Abstract
Bordetella bronchiseptica is one of the etiologic agents causing atrophic rhinitis and pneumonia in swine. It produces several purported virulence factors, including the dermonecrotic toxin (DNT), which has been implicated in the turbinate atrophy seen in cases of atrophic rhinitis. The purpose of these experiments was to clarify the role of this toxin in respiratory disease by comparing the pathogenicity in swine of two isogenic dnt mutants to their virulent DNT+ parent strains. Two separate experiments were performed, one with each of the mutant-parent pairs. One-week-old cesarean-derived, colostrum-deprived pigs were inoculated intranasally with the parent strain, the dnt mutant strain, or phosphate-buffered saline. Weekly nasal washes were performed to monitor colonization of the nasal cavity, and the pigs were euthanized 4 weeks after inoculation to determine colonization of tissues and to examine the respiratory tract for pathology. There was evidence that colonization of the upper respiratory tract, but not the lower respiratory tract, was slightly greater for the parent strains than for the dnt mutants. Moderate turbinate atrophy and bronchopneumonia were found in most pigs given the parent strains, while there was no turbinate atrophy or pneumonia in pigs challenged with the dnt mutant strains. Therefore, production of DNT by B. bronchiseptica is necessary to produce the lesions of turbinate atrophy and bronchopneumonia in pigs infected with this organism.
Bordetella bronchiseptica causes respiratory disease in many species (11). In particular, it is one of the etiologic agents of atrophic rhinitis and pneumonia in swine (10). The characteristic lesion of atrophic rhinitis is atrophy of the nasal turbinate bones. Microscopic lesions include mucosal infiltration by neutrophils, loss of cilia, metaplasia of the epithelium, and resorption of bone with replacement by fibrous tissue (9). Pulmonary lesions are characterized by infiltration of the airways with neutrophils, necrosis of the alveoli and blood vessels, hemorrhage, and, eventually, extensive fibrosis as the lesions become more chronic (8).
B. bronchiseptica, like its close relative Bordetella pertussis, produces virulence factors which are regulated by a two-component sensory transduction system encoded by the bvg locus (3, 28). Some of the virulence factors which are positively regulated by bvg include adhesins such as filamentous hemagglutinin, pertactin, and fimbriae and the adenylate cyclase-hemolysin toxin and dermonecrotic toxin (DNT). B. bronchiseptica DNT produces dermonecrosis when injected intradermally into many animals including guinea pigs, mice, and rabbits and is lethal for mice when given intravenously (15). In cell cultures, DNT stimulates DNA and protein synthesis and assembly of actin stress fibers while inhibiting cell division, resulting in polynucleation of cells (19, 20). It mediates these changes through modification and activation of the small GTP-binding protein, Rho (18). DNT has been implicated in the nasal turbinate atrophy seen in swine during infection with this organism, based on experiments using toxigenic and nontoxigenic field isolates of B. bronchiseptica (2, 26, 34). Although intriguing, the results of these experiments were not conclusive since it is unknown if there were other differences besides the production of DNT in these isolates which could be responsible for the differences in pathogenicity that were observed. The purpose of these experiments was to definitively determine a role for the DNT of B. bronchiseptica by comparing the pathogenicity of isogenic dnt mutants to their virulent DNT+ parent strains using a cesarean-derived, colostrum-deprived pig model.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. bronchiseptica strains B58 (26) and KM22 are virulent phase I isolates from swine herds with clinical atrophic rhinitis. B. bronchiseptica B58 and KM22 were cultured at 37°C for 40 h on Bordet-Gengou agar supplemented with 10% sheep’s blood (BG). Suspensions of these cultures were prepared in phosphate-buffered saline (PBS) for inoculation of the pigs. The dnt mutants of B58 (B58GP) and KM22 (KB24) were cultured under the same conditions with the addition of kanamycin (50 μg/ml) or gentamicin (100 μg/ml), respectively, to the medium. Dilutions of the B58 suspension used to inoculate pigs were plated on BG without kanamycin, and the titer was approximately 3 × 105 CFU/ml, with 100% of the colonies appearing to be Bvg+ based on colony morphology (domed colonies and hemolysis). Dilutions of the B58GP inoculum were plated on BG with kanamycin, and the titer was also approximately 3 × 105 CFU/ml. However, only 58% of the colonies appeared to be in the Bvg+ phase. Dilutions of the KM22 and KB24 inocula were plated on BG without or with gentamicin, respectively, and their titers were both approximately 106 CFU/ml. A total of 100% of the colonies of both appeared to be in the Bvg+ phase.
Preparation of the dnt mutant of B. bronchiseptica B58 (B58GP).
Plasmid pBHI, containing the B. bronchiseptica B58 DNT gene cloned in pBluescript (31), was digested with NsiI. The fragment consisting of the vector and part of the dnt gene was purified from a gel by electroelution, effectively removing a 1,854-nucleotide fragment from the coding region. The kanamycin resistance cassette (1.2 kb) was excised from pUC4K (Amersham Pharmacia) by digestion with PstI, and the fragment was electroeluted from an agarose gel. These two fragments were ligated (Fig. 1) and transformed into competent cells of Escherichia coli XLI-Blue. Colonies growing on plates containing ampicillin (100 μg/ml), tetracycline (20 μg/ml), and kanamycin (25 μg/ml) were selected. Plasmid DNA was prepared from these, and a clone containing one kanamycin resistance cassette was identified by restriction analysis. The insert from this was excised using BamHI followed by electroelution and was then ligated into pRTP1 (35) which had been cut with BamHI and treated with calf intestinal phosphatase. This plasmid was transformed into E. coli SM10, using ampicillin and kanamycin selection, and this was used as the donor for conjugation into a spontaneous nalidixic acid-resistant mutant of B. bronchiseptica B58. Transconjugants were selected and passaged once on BG containing 30 mM MgSO4 (to keep the strains locked in C mode and avoid in vitro selection for phase III bacteria), 40 μg of kanamycin per ml, and 40 μg of nalidixic acid per ml (22). Ampicillin-sensitive colonies from which the delivery plasmid had been eliminated were identified by replica plating onto BG with ampicillin and kanamycin. Potential dnt mutants were analyzed genetically by PCR. Genomic DNA was prepared from the potential mutants using the Wizard genomic DNA purification kit (Promega). A DNT fragment encompassing the mutation was amplified by standard procedures using primers DNTS (5′-GTTCGCCTACGACGAATTGG) and DNTAS (5′-CTCCTGCAGGTATCGATATG). A fragment of 1.7 kb was amplified from the mutant genomic DNA, whereas a fragment of 2.3 kb was obtained using pBHI as the template (Fig. 2). Therefore, the mutant (designated B58GP) had the expected deletion.
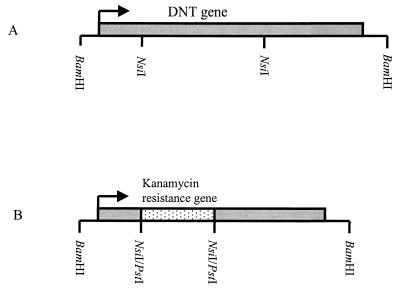
FIG. 1.
Construction of dnt mutant B58GP. (A) Map of the 5-kb insert of pBH1 containing the dnt gene. (B) Map showing the insert after replacement of the internal NsiI fragment (1.8 kb) with the kanamycin resistance cassette (1.2 kb).
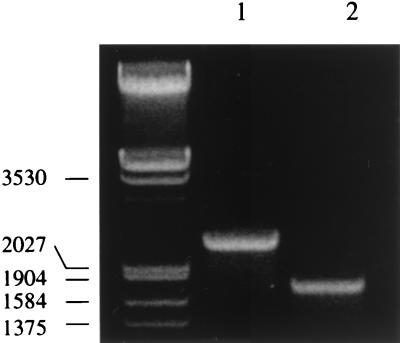
FIG. 2.
PCR products separated by agarose gel electrophoresis. Lanes: 1, pBHI control; 2, B58GP genomic DNA. The molecular size of the markers is indicated in base pairs on the left.
Preparation of the dnt mutant of B. bronchiseptica KM22 (KB24).
A KM22 dnt mutant was obtained by triparental matings as described previously (38) using the mutator plasmid pKEW42 and an E. coli strain containing the broad-host-range mobilizing plasmid pRK2013. The mutator plasmid contains a 1.8-kb NotI-BamHI fragment of the B. pertussis dnt gene. A 3.7-kb Genr/oriT cassette was inserted into a BglI site in the 1.8-kb fragment, permitting recombination into the chromosome and selection for recombinants. Colicin B, crudely purified from E. coli DM1178(pCLB1), was used for counterselection against the donor and mobilizing strains (5). Transconjugants were analyzed by Southern blotting to confirm a single insertion into the dnt gene. All transconjugants analyzed were found to also contain the mutator plasmid. One transconjugant found to be stable after 10 passages on antibiotic-free medium was selected for further study and designated KB24.
Phenotypic characterization of bacterial strains.
Bacterial protein expression was evaluated by immunoblotting. Proteins from whole-cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% polyacrylamide gels by the method of Doucet et al. (7). Following electrophoretic transfer to nitrocellulose, immunoblotting was carried out as reported previously (32). Expression of DNT, filamentous hemagglutinin (FHA), pertactin, and adenylate cyclase toxin was detected using previously described monoclonal antibodies AE9 (31), X3C (23), BPE3 (4), and 9D4 (13), respectively.
Lethality in mice was used as a correlate for expression and activity of B. bronchiseptica DNT. Toxicity testing for strains B58 and B58GP has been described and results have been reported previously (24). For strains KM22 and KB24, three mice each were injected intraperitoneally with 0.5 ml of sterile, cell-free sonicates, as previously described (25). Death within 24 h was interpreted as a positive result. Surviving mice were monitored for 3 days, at which time the experiment was terminated.
Experimental infection in swine.
Two similar experiments were performed, the first with B58 and B58GP and the second with KM22 and KB24. In each experiment, cesarean-derived, colostrum-deprived pigs were divided into three groups and inoculated intranasally at 1 week of age with 1 ml (0.5 ml/nostril) of a bacterial suspension of either the parent B. bronchiseptica or its dnt mutant or with 1 ml of sterile PBS. In the first experiment, there were six pigs per group; in the second experiment there were eight pigs in the groups that received KB24 and PBS and six pigs in the group that received KM22. Tonsillar and nasal swabs were obtained from all pigs prior to the start of the experiment, and no B. bronchiseptica was isolated. Clinical signs and rectal temperature were recorded daily, and body weight was recorded twice weekly. At 28 days after inoculation, the pigs were euthanized with an overdose of barbiturate and necropsies were performed.
Determination of colonization.
Nasal washes were performed at 1, 2, and 3 weeks (and at 4 weeks in the first experiment) after inoculation with B. bronchiseptica to estimate colonization of the nasal cavity. Nasal washes were performed by injecting 5 ml of PBS into the nasal cavity through one nostril and collecting the effluent into a beaker. Tenfold dilutions of the effluent were prepared and plated out on duplicate selective blood agar plates containing 20 μg of penicillin per ml, 10 μg of amphotericin B per ml, 10 μg of streptomycin per ml, and 10 μg of spectinomycin per ml. At 4 weeks after inoculation, when the pigs were euthanized, a weighed specimen of nasal turbinate, trachea, and lung from each pig was ground individually in PBS with a Ten Broeck grinder. The number of CFU of B. bronchiseptica per gram of tissue was determined by plating serial 10-fold dilutions of homogenates on duplicate selective blood agar plates. B. bronchiseptica was identified from primary isolation plates by a Bordetella-specific hybridization assay (33). Additionally, a countable dilution was plated on BG with kanamycin in the first experiment or gentamicin in the second experiment to compare colony counts, making sure that the pigs were colonized with either the parent or mutant strains.
Pathological evaluation.
At necropsy, an estimate of the percent gross lung involvement was assigned based on the percentage of each lung lobe affected and the percentage of total lung volume represented by each lobe. The percentage of the total lung volume contributed by each lobe was estimated as 5% for the left cranial, 6% for the left middle, 29% for the left caudal, 11% for the right cranial, 10% for the right middle, 34% for the right caudal, and 5% for the intermediate lung lobes.
Snouts were transversely sectioned at the level of the first premolar tooth, and each of the four scrolls of the ventral turbinates was assigned a score which ranged from 0 to 4: 0, normal; 1, more than half of the turbinate remaining; 2, half or less of the turbinate remaining; 3, turbinate is straightened with only a small portion left; 4, total atrophy. The nasal septum received a score of 0 to 2: 0, normal; 1, slight deviation; 2, severe deviation. The total snout score is the addition of the four turbinate scores plus the septal score and ranges from 0 to 18.
Sections from nasal turbinate, trachea, and lung were taken for microscopic evaluation. All tissues were fixed in 10% neutral buffered formalin for 24 h and then placed in 90% ethanol. Nasal turbinates were decalcified in EDTA decalcifying solution for an additional 24 h after fixation. All sections were routinely processed and embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Immunohistochemical staining for B. bronchiseptica.
B. bronchiseptica was detected in tissue sections from pigs in the second experiment (KM22 and KB24) using immunohistochemical techniques as previously described (1). Briefly, slides were incubated with a primary polyclonal rabbit anti-B. bronchiseptica antibody followed by a secondary biotinylated goat anti-rabbit antibody. The sections were then incubated with streptavidin-phosphatase and phosphatase substrate and counterstained with Mayer’s hematoxylin.
Statistical analysis.
A two-tailed, non-paired Student’s t test assuming unequal variance and a significance level of P < 0.05 was used to compare bacterial colonization levels, turbinate scores, and percent lung involvement between the groups inoculated with the parent and DNT− mutant strains of B. bronchiseptica.
RESULTS
Phenotypic analysis of bacterial strains.
All strains (B58, B58GP, KM22, and KB24) were confirmed to be phase I by their colony morphology. Comparison of immunoblottings carried out with whole-cell extracts demonstrated that all strains produced FHA, pertactin, and adenylate cyclase toxin but only B58 and KM22 produced DNT (Fig. 3). Cell-free sonic extracts of both parent strains, B58 and KM22, were lethal for mice, whereas extracts of both mutants, B58GP and KB24, were not.
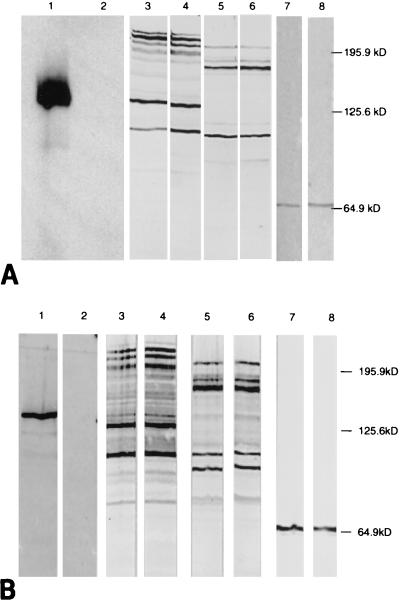
FIG. 3.
Expression of DNT, FHA, adenylate cyclase toxin, and pertactin. Proteins contained in bacterial cell extracts prepared from strain B58 (lanes 1, 3, 5, and 7) or B58GP (lanes 2, 4, 6, and 8) (A) or KM22 (lanes 1, 3, 5, and 7), or KB24 (lanes 2, 4, 6, and 8) (B) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. DNT, FHA, adenylate cyclase toxin, and pertactin were detected by incubation with monoclonal antibodies AE9 (lanes 1 and 2), X3C (lanes 3 and 4), 9D4 (lanes 5 and 6), and BPE3 (lanes 7 and 8), respectively.
Clinical response of pigs. (i) Clinical signs.
Pigs in both experiments which received B. bronchiseptica, either parent or dnt mutant, had clinical signs of Bordetella infection, including sneezing, coughing, nasal congestion, and ocular and nasal discharge. Based on the number of days these clinical signs were observed per pig, the groups which were infected with the parent strains appeared slightly more affected than the groups which received the dnt mutants. For example, in the second experiment, clinical signs were recorded for an average of 9 days per pig inoculated with the parent strain but for only 4 days per pig inoculated with the dnt mutant. In the first experiment, clinical signs in addition to those attributable to infection with B. bronchiseptica were observed in both groups which received B. bronchiseptica. These clinical signs included swollen joints with lameness and reluctance to move as well as increased lethargy and anorexia. One pig from the group infected with the parent B58 died on day 12 postinfection. In both experiments, pigs which were given only PBS showed no clinical signs.
(ii) Temperature response.
In the first experiment, pigs in both groups receiving B. bronchiseptica had febrile responses. The normal temperature for a pig is less than 40°C. The mean rectal temperature for the group given B58 was ≥40°C for 7 days, whereas the mean rectal temperature for the group given the dnt mutant B58GP was ≥40°C for only 2 days. The mean rectal temperature for the group given PBS was never ≥40°C. In the second experiment, one pig in the group given the parent KM22 strain of B. bronchiseptica had a rectal temperature of ≥40°C for 2 days; none of the pigs in the other groups had febrile responses.
(iii) Weight gain.
In the first experiment, both groups receiving B. bronchiseptica had a lower mean weight gain (3.61 and 4.02 kg for the groups infected with B58GP and B58, respectively) than did the group which received PBS (5.32 kg). The mean weight gain for the group infected with the mutant B58GP was statistically lower than for the PBS control group (P = 0.009). The mean weight gain for the group infected with B58 was not statistically lower than that for the control PBS group (P = 0.074), probably due to the greater variation in values and fewer numbers, since one pig in this group died before the end of the experiment. There was no statistical difference between the weight gains of the groups infected with B58 and B58GP (P = 0.62). In the second experiment, all groups gained weight at the same rate.
Colonization of the respiratory tract. (i) Nasal washes.
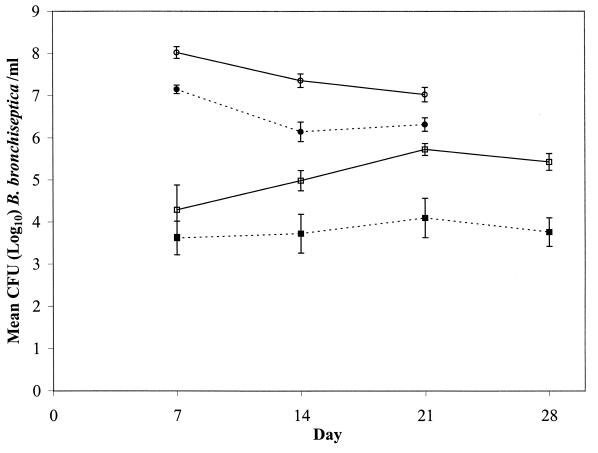
Figure 4 shows the level of colonization of the nasal cavity based on the nasal washes performed at weekly intervals. In both experiments, larger numbers were recovered from pigs infected with the parent strains than from pigs infected with the dnt mutant strains. This difference was statistically significant on days 14, 21, and 28 of the first experiment (with strains B58 and B58GP) and on days 7, 14, and 21 of the second experiment (with strains KM22 and KB24). The level of colonization was higher for strains KM22 and KB24 than for strains B58 and B58GP. B. bronchiseptica was not isolated from the nasal wash at any time point from any of the pigs given PBS.
FIG. 4.
Colonization of the nasal cavity of pigs inoculated with B58 (□), B58GP (▪), KM22 (○), or KB24 (•), as determined by nasal washes performed 7, 14, 21 (and 28 in the first experiment) days postinfection. The difference in the level of colonization between the parent and isogenic mutant strains was statistically significant for B58-B58GP on days 14 (P = 0.046), 21 (P = 0.016), and 28 (P = 0.003) and for KM22-KB24 on days 7 (P = 0.001), 14 (P = 0.001), and 21 (P = 0.01).
(ii) Tissue colonization.
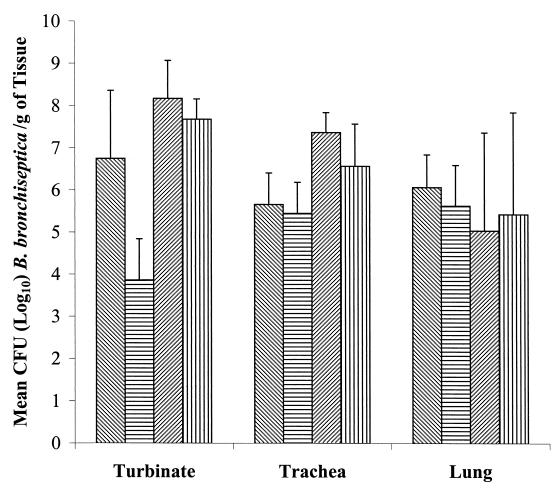
Figure 5 shows the colonization of the different respiratory tissues at necropsy, 4 weeks postinfection. Although the mean number of bacteria isolated per gram of tissue was generally higher for pigs inoculated with the parent strains than for those inoculated with the dnt mutant strains, this difference was statistically significant only for turbinate in the first experiment (P = 0.013). Again, the level of colonization was generally higher for strains KM22 and KB24 than for strains B58 and B58GP. B. bronchiseptica was not isolated from the tissues of any of the pigs given PBS.
FIG. 5.
Colonization of the turbinate, trachea, and lungs of pigs inoculated with B58( ), B58GP (▤), KM22 (
), B58GP (▤), KM22 ( ), or KB24 (▥) at 28 days postinfection. The difference in the level of colonization between B58 and B58GP was statistically significant for the turbinate (P = 0.013).
), or KB24 (▥) at 28 days postinfection. The difference in the level of colonization between B58 and B58GP was statistically significant for the turbinate (P = 0.013).
In the first experiment, random colonies recovered from nasal washes and tissues were inoculated on BG with kanamycin, and the subsequent growth confirmed that parent or mutant strains were recovered from animals inoculated with the corresponding strains. Similarly, in the second experiment, dilutions of countable numbers of B. bronchiseptica from nasal washes and tissues were plated on BG with gentamicin and confirmed that only parent or mutant bacteria colonized the pigs inoculated with the respective strains.
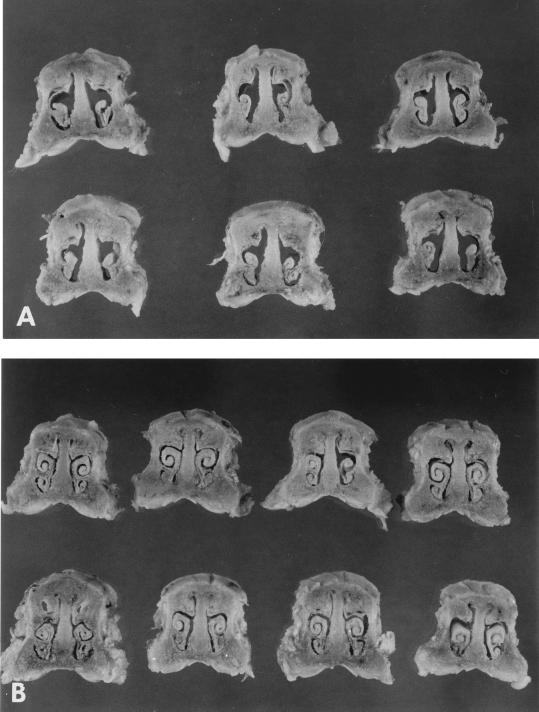
Pathology. (i) Turbinate.
At necropsy, the turbinates of pigs infected with the parent strains, B58 and KM22, showed signs of atrophy while the turbinates of pigs infected with the dnt mutants B58GP and KB24 filled the nasal cavity and were comparable to those of the noninfected control pigs (Fig. 6). The mean turbinate scores for pigs in the groups which were infected with the parent strains were statistically greater than the scores for pigs in the groups which were infected with the dnt mutant strains (Table 1). Microscopic changes in turbinates of pigs infected with the parent strains included rhinitis with variable mucopurulent intraluminal exudate, submucosal infiltrates of lymphocytes and plasma cells, and intraepithelial aggregates of neutrophils. Epithelial changes included loss of cilia, hyperplasia, and squamous metaplasia. There was resorption of bone, with an increase in the number of fibroblasts underlying the fibrocyte layer of the periosteum. Microscopic changes in the turbinates from pigs infected with the dnt mutant strains also had lesions of rhinitis with submucosal infiltrates of lymphocytes and plasma cells, intraepithelial aggregates of neutrophils, and mucopurulent intraluminal exudate, but the epithelial and bone changes were not seen.
FIG. 6.
Cross sections of snouts showing the degree of turbinate atrophy at 28 days postinfection in pigs inoculated with KM22 (A) or KB24 (B).
TABLE 1.
Mean nasal turbinate score and percentage of the lung affected by pneumonia at necropsy, 4 weeks after inoculation
| Expt | Mean turbinate score (range)a | Mean percentage of pneumoniab |
|---|---|---|
| 1 | ||
| B58 (DNT+) | 8.80 (3–13) | 14.2 |
| B58GP (DNT−) | 0.33 (0–1) | 0 |
| P | 0.011 | 0.071 |
| 2 | ||
| KM22 (DNT+) | 8.00 (8) | 16.8 |
| KB24 (DNT−) | 0.75 (0–4) | <1 |
| P | 0.00003 | 0.0031 |
Turbinate score: 0 to 2, normal; 3 to 6, mild atrophy; 7 to 10, moderate atrophy; 11 to 16, severe atrophy. See Materials and Methods for an explanation of the determination of scores.
Lung score: percentage of the lung grossly affected by pneumonia. See Materials and Methods for an explanation of the determination of the percentage.
(ii) Trachea.
Lesions were not seen in the tracheas of control pigs or pigs infected with the dnt mutants of B. bronchiseptica. Lesions were seen in the tracheas of about half of the pigs infected with the parent B. bronchiseptica strains and included variable attenuation of epithelium with loss of cilia, squamous metaplasia, and intraluminal mucopurulent exudate.
(iii) Lung.
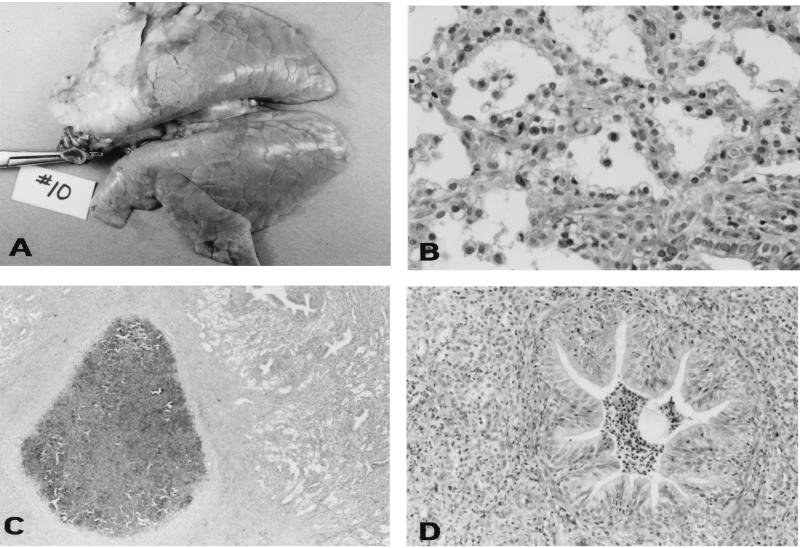
Neither gross nor microscopic lesions were seen in the lungs of any of the noninfected pigs. None of the pigs infected with the dnt mutant B58GP and only one pig infected with KB24 had gross and microscopic lesions of the lung. The one pig infected with KB24 which had lesions had a focal area of red consolidation affecting less than 2% of the lung. Microscopically there was mild histiocytic and neutrophilic pneumonitis. Of the pigs infected with the parent strains of B. bronchiseptica, four of six infected with B58 and six of six infected with KM22 had gross and microscopic pneumonia (Table 1). Grossly, the lesions appeared as firm, tan-colored consolidated lesions with a cranial ventral distribution (Fig. 7A). Microscopically, the lesions were characterized by fibroplasia with alveolar loss, alveolar spaces variably filled with macrophages and neutrophils, type II pneumocyte hyperplasia, and moderate thickening of alveolar septae with fibrin or collagen (Fig. 7B). Some sections had focal, well-demarcated, encapsulated abscesses composed of partially mineralized necrotic centers containing many necrotic neutrophils and bordered by a dense band of mature collagenous connective tissue (Fig. 7C). Bronchiolar changes included lumina variably filled with moderate numbers of neutrophils and mucus and bronchiolar epithelial hypertrophy and hyperplasia (Fig. 7D).
FIG. 7.
Pathological examination of the lungs of pigs 28 days after infection with KM22. (A) Gross photograph showing an area of tan, fibrous consolidation of the right cranial, middle, and part of the caudal lung lobes. (B) Photomicrograph showing moderate to marked thickening of the alveolar septa with fibrillar material, fibroblasts, and macrophages; marked type II pneumocyte hyperplasia and pneumocytes which contain abundant vacuolated cytoplasm; and alveolar lumina which contain variable quantities of fibrin, sloughed epithelium, and macrophages. Hematoxylin and eosin stain; 1 cm = 55 μm. (C) Photomicrograph showing abscessed lung lobule. Note the core of necrosis surrounded by a thick dense band of collagenous connective tissue. Hematoxylin and eosin stain. 1 cm = 350 μm. (D) Photomicrograph showing extensively focal obliteration of the alveolar lumina with infiltrates of large numbers of neutrophils and neutrophilic bronchiolitis. Note the infolding of the bronchiole mucosa and epithelial hyperplasia. Hematoxylin and eosin stain. 1 cm = 110 μm.
(iv) Immunohistochemistry.
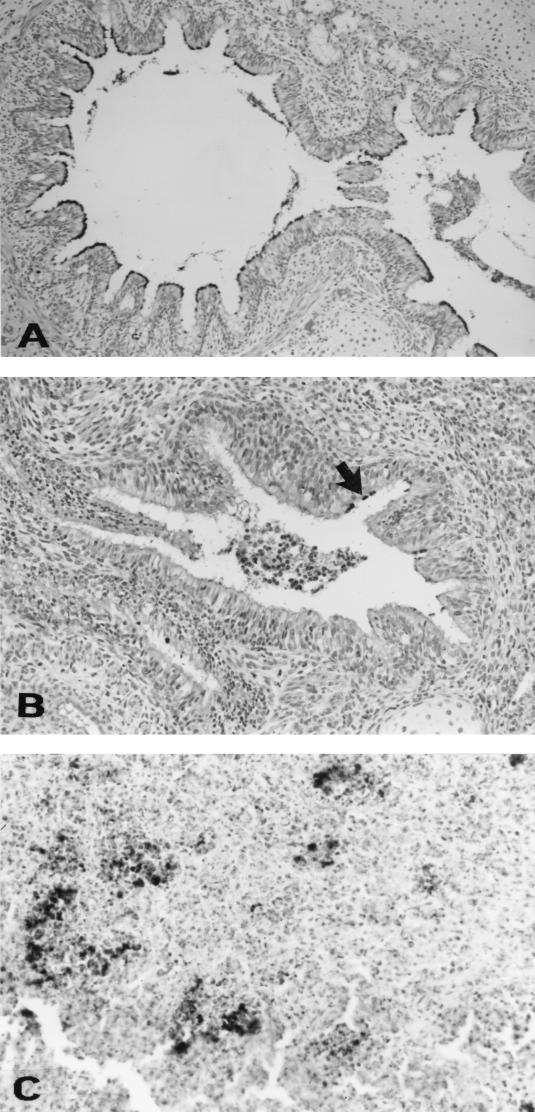
B. bronchiseptica as detected by polyclonal antiserum was present in the ciliary areas of epithelial cells in sections of turbinate, trachea, and bronchus from pigs in groups infected with KM22 and KB24. B. bronchiseptica was also detected in the abscessed areas of lungs in pigs infected with the parent strain KM22 (Fig. 8).
FIG. 8.
B. bronchiseptica immunoreactivity in the cilia of epithelial cells of a bronchus in a pig infected with KB24 (A) and in the cilia of epithelial cells of a bronchus (arrow) (B) as well as an area of abscessation of the lung (C) in a pig infected with KM22.
(v) Other lesions.
In experiment 1, a fibrinous polyserositis was seen in four of six pigs in both groups infected with B. bronchiseptica. These lesions were not seen in any of the noninfected pigs. Bacteria were not isolated from the peritoneal, pericardial, or joint fluid of the affected pigs.
DISCUSSION
Members of the genus Bordetella produce many virulence factors which are under the regulatory control of the bvg locus. One of these is DNT, an intracytoplasmic, heat-labile toxin that causes dermonecrosis when injected intradermally and has lethal, vasoconstrictive, and splenatrophic activities (6, 30). The DNT for the species B. pertussis, B. parapertussis, and B. bronchiseptica are very similar genetically and biologically (38). Purified B. bronchiseptica DNT induces mucosal damage in swine nasal tissue in vitro and causes degenerative changes of osteoblasts and the periosteum when injected into the subcutaneous tissue overlying the calvariae of neonatal rats (17, 29). Previous studies have suggested that the DNT of B. bronchiseptica may play a role in the pathogenesis of nasal turbinate atrophy and pneumonia seen in pigs infected with this organism (2, 26, 34). The results of the experiments reported here confirm that the production of DNT is necessary for the induction of these lesions in swine. Turbinate atrophy and pneumonia developed only in pigs infected with the DNT-producing parent strains.
These results do not rule out the possibility that other factors along with DNT are involved, at least indirectly, in the pathogenesis of these lesions in swine and that other factors may be responsible for lesions seen in other species. Although the dnt mutants were much reduced in their ability to cause turbinate atrophy and pneumonia, they were not completely avirulent. Pigs infected with the mutants did experience clinical signs of sneezing, coughing, and inflammation in the nasal cavity. Adhesins are most probably required to initiate infection, and other toxins may enable persistence of the bacteria in the host. The adenylate cyclase-hemolysin and pertussis toxins of B. pertussis lead indirectly to pulmonary lesions of B. pertussis in the mouse model by promoting increased colonization or inhibiting clearance (21). DNT apparently does not play an important role in virulence in the lethal mouse model, since DNT-negative strains of B. bronchiseptica and B. pertussis were as virulent as DNT-producing strains (12, 25, 39).
The increased febrile response and decreased weight gain seen in pigs infected with B. bronchiseptica in the first experiment might result from strain differences or, alternatively, might be caused by coinfection with another pathogen. No other agent was cultured from these pigs, but it is typically difficult to isolate organisms from these types of lesions. There are no reports of B. bronchiseptica causing polyserositis, and we did not see polyserositis lesions in any of the control pigs. B. bronchiseptica predisposes to infection with Streptococcus suis, which can cause lesions of polyserositis in pigs (36, 37).
Differences were observed in our experiments when the levels of parent and dnt mutant strains of B. bronchiseptica in the nasal cavity were compared. Thus, production of DNT may positively affect colonization of the upper respiratory tract. This is unlikely to be a direct effect, since DNT has not been reported to have adhesive properties, and it is possible that damage to the respiratory epithelium leads indirectly to increased colonization. One might argue that the decreased levels of colonization in the nasal cavity may partially explain the differences in atrophy induced by the parents and the mutants. This is not probable, because the highest colonization levels of the nasal cavities for pigs infected with the mutants overlapped with the lowest colonization levels for pigs infected with the parents and there were still significant differences in the amount of turbinate atrophy between pigs from the two groups with similar colonization levels.
The main lesions seen in the turbinates were inflammation (which occurred to some extent in pigs infected with the dnt mutant strains as well), epithelial changes, and fibrosis in the lamina propria and bony core, findings that are consistent with previous reports (9). Typical lesions of the lung reported here, as well as from other experiments describing lesions induced by B. bronchiseptica, are early vascular and inflammatory lesions followed by fibrosis (8). The mechanism by which DNT induces these lesions is not completely understood. B. bronchiseptica DNT affects cultured cells; in particular, it inhibits alkaline phosphatase activity and reduces the type I collagen accumulation, both of which are linked to osteoblastic differentiation in an osteoblastic cell line, MC3T3-E1. Thus, impairment of the ability of osteoprogenitor cells to differentiate may result in decreased bone formation and may at least partially explain the nasal turbinate atrophy seen (16). DNT also stimulates DNA and protein synthesis in MC3T3-E1 cells but inhibits cell division, resulting in the formation of polynucleated cells (19, 20). DNT modifies members of the Rho family of small G-proteins either by deamidation or polyamination. Either modification activates the protein, leading to aberrant activation of downstream signaling cascades (14, 27). How these actions lead to the pathology seen is as yet unclear. Inflammation and fibrosis may be due to the induction of cytokine release as the result of the altered signal transduction pathways. It would be interesting to examine whether increased production of proinflammatory cytokines, vasoactive compounds, and/or fibrosis-inducing factors, such as transforming growth factor β, occurs in response to B. bronchiseptica in general and DNT in particular.
Acknowledgments
We thank Kim Driftmier, Pamala Beery, and Don Hackbarth for technical assistance.
This work was supported, in part, by grants OTKA T025536 and BBSRC 18/CR07622.
Editor: R. N. Moore
REFERENCES
- 1.Ackermann, M. R., K. B. Register, C. Gentry-Weeks, S. M. Gwaltney, and T. Magyar. 1997. A porcine model for the evaluation of virulence of Bordetella bronchiseptica. J. Comp. Pathol. 116:55–61. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann, M. R., R. B. Rimler, and J. R. Thurston. 1991. Experimental model of atrophic rhinitis in gnotobiotic pigs. Infect. Immun. 59:3626–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arico, B., V. Scarlato, D. M. Monack, S. Falkow, and R. Rappouli. 1991. Structural and genetic analysis of the bvg locus in Bordetella species. Mol. Microbiol. 5:2481–2491. [DOI] [PubMed] [Google Scholar]
- 4.Brennan, M. J., Z. M. Li, J. L. Cowell, M. E. Bisher, A. C. Steven, P. Novotny, and C. R. Manclark. 1988. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect. Immun. 56:3189–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullock, J., S. K. Armstrong, J. L. Shear, D. P. Leis, and M. A. MeIntosh. 1990. Formation of ion channels by colicin B in planar lipid bilayers. J. Membr. Biol. 114:79–95. [DOI] [PubMed] [Google Scholar]
- 6.Cowell, J. L., E. L. Hewlett, and C. R. Manclark. 1979. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect. Immun. 25:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet, J. P., B. J. Murphy, and B. S. Tuana. 1990. Modification of a discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide gel system for minigel electrophoresis. Anal. Biochem. 190:209–211. [DOI] [PubMed] [Google Scholar]
- 8.Duncan, J. R., F. K. Ramsey, and W. P. Switzer. 1966. Pathology of experimental Bordetella bronchiseptica infection in swine: pneumonia. Am. J. Vet. Res. 27:467–472. [PubMed] [Google Scholar]
- 9.Duncan, J. R., R. F. Ross, W. P. Switzer, and F. K. Ramsey. 1966. Pathology of experimental Bordetella bronchiseptica infection in swine: atrophic rhinitis. Am. J. Vet. Res. 27:457–466. [PubMed] [Google Scholar]
- 10.Giles, C. J. 1992. Bordetellosis, p.436–445. In A. D. Leman, B. E. Straw, W. L. Mengeling, S. D’Allaire, and D. J. Taylor (ed.), Diseases of swine. Iowa State University Press, Ames.
- 11.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gueirard, P., and N. Guiso. 1993. Virulence of Bordetella bronchiseptica: role of adenylate cyclase-hemolysin. Infect. Immun. 61:4072–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewlett, E. L., V. M. Gordon, J. D. McCaffery, W. M. Sutherland, and M. C. Gray. 1989. Adenylate cyclase toxin from Bordetella pertussis: identification and purification of the holotoxin molecule. J. Biol. Chem. 264:19379–19384. [PubMed] [Google Scholar]
- 14.Horiguchi, Y., N. Inoue, M. Masuda, T. Kashimoto, J. Katahira, N. Sugimoto, and M. Matsuda. 1997. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl. Acad. Sci. USA 94:11623–11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiguchi, Y., T. Nakai, and K. Kume. 1989. Purification and characterization of Bordetella bronchiseptica dermonecrotic toxin. Microb. Pathog. 6:361–368. [DOI] [PubMed] [Google Scholar]
- 16.Horiguchi, Y., T. Nakai, and K. Kume. 1991. Effects of Bordetella bronchiseptica dermonecrotic toxin on the structure and function of osteoblastic clone MC3T3–E1 cells. Infect. Immun. 59:1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiguchi, Y., T. Okada, N. Sugimoto, Y. Morikawa, J. Katahira, and M. Matsuda. 1995. Effects of Bordetella bronchiseptica dermonecrotizing toxin on bone formation in calvaria of neonatal rats. FEMS Immunol. Med. Microbiol. 12:29–32. [DOI] [PubMed] [Google Scholar]
- 18.Horiguchi, Y., T. Senda, N. Sugimoto, J. Katahira, and M. Matsuda. 1995. Bordetella bronchiseptica dermonecrotizing toxin stimulates assembly of actin stress fibers and focal adhesions by modifying the small GTP-binding protein rho. J. Cell Sci. 108:3243–3251. [DOI] [PubMed] [Google Scholar]
- 19.Horiguchi, Y., N. Sugimoto, and M. Matsuda. 1993. Stimulation of DNA synthesis in osteoblastic-like MC3T3–E1 cells by Bordetella bronchiseptica dermonecrotic toxin. Infect. Immun. 61:3611–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiguchi, Y., N. Sugimoto, and M. Matsuda. 1994. Bordetella bronchiseptica dermonecrotizing toxin stimulates protein synthesis in an osteoblastic clone, MC3T3–E1 cells. FEMS Microbiol. Lett. 120:19–22. [DOI] [PubMed] [Google Scholar]
- 21.Khelef, N., C. M. Bachelet, B. B. Vargaftig, and N. Guiso. 1994. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect. Immun. 62:2893–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lax, A. J. 1985. Is phase variation in Bordetella caused by mutation and selection? J. Gen. Microbiol. 131:913–917. [DOI] [PubMed] [Google Scholar]
- 23.Leininger, E., P. G. Probst, M. J. Brennan, and J. G. Kenimer. 1993. Inhibition of Bordetella pertussis filamentous hemaglutinin-mediated cell adherence with monoclonal antibodies. FEMS Microbiol. Lett. 106:31–38. [DOI] [PubMed] [Google Scholar]
- 24.Magyar, T., R. Glavits, G. D. Pullinger, and A. J. Lax. 2000. The pathological effect of the Bordetella dermonecrotic toxin in mice. Acta Vet. Hung. 48:397–406. [DOI] [PubMed] [Google Scholar]
- 25.Magyar, T. 1990. Virulence and lienotoxicity of Bordetella bronchiseptica in mice. Vet. Microbiol. 25:199–207. [DOI] [PubMed] [Google Scholar]
- 26.Magyar, T., N. Chanter, A. J. Lax, J. M. Rutter, and G. A. Hall. 1988. The pathogenesis of turbinate atrophy in pigs caused by Bordetella bronchiseptica. Vet. Microbiol. 18:135–146. [DOI] [PubMed] [Google Scholar]
- 27.Masuda, M., L. Betancourt, T. Matsuzawa, T. Kashimoto, T. Takao, Y. Shimonishi, and Y. Horiguchi. 2000. Activation of Rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 19:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monack, D. M., B. Arico, R. Rappuoli, and S. Falkow. 1989. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol. Microbiol. 3:1719–1728. [DOI] [PubMed] [Google Scholar]
- 29.Nakai, T., K. Kume, H. Yoshikawa, T. Oyamada, and T. Yoshikawa. 1988. Adherence of Pasteurella multocida or Bordetella bronchiseptica to the swine nasal epithelial cell in vitro. Infect. Immun. 56:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakai, T., A. Sawata, and K. Kume. 1985. Intracellular locations of dermonecrotic toxins in Pasteurella multocida and Bordetella bronchiseptica. Am. J. Vet. Res. 46:870–874. [PubMed] [Google Scholar]
- 31.Pullinger, G. D., T. E. Adams, P. B. Mullan, T. I. Garrod, and A. J. Lax. 1996. Cloning, expression, and molecular characterization of the dermonecrotic toxin gene of Bordetella spp. Infect. Immun. 64:4163–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Register, K. B., and M. R. Ackermann. 1997. A highly adherent phenotype associated with virulent Bvg+ phase swine isolates of Bordetella bronchiseptica grown under modulating conditions. Infect. Immun. 65:5295–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Register, K. B., M. R. Ackermann, and D. W. Dyer. 1995. Nonradioactive colony lift-hybridization assay for detection of Bordetella bronchiseptica infection in swine. J. Clin. Microbiol. 33:2675–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roop, R. M., II, H. P. Veit, R. J. Sinsky, S. P. Veit, E. L. Hewlett, and E. T. Kornegay. 1987. Virulence factors of Bordetella bronchiseptica associated with the production of infectious atrophic rhinitis and pneumonia in experimentally infected neonatal swine. Infect. Immun. 55:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stibitz, S., W. Black, and S. Falkow. 1986. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene 50:133–140. [DOI] [PubMed] [Google Scholar]
- 36.Vecht, U., J. P. Arends, E. J. Van der Molen, and L. A. Leengoed. 1989. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am. J. Vet. Res. 50:1037–1043. [PubMed] [Google Scholar]
- 37.Vecht, U., H. J. Wisselink, J. E. Van Dijk, and H. E. Smith. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect. Immun. 60:550–556. [DOI] [PMC free article] [PubMed]
- 38.Walker, K. E., and A. A. Weiss. 1994. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect. Immun. 62:3817–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss, A. A., and M. St. M. Goodwin. 1989. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect. Immun. 57:3757–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]