were performed essentially as described previously (12). Briefly, 200 μl of heparinized whole blood freshly taken from healthy volunteers was diluted fivefold with isotonic sodium chloride solution. After addition of the bacterial stimuli, the samples were incubated in polypropylene vials (Eppendorf, Hamburg, Germany) in the presence of 5% CO2 at 37°C for 18 h. Then, after shaking, the cells were pelleted by centrifugation (400 × g, 2 min) and the cell supernatants were stored at −70°C for cytokine determination. Endotoxin from Salmonella abortus equi (Sigma) served as the control stimulus.
Cytokines were measured by commercial ELISA for interleukin 1 beta (IL-1β) (Quantikine; DPC Biermann, Bad Nauheim, Germany) or with ELISAs based on antibody pairs against human tumor necrosis factor alpha (TNF-α), IL-10, and IL-6 (Pharmingen, Hamburg, Germany). Binding of biotinylated antibody was quantified using streptavidin-peroxidase (Jackson Immuno Research, West Grove, Pa.) and the substrate TMB (3,3",5,5"-tetramethylbenzidine) (Sigma). Recombinant human cytokines serving as standards were from G. Adolf (Bender, Vienna, Austria) (TNF-α), Pharmingen (IL-10), and Genzyme (Ruesselsheim, Germany) (IL-6).
Human recombinant soluble CD14 employed in some incubations at 10 μg/ml was a generous gift of H. Lichenstein (Amgen, Boulder, Colo).
Statistics.
Statistical analysis was performed using the GraphPad InStat program (GraphPad Software, San Diego, Calif.). Data are given as means ± standard errors of the means. Cytokine levels are given per milliter of blood, i.e., corrected for the dilution factor 5 in the 20% blood incubation.
RESULTS
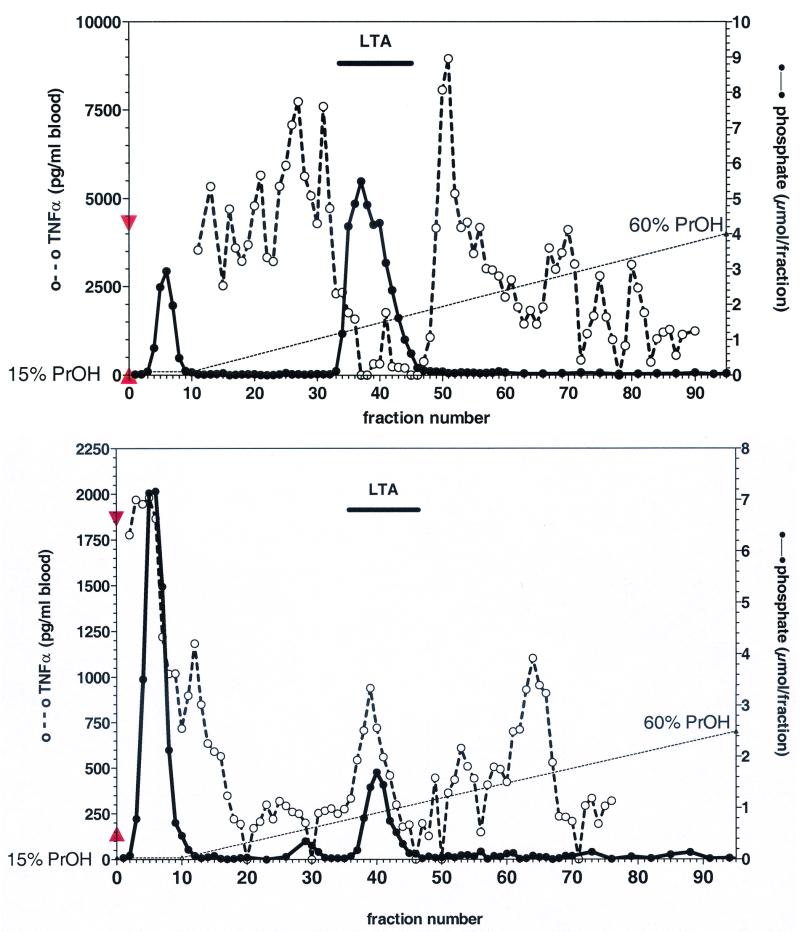
Early reports describe potent immunostimulatory activity of commercial LTA preparations from S. aureus (4, 18), while highly purified preparations were found to be essentially inactive in vitro (1, 18, 20). We performed a HIC of commercial S. aureus LTA. The elution profile of TNF-α stimulatory activity in human whole blood and phosphate content is depicted in Fig. 1A. LTA eluted, as indicated by the second phosphate peak, in a typical manner between propanol concentrations of 22 and 32%. In contrast, TNF-α stimulatory activity eluted in earlier and later fractions. It is noteworthy that considerable variations in repeated experiments were found using different lots of this commercial LTA material. This LTA, purified from the commercial sample, contained N-acetyl glucosamine, d-alanine, and hydroxyl groups in molar ratios to glycerol of 0.05, 0.20, and 0.75, respectively. The integral ratio of glycerol and the α-methylene group identified an average chain length of n = 25. Comparing LTA from S. aureus isolated according to the improved preparation method (24) with commercial LTA shows a different composition pattern (Table 1).
FIG. 1.
Elution profile of phosphate content and TNF-α stimulatory activity after HIC purification of commercial LTA from S. aureus and B. subtilis. Twenty-five milligrams of commercial LTA preparations from S. aureus (Sigma, lot 86H4085) (A) or B. subtilis (Sigma, lot 17H4021) (B) were subjected to HIC. Fractions were analyzed for phosphate content (•), indicative of LTA in hydrophobic fractions, and for their TNF-α stimulatory activity (○) by incubating 1 ml of 20% blood in the presence of 10 μl of eluate for 24 h. ▴, TNF-α release of unstimulated blood; ▾, TNF-α release induced by 100 ng of LPS/ml.
TABLE 1.
Comparison of chain length and substituents of commercial and butanol-extracted LTA preparations from S. aureus and B. subtilisa
| LTA type | GN (%) | d-Ala (%) | d-Ala/GN ratio | n |
|---|---|---|---|---|
| S. aureus (Sigma) | 5 | 20 | 2.5 | 25 |
| S. aureus (butanol-extracted) | 15 | 70 | 4.8 | 48 |
| B. subtilis (Sigma) | 20 | 5 | 0.4 | ND |
| B. subtilis (butanol-extracted) | 25 | 25 | 1.0 | 22 |
Degrees of substitutions (n, chain length of LTA-glycerophosphate) were determined from the 1H NMR integrals of LTA. The high degree of nucleic acid contamination prevented chain length determination (ND, not determined) by NMR in case of the commercial B. subtilis LTA.
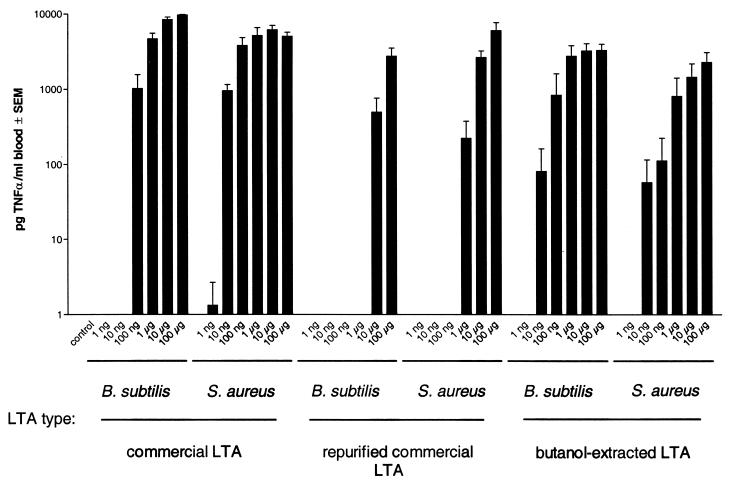
After purification, the commercial LTA from S. aureus, representing about 75% of the original preparation as measured by the phosphate determination, was essentially ineffective in inducing cytokines in human whole blood cultures. Its concentration-response curve for monokine (TNF-α) induction was shifted to the right by more than 2 orders of magnitude compared to the original commercial material or to butanol-extracted LTA from S. aureus (Fig. 2). The more hydrophobic fractions 49 to 80 in Fig. 1A containing no measurable amounts of phosphate, and thus no LTA, significantly induced TNF-α release in human whole blood. Those fractions tested negative in the LAL assay (< 1 pg of LPS/ml), and the TNF-α induction by these fractions was abolished in the presence of sCD14 (data not shown). Thus, the unknown stimulus represents neither LPS nor LTA but most probably a ligand of membrane CD14, since sCD14 is competing with activation.
FIG. 2.
Concentration-dependent induction of TNF-α in human whole blood by commercial, commercial HIC-purified, and butanol-extracted LTAs. Twenty percent whole blood from three donors was incubated in the presence of the concentrations of different LTA indicated for 24 h. TNF-α was measured in the cell supernatant by ELISA. Commercial LTA from B. subtilis and LTA from S. aureus were purified by HIC, pooling the phosphate-containing hydrophobic fractions. The start material was compared to this preparation as well as a butanol-extracted LTA from the same species prepared in our laboratory.
In a series of similar experiments, a commercial LTA from B. subtilis was analyzed. The different lots tested contained about 16% ± 1% (n = 3) LTA according to phosphate determination. The HIC of one typical sample is depicted in Fig. 1B. In this case, TNF-α stimulatory activity eluted together with LTA, but considerable activity was also found in earlier and later phosphate-free fractions. The dose-response curves for commercial, commercial repurified, and butanol-extracted LTA from B. subtilis were similar to those for S. aureus (Fig. 2).
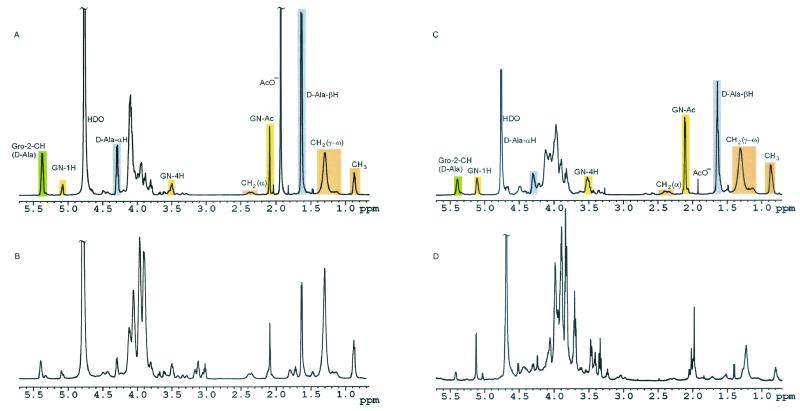
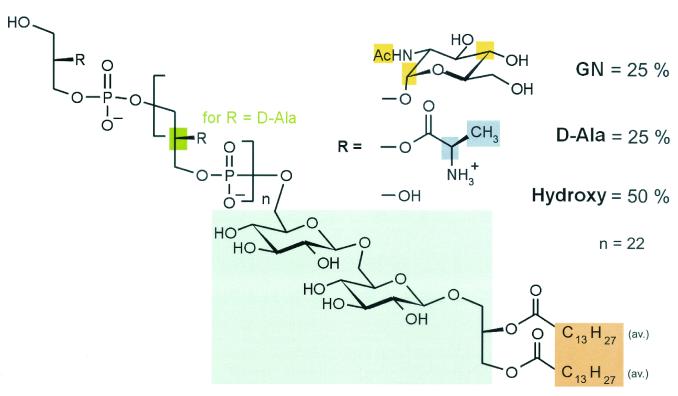
Furthermore, the commercial LTA from B. subtilis was contaminated with DNA (typical unstructured 1H NMR signal between 5.5 and 6 and between 7.5 and 8.5 ppm) as shown in Fig. 3. Table 1 shows differences in the degree of substitution of butanol-extracted and commercial LTA of B. subtilis. A similar pattern was found for commercial LTA from S. pyogenes (data not shown). NMR of the butanol-extracted LTA from B. subtilis allowed the deduction of its structure (Fig. 4), which was similar to that reported previously for S. aureus (24). Beside the differences in the polyglycerophosphate backbone (Table 1), the most obvious difference was the shorter fatty acids of the lipid anchor (mean C14 compared to C16-18 for S. aureus). In both LTA, gentiobiose represented the carbohydrate of the glycolipid anchor.
FIG. 3.
A-D 1H NMR spectra of commercial and butanol-extracted LTA preparations. The 1H NMR spectra of LTA from S. aureus (butanol-extracted) (A), LTA from S. aureus (Sigma) (B), LTA from B. subtilis (butanol extracted) (C), or LTA from B. subtilis (Sigma) (D) are shown.
FIG. 4.
Structure of B. subtilis LTA. The structure was deduced from the NMR analysis of butanol-extracted LTA from B. subtilis shown in Fig. 3.
When the commercial LTA preparations were subjected to a LAL test, a reactivity comparable to that for 10 to 100 ng of reference endotoxin was found per mg of LTA. In contrast, HIC-purified LTA from S. aureus as well as B. subtilis was essentially negative in the LAL assay, i.e., <30 pg of LPS equivalent/mg of LTA. These data suggest that the commercial preparations are contaminated with a relevant endotoxin contamination, e.g., stimulation of whole blood with 10 μg of LTA/ml will include more than 100 pg of contaminating endotoxin/ml, while the threshold of cytokine induction is around 3 pg of LPS/ml in this system.
These data suggest that at 10 μg of commercial LTA/ml, 100 pg to 1 ng of LPS is present, which suffices to explain the TNF-α induction observed. Therefore, the LAL-negative 10 μg of butanol-extracted LTA/ml was added to the whole-blood incubations to characterize the pattern of further cytokines induced.
Both LTA stimulated whole blood to release cytokines in addition to TNF-α; the release of the cytokines IL-1β, IL-6, and IL-10 is shown in Table 2. Ten micrograms of purified LTA or LPS/ml induced these cytokines at concentrations in the same order of magnitude after 24 h of incubation. These data indicate that only about 1% of the immunostimulatory activity of commercial preparation can be attributed to LTA itself. For both bacterial species, the novel preparation method resulted in highly purified, essentially LPS-free LTA more potent than the commercial preparation. In comparing the repurified commercial LTA from B. subtilis with the butanol-extracted one, a 1,000-fold difference in potency was found. In conclusion, appropriately purified LTA have the potential to induce a variety of inflammatory mediators from human monocytes.
TABLE 2.
Induction of monokine release in human whole blood by LPS and LTAa
| Monokine | Response (ng/ml) to:
|
||
|---|---|---|---|
| LPS S. a. e. (HIC-purified) | LTA (S. aureus) | LTA (B. subtilis) | |
| IL-1β | 22.6 ± 5 | 2.2 ± 0.82** | 18.2 ± 3.7 |
| IL-6 | 5.5 ± 1.5 | 4.3 ± 2.1 | 2.2 ± 0.5 |
| IL-10 | 0.6 ± 0.2 | 0.2 ± 0.1* | 0.9 ± 0.1 |
LPS from Salmonella abortus equi (S. a. e.) (Sigma) was repurified by HIC and compared to butanol-extracted LTAs from S. aureus and B. subtilis. All stimuli were incubated at a concentration of 10 μg/ml in whole blood (n = 6). The cytokine response after 24 h of incubation was measured by ELISA. The data are means ± standard errors of the means. In the absence of stimuli, no significant release of these monokines was found. The paired raw data were tested against results with LPS for significance. Single asterisk, P < 0.05. Double asterisk, P < 0.01.
DISCUSSION
Conflicting results gained with commercial and highly purified LTA preparations with regard to cytokine induction were reported previously by Keller et al. (18) and Gao et al. (9). The most striking contradiction was observed with regard to LTA from S. aureus, which was a very potent stimulus for immune cells in some studies but failed to induce effects in others. In line with reports from Gao et al. (9) and Kusunoki et al. (20), we demonstrated that HIC purification of S. aureus LTA from the potent commercial material abolished the immunostimulatory potential. We have recently shown that the immunostimulatory activity of LTA from S. aureus is lost due to decomposition during phenol extraction (24).
In line with this report, the commercial phenol-extracted LTA showed decomposition of LTA characterized by the loss of glycerophosphate repeats (S. aureus; mean n = 48, down to 25). In parallel, a dramatic loss of d-alanine and N-acetylglucosamine substituents was observed. The same observation was made in comparing commercial and butanol-extracted LTA from B. subtilis. All batches of the three commercial LTA contained considerable contamination by non-LTA (no phosphate), non-LPS (no LAL reactivity) immunostimulatory contaminations in HIC fractions not typical for either LTA or LPS. NMR analysis suggests, at least for B. subtilis LTA, a major contamination by bacterial DNA, which has well-known immunostimulatory effects. This contamination impaired the determination of chain length by NMR of the commercial LTA from B. subtilis.
Our findings raise doubts as to the purity and quality of the commercial preparations. In view of these observations, results obtained with such preparations should be considered with caution. Especially, this might explain why Cleveland et al. found a CD14-dependent induction of IL-12 in human monocytic THP-1 cells by using this commercial LTA preparation (2), while highly purified LTA from the same bacterial species, in contrast to endotoxins, failed to induce any IL-12 in human whole blood in our hands (15). The immunostimulatory activities of the commercial preparation of S. aureus have to be attributed to yet-unidentified components other than LTA and LPS contaminations. However, reports on the potent activity of heat-killed S. aureus (16) support the existence of immunostimulatory surface components in line with our previous report (24).
The fact that LTA derived from phenol extraction had lost both glycerophosphate units and substituents shows that repurification of commercial LTA (19, 20) does not abolish the limitations of these preparations. In contrast to LTA from S. aureus, which loses most of its immunostimulatory activity when isolated by phenol, this does not hold true for LTA in general. In a recent study, phenol-extracted LTA preparations from 13 other bacterial species of purity comparable to that of our butanol-extracted preparations were found to be activators of monokine release (15), although in general, higher concentrations of LTA are required to initiate monokine release comparable with the potent LPS from Escherichia coli and Salmonella species. Nevertheless, the potency of LTA was comparable to that of LPS from Pseudomonas species (24).
The contamination by endotoxin and the crude nature of the extracts might explain current conflicting results concerning the involvement of toll-like receptors in the signaling of LTA: recently published data indicate a role of toll-like receptor 2 (tlr-2) in recognition of whole gram-positive bacteria and a commercial LTA preparation (30, 37, 39), but macrophages from tlr-4 knockout mice lacked any response to commercial LTA (36). Earlier reports on a contribution of tlr-4 (35, 36, 38) might have resulted from the poor quality and LPS contamination of the commercial LTA preparations employed. For our butanol-extracted LTA from both S. aureus and B. subtilis we have shown exclusive signaling via tlr-2 (22, 23, 27).
Taken together, the heterogeneity of commercially available LTA preparations does not allow characterization of immunostimulatory activities of LTA. The crude nature of the extracts, LPS contaminations, and the nonappropriate phenol extraction resulting in partial decomposition, especially in the case of LTA from S. aureus, make this material unsuitable for study of immune activation by LTA.
Acknowledgments
We are indebted to W. Fischer, Erlangen, Germany, who introduced us to the preparation and analysis of LTA. Furthermore, we are grateful for the excellent technical assistance of A. Biedermann, G. Pinski, and L. Cobianchi and the prompt help of L. Hareng. We thank S. von Aulock for help with the manuscript.
Editor: J. T. Barbieri
REFERENCES
- 1.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleveland, M. G., J. D. Gorham, T. L. Murphy, E. Tuomanen, and K. M. Murphy. 1996. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 64:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danforth, J. M., R. M. Strieter, S. L. Kunkel, D. A. Arenberg, G. M. van Otteren, and T. J. Standiford. 1995. Macrophage inflammatory protein-1α expression in vivo and in vitro: the role of lipoteichoic acid. Clin. Immunol. Immunopathol. 74:77-83. [DOI] [PubMed] [Google Scholar]
- 4.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan, X., F. Stelter, R. Menzel, R. Jack, I. Spreitzer, T. Hartung, and C. Schütt. 1999. Structures in Bacillus subtilis are recognized by CD14 in a lipopolysaccharide binding protein-dependent reaction. Infect. Immun. 67:2964-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer, W., H. U. Koch, and R. Haas. 1983. Improved preparation of lipoteichoic acids. Eur. J. Biochem. 133:523-530. [DOI] [PubMed] [Google Scholar]
- 7.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, W. 1993. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography, exemplified with lipoteichoic acids. Anal. Biochem. 208:49-56. [DOI] [PubMed] [Google Scholar]
- 9.Gao, J. J., Q. Xue, E. G. Zuvanich, K. R. Haghi, and D. C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunfeld, C., M. Marshall, J. K. Shigenaga, A. H. Moser, P. Tobias, and K. R. Feingold. 1999. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J. Lipid Res. 40:245-252. [PubMed] [Google Scholar]
- 11.Hartung, T., W.-D. Döcke, F. Gantner, G. Krieger, A. Sauer, P. Stevens, H.-D. Volk, and A. Wendel. 1995. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood 85:2482-2489. [PubMed] [Google Scholar]
- 12.Hartung, T., and A. Wendel. 1996. Detection of pyrogens using human whole blood. In Vitro Toxicol. 9:353-359. [Google Scholar]
- 13.Hashimoto, M., Y. Imamura, J. Yasuoka, S. Kotani, S. Kusumoto, and Y. Suda. 1999. A novel cytokine-inducing glycolipid isolated from the lipoteichoic acid fraction of Enterococus hirae ATCC 9790: a fundamental structure of the hydrophilic part. Glycoconj. J. 16:213-221. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto, M., J. Yasuoka, Y. Suda, H. Takada, T. Yoshida, S. Kotani, and S. Kusumoto. 1997. Structural feature of the major but not cytokine-inducing molecular species of lipoteichoic acid. J. Biochem. (Tokyo) 121:779-786. [DOI] [PubMed] [Google Scholar]
- 15.Hermann, C., I. Spreitzer, N. W. J. Schröder, S. Morath, M. D. Lehner, W. Fischer, C. Schütt, R. R. Schumann, and T. Hartung. Cytokine induction by purified lipoteichoic acids from various bacterial species---Role of LBP, sCD14, CD14 and failure to induce interleukin-12 and subsequent IFNγ release. Eur. J. Immunol., in press. [DOI] [PubMed]
- 16.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamura, N., N. Imanishi, H. Koike, H. Nakahara, L. Phillips, and S. Morooka. 1995. Lipoteichoic acid-induced neutrophil adhesion via E-selectin to human umbilical vein endothelial cells (HUVECs). Biochem. Biophys. Res. Commun. 217:1208-1215. [DOI] [PubMed] [Google Scholar]
- 18.Keller, R., W. Fischer, R. Keist, and S. Bassetti. 1992. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 60:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusunoki, T., E. Hailman, T. S.-C. Juan, H. S. Lichenstein, and S. D. Wright. 1995. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 182:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusunoki, T., and S. D. Wright. 1996. Chemical characteristics of Staphylococcus aureus molecules that have CD14-dependent cell-stimulating activity. J. Immunol. 157:5112-5117. [PubMed] [Google Scholar]
- 22.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 23.Michelsen, K. S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C. J. Kirschning, and R. R. Schumann. 2001. The role of Toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 24.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohshima, Y., J. Beuth, A. Yassin, H. L. Ko, and G. Pulverer. 1988. Stimulation of human monocyte chemiluminescence by staphylococcal lipoteichoic acid. Med. Microbiol. Immunol. 177:115-121. [DOI] [PubMed] [Google Scholar]
- 26.Ohshima, Y., H. L. Ko, J. Beuth, H. Burrichter, K. Oette, and G. Pulverer. 1991. Activation of mononuclear immune cells in response to staphylococcal lipoteichoic acid. Zentbl. Bakteriol. 275:374-381. [DOI] [PubMed] [Google Scholar]
- 27.Opitz, B., N. W. Schroder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U. B. Gobel, and R. R. Schumann. 2001. Toll-like receptor (TLR)-2 mediates treponema glycolipid and lipoteichoic acid (LTA)-induced NF-kappa B translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 28.Rietschel, E. T., H. Brade, O. Holst, L. Brade, S. Müller-Loennies, U. Mamat, U. Zähringer, F. Beckmann, U. Seydel, K. Brandenburg, A. J. Ulmer, T. Mattern, H. Heine, J. Schletter, H. Loppnow, U. Schönbeck, H.-D. Flad, S. Hauschildt, U. F. Schade, F. di Padova, and S. S. Kusumoto. 1996. Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification, p. 40-69. In E. T. Rietschel and H. Wagner (ed.), Pathology of septic shock, vol. 216. Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 29.Schnitger, H., K. Papenberg, E. Ganse, R. Czok, T. Bücher, and H. Adam. 1959. Chromatographie phosphathaltiger Metabolite eines menschlichen Leberpunktats. Biochem. Z. 332:167-185. [PubMed] [Google Scholar]
- 30.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 31.Suda, Y., H. Tochio, K. Kawano, H. Takada, T. Yoshida, S. Kotani, and S. Kusumoto. 1995. Cytokine-inducing glycolipids in the lipoteichoic acid fraction from Enterococcus hirae ATCC 9790. FEMS Immunol. Med. Microbiol. 12:97-112. [DOI] [PubMed] [Google Scholar]
- 32.Sugawara, S., R. Arakaki, H. Rikiishi, and H. Takada. 1999. Lipoteichoic acid acts as an antagonist and an agonist of lipopolysaccharide on human gingival fibroblasts and monocytes in a CD14-dependent manner. Infect. Immun. 67:1623-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama, A., R. Arakaki, T. Ohnishi, N. Arakaki, Y. Daikuhara, and H. Takada. 1996. Lipoteichoic acid and interleukin 1 stimulate synergistically production of hepatocyte growth factor (scatter factor) in human gingival fibroblasts in culture. Infect. Immun. 64:1426-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada, H., Y. Kawabata, R. Arakaki, S. Kusumoto, K. Fukase, Y. Suda, T. Yoshimura, S. Kokeguchi, K. Kato, T. Komuro, et al. 1995. Molecular and structural requirements of a lipoteichoic acid from Enterococcus hirae ATCC 9790 for cytokine-inducing, antitumor, and antigenic activities. Infect. Immun. 63:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi, O., and S. Akira. 2001. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 1:625-635. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 37.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 38.Yang, S., R. Tamai, S. Akashi, O. Takeuchi, S. Akira, S. Sugawara, and H. Takada. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 69:2045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]