Abstract
Yersinia pseudotuberculosis mutants that overproduce the DNA adenine methylase (Dam) are highly attenuated, confer fully protective immune responses, and secrete several Yersinia virulence proteins (Yersinia outer proteins [Yops]) under conditions that are nonpermissive for secretion in wild-type strains. We examined here the effects of Dam overproduction on Yersinia virulence determinant expression and secretion, as well as the host immune response to Yersinia antigens. Western blot analysis with convalescent antisera identified several low-calcium-responsive antigens whose synthesis was affected by Dam overproduction. One of these antigens was shown to be the type III secretion effector protein, YopE, a cytotoxin involved in antiphagocytosis. Dam overproduction disrupted both the thermal and calcium regulation of YopE synthesis and relaxed the thermal but not the calcium dependence of YopE secretion. Altered expression and/or secretion of Yersinia proteins in Dam-overproducing strains may contribute to the decreased virulence and heightened immunity observed in vaccinated hosts and may provide a means by which to deliver heterologous antigens and/or immune modulators of the inflammatory response.
Yersinia spp. are human and animal pathogens with a clear tropism for lymphoid tissue. Yersinia pestis is usually transmitted by fleas and is the causative agent of plague, which is often fatal (4, 5). Y. pseudotuberculosis and Y. enterocolitica are enteropathogens causing self-limiting infections in humans, including gastroenteritis and mesenteric adenitis. Yersinia spp. pathogenesis is dependent on virulence proteins called Yops (for Yersinia outer proteins) (7, 9, 11, 30) which, upon host contact, are injected directly into the host cell cytoplasm via type III secretion machinery, where they act as effectors to inhibit phagocytosis and proinflammatory cytokine release (3, 5, 6, 8, 12, 25, 26, 29, 31, 35). The secretion of Yops is under strict regulatory control by the low calcium response, whereby Yop secretion only occurs in vitro under conditions of low calcium (Ca2+) and high temperature (37°C[32, 33]). We recently showed that overproduction of Dam in Y. pseudotuberculosis relaxed the temperature but not the low calcium dependence of Yop secretion (18). Moreover, such Dam-overproducing Yersinia strains were avirulent and elicited protective immune responses in vaccinated mice. Here we examined the effects of Dam overproduction on protein expression and secretion, as well as the humoral response to Yersinia antigens.
Yersinia spp. overproducing Dam efficiently colonize mucosal but not systemic tissues.
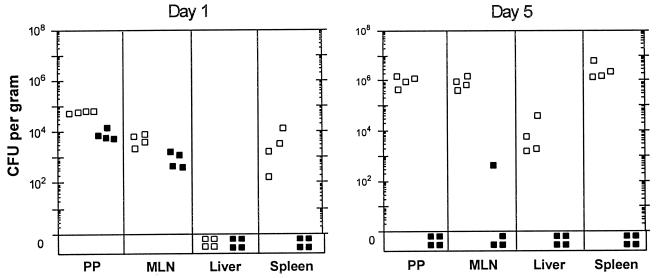
To understand the mechanism by which Dam-overproducing Yersinia spp. are attenuated for virulence yet elicit protective immune responses, the survival rates of wild-type (Dam+) and Dam-overproducing yersiniae were compared in mouse tissue sites after oral infection. Dam-overproducing yersiniae survive near wild-type levels in Peyer’s patches of the mouse small intestine and mesenteric lymph nodes for at least 24 h. However, at day 5, >105-fold fewer Dam-overproducing yersiniae were observed in the Peyer’s patches and mesenteric lymph nodes, and 103- to 106-fold fewer Dam-overproducing yersiniae were observed in the liver and spleen, respectively, compared to Dam+ bacteria (Fig. 1). These data suggest that Dam-overproducing yersiniae are proficient in the targeting and colonization of mucosal but not deep systemic tissues, which may result in the elicitation of host immune responses without acute disease manifestations.
FIG. 1.
Colonization of mouse tissue sites by Dam-overproducing Y. pseudotuberculosis. Six- to eight-week-old BALB/c mice were infected via gastrointubation at a dose of ca. 2.5 × 1010 Dam+ (□) or Dam-overproducing (▪) Y. pseudotuberculosis. At 1 or 5 days postinfection, mice were sacrificed and bacteria were recovered from the host tissues indicated. Abbreviations: PP, Peyer’s patches (the four Peyer’s patches proximal to the ileal-cecal junction); MLN, mesenteric lymph nodes.
Dam-overproducer Y. pseudotuberculosis synthesizes and secretes YopE under conditions nonpermissive for the wild type.
Recently, we showed that the strict regulatory control of Yop secretion is disrupted in Dam-overproducing Yersinia mutants (15). These mutants secrete Yops at low Ca 2+ and low temperature, which are nonpermissive conditions for Yop secretion in wild-type Yersinia. Here we wanted to test whether the synthesis and cellular localization of Yops was also disrupted in Dam overproducer conditions. For these experiments, we focused our efforts on YopE, a 23-kDa Yersinia cytotoxin that is secreted under low-calcium conditions (1, 2, 34) and is also known to be antigenic (16, 20).
Analysis of the effect of Dam on expression of Y. pseudotuberculosis antigens.
To begin to characterize the humoral response conferred by Yersinia Dam-overproducing strains, we examined protein expression profiles of Dam+ and Dam-overproducing strains (Table 1). Proteins derived from Dam+ and Dam-overproducing strains grown under laboratory conditions (in vitro) were subjected to Western analysis with convalescent-phase antisera derived from mice infected with either wild-type (Fig. 3) or Dam-overproducing (Fig. 3B) Y. pseudotuberculosis.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Y. pseudotuberculosis | ||
| YPIIIpYV | Wild type | Stanley Falkow |
| MT2294 | dam::Kn + pTP166-Cm | 15 |
| MT2365 | ΔyopE (in-frame deletion) | This work |
| MT2366 | Dgr;yopE dam::Kn + pTP166-Cm | This work |
| Plasmid pTP166-Cm | E. coli dam under tac promoter control; chloramphenicol-resistant derivative of pTP166 | 15,19 |
FIG. 3.
Mice vaccinated with Dam-overproducing Yersinia strains show an altered humoral response compared to mice infected with wild-type Yersinia strains. Whole-cell protein extracts from wild-type (WT) and Dam-overproducing (OP) Y. pseudotuberculosis grown under the indicated temperature and calcium conditions served as the antigen source. Whole-cell protein extracts derived from 2.0 × 106 cells (∼20 μg of protein/well) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane (Pierce), and probed with Dam+ (A) or Dam-overproducing (B) pooled convalescent antisera at a 1/15,000 or a 1/7500 dilution of antibody, respectively. Peroxidase-conjugated sheep anti-mouse immunoglobulin G (Amersham Life Sciences) was used as the secondary antibody at 1/40,000 dilution, and hybridization was detected by using Supersignal West Femto Maximum Sensitivity Substrate (Pierce). Group 1 antigens were produced by wild-type and Dam-overproducing strains in vitro and recognized by both wild-type and Dam-overproducing convalescent-phase antisera (arrows 1). Group 2 antigen was produced by wild-type and Dam-overproducing strains in vitro and preferentially recognized by wild-type convalescent-phase antisera (arrows 2). Group 3 antigens were expressed under Yop inducing conditions (low Ca2+ and high temperature) and were preferentially produced by either Dam+ or Dam-overproducing cells in vitro (arrows 3). Numbers refer to protein sizes in kilodaltons. To generate the wild-type and Dam-overproducing convalescent-phase sera, 6- to 8-week-old BALB/c mice were gastrointubated with 2.5 × 107 wild-type Y. pseudotuberculosis (the lethal dose required to kill 50% of the animals [21]) or 2.5 × 1010 Dam-overproducing Y. pseudotuberculosis bacteria. At 5 weeks postimmunization, whole blood from four to six mice was collected by cardiac puncture, and the sera were pooled and stored in 20% glycerol at −20°.
In order to characterize the expression, localization, and secretion profiles of YopE in response to Dam overproduction, whole-cell, membrane, and supernatant fractions of Dam+ and Dam-overproducing cells grown under Yop-inducing and noninducing conditions were analyzed by immunoblotting. In contrast to the wild type, Dam-overproducing Yersinia strains synthesized YopE under all three nonpermissive conditions (high calcium and low temperature, high calcium and high temperature, and low calcium and low temperature) (Fig. 2, whole-cell fraction). However, the localization of YopE to the membrane or supernatant fractions required low calcium at either permissive or nonpermissive temperatures (Fig. 2). Thus, YopE is synthesized under all nonpermissive conditions in Dam-overproducing strains, but its export from the cytoplasm still requires a low calcium signal for secretion. These data suggest that Dam overproduction disrupts both thermal and calcium regulation of YopE synthesis and, in addition, relaxes the thermal but not the calcium dependence of YopE secretion. Alternatively, Dam overproduction may lead to YopE overexpression coupled with increased YopE secretion (only at low Ca2+ and a low temperature) simply as a consequence of an increased amount of protein in the cell. The proposed altered expression or secretion of YopE and the possible ectopic expression or secretion of other bacterial antigens may contribute to the heightened immune response in hosts vaccinated with Dam-overproducing Yersinia mutants.
FIG. 2.
Dam overproduction results in YopE synthesis and secretion under conditions nonpermissive for the wild type. Whole-cell (WC), membrane (Memb), and supernatant (Sup) fractions were prepared from wild-type (WT) and Dam-overproducing (OP) Y. pseudotuberculosis grown under the indicated conditions. For each growth condition, total protein corresponding to 2.0 × 106 cells was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and probed with convalescent-phase antisera derived from BALB/c mice infected with wild-type Y. pseudotuberculosis as a source of anti-YopE antibody. Western analysis with YopE antibody confirmed that the 23-kDa low-calcium-responsive protein was YopE. Numbers refer to protein sizes (in kilodaltons).
The effect of Dam overproduction on YopE synthesis is comparable to the phenotype of Yersinia mutants defective in LcrQ, a negative regulator of Yop synthesis (27). Wild-type Yersinia secretes LcrQ out of the bacterial cell under the permissive conditions of low calcium and high temperature, resulting in a decreased intracellular concentration of LcrQ and increased expression of Yops. LcrQ mutants are disrupted for the normally strict thermal and calcium regulation of Yop synthesis; however, low calcium is still required as a signal for the type III secretion-dependent delivery of most Yops (28). Similarly, Dam overproduction relaxes the temperature and calcium regulation of YopE synthesis, but export of YopE from the cytoplasm remains dependent on the low-calcium secretion signal.
At least three groups of antigens were identified. Group 1 antigens were produced by wild-type and Dam-overproducing strains in vitro and were recognized by convalescent-phase antisera derived from mice infected with wild type or Dam-overproducing strains infected mice (Fig. 3, arrows labeled 1). Group 2 antigen was produced by wild-type and Dam-overproducing strains in vitro and was preferentially recognized by convalescent antisera derived from wild type-infected mice (Fig. 3, arrows labeled 2). Group 3 antigens were expressed under Yop inducing conditions (low Ca2+ and high temperature) and were often preferentially expressed by either wild-type or Dam-overproducing strains in vitro (Fig. 3, arrows labeled 3).
These data show that Dam overproduction affects the humoral response but not the in vitro synthesis of the group 2 antigen. This suggests that, although the group 2 antigen was produced at wild-type levels in Dam-overproducing cells in vitro, it is not produced in sufficient quantity or for sufficient duration, nor is it presented to the appropriate immune cells, during Dam-overproducing infection. Alternatively, Dam-overproducing cells may inhibit the humoral response to the group 2 antigen. Such differential expression of an in vivo-induced antigen or altered immune response to an in vivo expressed antigen can have profound effects on the immunity conferred by Dam-overproducing Yersinia strains.
The role of Dam in virulence and in the elicitation of protective immune responses may be attributed to its capacity as a global regulator of gene expression (10, 13, 14, 17–19). Overproduction of Dam activity in Yersinia strains alters the expression and/or secretion of low-calcium-responsive proteins (Fig. 2 and 3) (15) and possibly other virulence functions required for pathogenesis. Additionally, Dam overproduction may contribute to the elicitation of protective responses by the elaboration of an expanded repertoire of antigens and/or immune modulators of host inflammatory activities. Although not known to be subject to Dam regulation, LcrV is a Yersinia virulence protein responsive to low calcium that suppresses inflammatory cytokines during infection (23, 24) and induces high levels of protection when delivered as a subunit vaccine (5, 22). Similarly, dysregulation of Dam activity may result in altered expression and/or secretion of functions required for virulence, immune modulation, and the elicitation of protection immune responses. Such a delivery strategy may also provide a means to deliver heterologous antigens and modulators of the inflammatory response (e.g., for suppression of inflammatory cytokines).
Acknowledgments
We thank Greg Plano for the generous gift of YopE antibody and Norris Allen and Robert Brubaker for critically reading the mauscript.
This work was supported by private donations from Jim and Deanna Dehlsen, the University of California Biotech Program, the Santa Barbara Cottage Hospital Research Program, a USDA grant, 2000-02539 (to M.J.M), a National Institutes of Health grant, AI23348 (to D.A.L.), and a postdoctoral grant from the Cancer Center of Santa Barbara (to D.M.H.).
Editor: A. D. O’Brien
REFERENCES
- 1.Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 3:301–310. [DOI] [PubMed] [Google Scholar]
- 2.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515–527. [DOI] [PubMed] [Google Scholar]
- 3.Bleves, S., and G. R. Cornelis. 2000. How to survive in the host: the Yersinia lesson. Microbes Infect. 2:1451–1460. [DOI] [PubMed] [Google Scholar]
- 4.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brubaker, R. R. 2000. Yersinia pestis and bubonic plague. Springer-Verlag, New York, N.Y. [Online.]
- 6.Cheng, L. W., and O. Schneewind. 2000. Type III machines of gram-negative bacteria: delivering the goods. Trends Microbiol. 8:214–220. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis, G. R. 2000. Type III secretion: a bacterial device for close combat with cells of their eukaryotic host. Philos. Trans. R. Soc. London B Biol. Sci. 355:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect. Immun. 69:7950–7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, K. L., and J. E. Dixon. 1990. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science 249:553–556. [DOI] [PubMed] [Google Scholar]
- 12.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:14361446. [DOI] [PubMed] [Google Scholar]
- 13.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967–970. [DOI] [PubMed] [Google Scholar]
- 15.Julio, S. M., D. M. Heithoff, D. Provenzano, K. E. Klose, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. DNA adenine methylase is essential for viability and plays a role in the pathogenesis of Yersinia pseudotuberculosis and Vibrio cholerae. Infect. Immun. 69:7610–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leary, S. E., K. F. Griffin, E. E. Galyov, J. Hewer, E. D. Williamson, A. Holmstrom, Forsberg, and R. W. Titball. 1999. Yersinia outer proteins (YOPS) E, K and N are antigenic but non-protective compared to V antigen, in a murine model of bubonic plague. Microb. Pathog. 26:159–169. [DOI] [PubMed] [Google Scholar]
- 17.Low, D. A., N. J. Weyand, and M. J. Mahan. 2001. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 69:7197–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahan, M., and D. Low. 2001. 0 DNA methylation regulates bacterial gene expression and virulence. ASM News 67:356–361. [Google Scholar]
- 19.Marinus, M. G., A. Poteete, and J. A. Arraj. 1984. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene 28:123–125. [DOI] [PubMed] [Google Scholar]
- 20.Mazza, G., A. E. Karu, and D. T. Kingsbury. 1985. Immune response to plasmid- and chromosome-encoded Yersinia antigens. Infect. Immun. 48:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953–965. [DOI] [PubMed] [Google Scholar]
- 26.Persson, C., R. Nordfelth, A. Holmstrom, S. Hakansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135–150. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231–1233. [DOI] [PubMed] [Google Scholar]
- 28.Rimpilainen, M., A. Forsberg, and H. Wolf-Watz. 1992. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J. Bacteriol. 174:3355–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosqvist, R., I. Bolin, and H. Wolf-Watz. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657–667. [DOI] [PubMed] [Google Scholar]
- 31.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straley, S. C., and R. D. Perry. 1995. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 3:310–317. [DOI] [PubMed] [Google Scholar]
- 33.Straley, S. C., G. V. Plano, E. Skrzypek, P. L. Haddix, and K. A. Fields. 1993. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol. Microbiol. 8:1005–1010. [DOI] [PubMed] [Google Scholar]
- 34.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737–748. [DOI] [PubMed] [Google Scholar]
- 35.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]