Abstract
The sequence of the Mycobacterium leprae homologue of ESAT-6 shows only 36% amino acid correspondence to that from Mycobacterium tuberculosis. Anti-M. leprae ESAT-6 polyclonal and monoclonal antibodies and T-cell hybridomas reacted only with the homologous protein and allowed identification of the B- and T-cell epitopes. The protein is expressed in M. leprae and appears in the cell wall fraction. Thus, M. leprae ESAT-6 shows promise as a specific diagnostic agent for leprosy.
The global implementation of effective chemotherapy for leprosy has resulted in the diminution of cases from approximately 10 million in 1985 to about 800,000 today (27). However, there is no evidence as yet of any reduction in the number of new cases (28), and we know little about the transmission of leprosy or the time elapsed between infection and disease. The single greatest need from leprosy research is definitive diagnostic tools to help understand transmission and allow early detection of disease. The recent completion of the sequencing of the genomes of Mycobacterium tuberculosis (4) and Mycobacterium leprae (5) provides the opportunity to identify leprosy-specific antigens. An analogous approach applied to Mycobacterium bovis BCG allowed the identification of deleted genes and the development of antigens that can distinguish between M. tuberculosis infection and vaccination with BCG (17). Among those antigens were two low-molecular-weight M. tuberculosis culture filtrate proteins, ESAT-6 and CFP10 (2, 10), both encoded by genes in the RD1 region, a genetic segment that has been deleted from all strains of BCG. When tested together in a gamma interferon assay of peripheral blood mononuclear cells from M. tuberculosis-infected and BCG-vaccinated individuals, the sensitivity and specificity of the response were 84 and 100%, respectively, with no responses in purified protein derivative-negative individuals (1).
Although previous studies have identified a number of M. leprae proteins (7, 11, 19) and peptides (6, 26) capable of inducing gamma interferon responses in leprosy patients, a comparative analysis of the M. tuberculosis and M. leprae genomes should reveal new specific antigens, potential diagnostic and epidemiological tools for leprosy. In this report, comparative analysis of the M. leprae and M. tuberculosis ESAT-6 homologues suggests that the M. leprae product holds promise in this respect.
Comparison of the sequences of ESAT-6 from M. leprae and M. tuberculosis.
Whereas M. tuberculosis contains 14 members of the ESAT-6 family (23), the M. leprae genome shows evidence of only 4 (5, 8). A comparison of the alignment of the sequences of the 95-amino-acid (aa)-length ESAT-6 protein from M. tuberculosis (22) with its counterpart from M. leprae showed 36% homology overall (Fig. 1). Although there was identity between 9 out of 13 amino acids (69% homology) in the region bounded by aa 34 and 46, this is the only instance with more than 4 consecutive, identical amino acids. The rest of the sequence shows only one or two identical amino acids, interrupted by conserved and nonconserved stretches.
FIG. 1.
Sequence alignment of M. leprae and M. tuberculosis ESAT-6. Identical amino acids at each position are shown in bold, conservative substitutions are shown with one dot, and nonconservative substitutions are shown with a space.
Cloning and production of recombinant M. leprae ESAT-6.
The DNA sequence encoding the full-length M. leprae ESAT-6 protein (designated ML0049) (5, 8) was cloned from M. leprae genomic DNA using Vent Pfu DNA polymerase (Promega, Madison, Wis.). PCR amplification was carried out with the forward primer 5"-CATATGATACAGGCGTGGCAC-3" and reverse primer 5"-AAGCTTCCCGGTGAACATACT-3" designed to introduce NdeI and HindIII sites to the 5" and 3" ends of the open reading frame. The pBluescript vector (Stratagene, La Jolla, Calif.) was digested with the restriction endonucleases NdeI and HindIII, ligated to the PCR products, and transformed into competent Escherichia coli TOP10 cells. The esx gene was subcloned into the expression vector PET 23b(+) (Novagen, Madison, Wis.) and transformed into BL21(DE3) pLys S cells by the heat shock method. Single colonies expressing ESAT-6 were grown in Luria-Bertani medium with ampicillin and induced with isopropyl β-d-thiogalactopyranoside. Recombinant ESAT-6 (rESAT-6) found in inclusion bodies was solubilized in 8 M urea in 20 mM Tris-0.1 M NaH2PO4 (pH 8.0) buffer, loaded onto a nickel-nitrilotriacetic acid resin column, and eluted with imidazole (20). The purity of the recombinant protein was established by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (16); lipopolysaccharide content was less than 0.2 ng/mg. Recombinant M. tuberculosis ESAT-6 was prepared under similar conditions.
Antibody binding specificity of M. leprae ESAT-6.
Comparison of the sequences of ESAT-6 from M. leprae and M. tuberculosis (Fig. 1) suggested few conserved regions capable of eliciting cross-reactive antibodies. To prove the point, BALB/c mice were immunized with an emulsion containing 50 μg of either M. leprae or M. tuberculosis rESAT-6 in a 1:1 ratio of phosphate-buffered saline and incomplete Freund's adjuvant. Monoclonal antibodies (MAbs) were produced (29) using the myeloma B-cell line SP2/0 (21). MAbs 1C7.2F1 (immunoglobulin G1 [IgG1]), 2F4.2C4 (IgG2a), 7B10.2B2 (IgG1), 7G7.2A5 (IgG1), and 8C9.2B5 (IgG1) were produced as cell culture supernatant or purified over a protein G-Sepharose affinity column. The reactivity of the antisera to the homologous and heterologous proteins confirmed the lack of cross-reactivity (Fig. 2). Neither antiserum reacted to any significant degree with the heterologous antigen by enzyme-linked immunosorbent assay (ELISA) or Western blotting (Fig. 3). Overlapping peptides covering the entire M. leprae protein, and the two immunodominant M. tuberculosis ESAT-6 peptides (3) were synthesized (Table 1) and reacted with the polyclonal and monoclonal antibodies (Table 2). Polyclonal antisera specific for the entire M. leprae ESAT-6 did not react to the homologous N-terminal peptide (p1, aa 1 to 20) or to the last two C-terminal peptides (p8, aa 71 to 90; p9, aa 81 to 95) or to either of the heterologous M. tuberculosis immunodominant peptides (p1, aa 1 to 20; p6, aa 50 to 71). It reacted most intensely to p2 (aa 11 to 30), slightly more weakly to p3 through p6 (p3, aa 21 to 40; p4, aa 31 to 50; p5, aa 41 to 60; p6, aa 51 to 70), and weakly to p7 (aa 61 to 80). These data are in accord with analysis of the M. leprae ESAT-6 peptides using the Jameson-Wolf antigenic index (12) and the Kyte-Doolittle hydrophilicity plot (15). The M. tuberculosis ESAT-6-specific polyclonal sera showed no reactivity to any of the M. leprae ESAT-6 peptides but reacted well to both of the homologous ESAT-6 peptides (p1 and p6) (Table 2). Of the five MAbs produced, all of which reacted strongly to the homologous ESAT-6 as determined by Western blotting, four (1C7.2F1, 2F4.2C4, 7G7.2A5, and 8C9.2B5) recognized the single (p2) peptide, suggesting that p2 contains the dominant B-cell epitope.
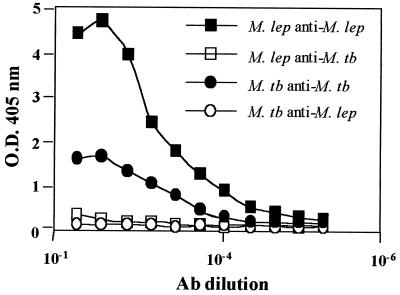
FIG. 2.
Reactivity of polyclonal anti-M. leprae and anti-M. tuberculosis rESAT-6 sera to the homologous and heterologous forms of rESAT-6 determined by ELISA. Polyclonal anti-M. leprae ESAT-6 serum had a 50% titer of 1:5,000 to the homologous protein (▪) but showed no cross-reactivity to M. tuberculosis ESAT-6 (□). Polyclonal anti-M. tuberculosis ESAT-6 serum had a 50% titer of 1:2,500 to the homologous protein (•) but showed no cross-reactivity to M. leprae ESAT-6 (○).
FIG. 3.
Western blot of M. leprae (even-numbered lanes) and M. tuberculosis (odd-numbered lanes) forms of purified rESAT-6 with monoclonal and polyclonal antibodies. Lanes 1 and 2, reaction with polyclonal antiserum raised against M. leprae rESAT-6 (1:1,000 dilution). Lanes 3 and 4, reaction with MAb 2F4.2C4 (1:20 dilution of culture supernatant) specific for the B-cell epitope p2 (aa 11 to 30) of M. leprae ESAT-6. Lanes 5 and 6, reaction with polyclonal antiserum raised against M. tuberculosis ESAT-6 (1:1,000 dilution). Lanes 7 and 8 show the equivalent amount of each protein run on a Tricine gel by silver staining. The additional band migrating at approximately 12 kDa in lanes 2 and 4 is a dimer of M. leprae rESAT-6, while lower-molecular-weight bands in lane 5 are breakdown products of M. tuberculosis rESAT-6.
TABLE 1.
Synthetic ESAT-6 peptides
| Peptide | Sequencec |
|---|---|
| M. lepraea | |
| p1 | 1MIQAWHFPALQGAVNELQGS20 |
| p2 | 11QGAVNELQGSQSRIDALLEQ30 |
| p3 | 21QSRIDALLEQCQESLTKLQS40 |
| p4 | 31CQESLTKLQSSWHGSGNESY50 |
| p5 | 41SWHGSGNESYSSVQRRFNQN60 |
| p6 | 51SSVQRRFNQNTEGINHALGD70 |
| p7 | 61TEGINHALGDLVQAINHSAE80 |
| p8 | 71LVQAINHSAETMQQTEAGVM90 |
| p9 | 81TMQQTEAGVMSMFTG95 |
| M. tuberculosisb | |
| p1 | 1MTEQQWNFAGIEAAASAIQG20 |
| p6 | 51YQGVQQKWDATATELNNALQ70 |
Overlapping peptides.
Immunogenic peptides.
Boldface type indicates amino acid identity between M. leprac and M. tuberculosis ESAT-6.
TABLE 2.
Reactivity of MAbs and polyclonal sera to M. leprae and M. tuberculosis whole ESAT-6 and peptidesa
| Antibody | Reactivity to peptide
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
M. leprae
|
M. tb.
|
rESAT-6
|
||||||||||||
| p1 | p2 | p3 | p4 | p5 | p6 | p7 | p8 | p9 | p1 | p6 | M. l. | M. tb. | ||
| 1C7.2F1 | - | ++ | - | - | - | - | - | - | - | - | - | +++ | - | |
| 2F4.2C4 | - | ++ | - | - | - | - | - | - | - | - | - | +++ | - | |
| 7B10.2B2 | - | - | - | - | - | - | - | - | - | - | - | +++ | - | |
| 7G7.2A5 | - | ++ | - | - | - | - | - | - | - | - | - | +++ | - | |
| 8C9.2B5 | - | ++ | - | - | - | - | - | - | - | - | - | +++ | - | |
| α-M. l. ESAT | - | +++ | ++ | ++ | ++ | ++ | + | - | - | - | - | +++ | - | |
| α-M. tb. ESAT | - | - | - | - | - | - | - | - | - | ++ | ++ | - | +++ | |
+++, Optical density (OD) by ELISA of >4.00; ++, OD of 1.00 to 4.00; +, OD of 0.50 to 1.00; -, no reaction, OD of <0.25. α-, anti; M. tb., M. tuberculosis; M. l., M. leprae.
Detection of native M. leprae ESAT-6 in subcellular fractions of M. leprae.
M. tuberculosis ESAT-6 is found mainly as a secreted protein in culture filtrate (22). However, M. leprae is derived from the spleens and livers of experimentally infected armadillos, rendering it very difficult to detect the protein in the tissue milieu. Instead, the subcellular fractions of M. leprae (18) were solubilized, and the proteins were subjected to Western blotting (Fig. 4). ESAT-6 was observed in the cell wall but not in the cytosol or membrane fractions.
FIG. 4.
Western blot of the native subcellular fractions of M. leprae, including cytosol (lane 1), membrane (lane 2), and cell wall (lane 3) (20 μg per lane), compared to 1 μg of rESAT-6 (lane 4), reacted with polyclonal antiserum (1:1,000 dilution) specific for whole M. leprae ESAT-6.
Identification of T-cell epitopes on M. leprae ESAT-6.
BALB/c mice (H-2d) were immunized into the hind footpad with 40 μg of M. leprae rESAT-6 in incomplete Freund's adjuvant, and lymph node cells were restimulated in vitro with dendritic cells pulsed with 0.5 μg of rESAT-6/ml, fused with the T-cell fusion partner BWα-β (25), and plated. Clones that grew in individual wells were screened using dendritic cell antigen-presenting cells with 0.5 μg of rESAT-6/well. Hybrids were tested for the production of interleukin 2 (IL-2) (13), and positive hybridomas were further tested for their response to the nine overlapping peptides (p1 to p9) of M. leprae ESAT-6. Clone 2A3 responded to peptide p4, and clones 3D8 and 6B7 responded to the C-terminal peptide, p9. None of the M. leprae ESAT-6-specific-T-cell hybridomas responded to either of the two M. tuberculosis ESAT-6 peptides, p1 or p6. Using B-cell lymphoma lines bearing either I-Ad (M12.B5), I-Ed (M12.A2) (9), or both (A20) (14), all three of the T-cell hybridomas were shown to recognize their respective peptides using the I-Ad restriction element (Table 3). In contrast, with M. tuberculosis ESAT-6, only one peptide was shown to stimulate T cells from the H-2d haplotype (BALB/c strain) mice, the N-terminal peptide (aa 1 to 20), whereas mice of H-2k and H-2a haplotypes recognized a separate internal peptide, aa 51 to 70 (3).
TABLE 3.
Presentation of peptides p4 and p9 by class II major histocompatibility complex I-Ad haplotype on APC to M. leprae ESAT-6-specific-T-cell hybridomas
| Hybridoma | Peptide | Results with APCb
|
||
|---|---|---|---|---|
| A20 (I-Ad I-Ed) | M12.B5 (I-Ad) | M12.A2 (I-Ed) | ||
| 2A3 | p4 | +a | + | - |
| 3D8 | p9 | + | + | - |
| 6B7 | p9 | + | + | - |
Positive responses indicate IL-2 production as detected by cytokine ELISA. Optimal stimulation of T-cell hybridomas with 1 μg of peptide induced >500 pg of IL-2/ml, while no peptide or inappropriate peptide controls gave background levels corresponding to <20 pg of IL-2/ml (−).
APC, antigen-presenting cells.
Conclusion.
Comparative analysis of the complete genome sequences of M. leprae and M. tuberculosis established that gene deletion and decay have resulted in the formation of 1,116 nonfunctional pseudogenes, resulting in an elimination of many key metabolic activities of M. leprae (5). Thus, M. leprae barely maintains its existence with a minimal gene set (24, 30). There are an estimated 135 functional coding sequences in the M. leprae genome that show no similarity to any known genes, and some of these, if found to actually produce a functional immunogenic protein, may be useful in the development of new epidemiological and diagnostic tools. The M. leprae version of ESAT-6, in light of its exceptional specificity, may qualify, although its promise was revealed by pursuing a different principle: proteins that share sizable correspondence to the M. tuberculosis counterpart but prove to have immunological specificity.
Acknowledgments
This work was supported by NIH NIAID contract NO1 AI-55262.
We thank John Belisle for the gift of M. tuberculosis ESAT-6 (generated through NIH NIAID contract NO1 AI-75320) and Alessandro Sette and Morten Harboe for key discussions. We thank Elisa French, Julia Granowski, and Rick Heimbichner for technical assistance and Marilyn Hein for preparation of the manuscript.
Editor: J. M. Mansfield
REFERENCES
- 1.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. V. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. M. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 2.Berthet, F.-X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, L., T. Oettinger, A. Holm, A. B. Andersen, and P. Andersen. 1996. Key epitopes on the ESAT-6 antigen recognized in mice during recall of protective immunity to Mycobacterium tuberculosis. J. Immunol. 157:3527-3533. [PubMed] [Google Scholar]
- 4.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, S. Squares, R. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 6.Dockrell, H. M., S. Brahmbhatt, B. D. Robertson, S. Britton, U. Fruth, N. Gebre, M. Hunegnaw, R. Hussain, R. Manandhar, L. Murillo, M. C. V. Pessolani, P. Roche, J. L. Salgado, E. Sampaio, F. Shahid, J. E. R. Thole, and D. B. Young. 2000. A postgenomic approach to identification of Mycobacterium leprae-specific peptides as T-cell reagents. Infect. Immun. 68:5846-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dockrell, H. M., S. K. Young, K. Britton, P. J. Brennan, B. Rivoire, M. F. R. Waters, S. B. Lucas, F. Shahid, M. Dojki, T. J. Chang, Q. Ehsan, K. P. W. J. McAdam, and R. Hussain. 1996. Induction of Th1 cytokine responses by mycobacterial antigens in leprosy. Infect. Immun. 64:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiglmeier, K., N. Honore, S. A. Woods, B. Caudron, and S. T. Cole. 1993. Use of an ordered cosmid library to deduce the genomic organization of Mycobacterium leprae. Mol. Microbiol. 7:197-206. [DOI] [PubMed] [Google Scholar]
- 9.Griffith, I. J., N. Nabavi, Z. Ghogawala, C. G. Chase, M. Rodriquez, D. J. McKean, and L. H. Glimcher. 1988. Structural mutation affecting intracellular transport and cell surface expression of murine class II molecules. J. Exp. Med. 167:541-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter, S. W., B. Rivoire, V. Mehra, B. R. Bloom, and P. J. Brennan. 1990. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265:14065-14068. [PubMed] [Google Scholar]
- 12.Jameson, B. A., and H. Wolf. 1988. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 4:181-186. [DOI] [PubMed] [Google Scholar]
- 13.Kappler, J. W., B. Skidmore, J. White, and P. Marrack. 1981. Antigen-inducible, H-2 restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J. Exp. Med. 153:1198-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, K. J., C. Kanellopoulos-Langevin, R. Merwin, D. Sachs, and R. Asofsky. 1979. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 122:549-554. [PubMed] [Google Scholar]
- 15.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques, M. A. M., S. Chitale, P. J. Brennan, and M. C. V. Pessolani. 1998. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect. Immun. 66:2625-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra, V., B. R. Bloom, A. C. Bajardi, C. L. Grisso, P. A. Dieling, D. Alland, J. Convit, X. Fan, S. H. Hunter, P. J. Brennan, T. H. Rea, and R. L. Modlin. 1992. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock protein. J. Exp. Med. 175:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porathe, J., J. Carlsson, I. Olsson, and G. Belfrage. 1975. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 258:598-599. [DOI] [PubMed] [Google Scholar]
- 21.Shulman, M., C. D. Wilde, and G. Kohler. 1978. A better cell line for making hybridomas secreting specific antibodies. Nature 276:269-270. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tekaia, F., S. V. Gordon, T. Garnier, R. Brosch, B. G. Barrell, and S. T. Cole. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79:329-342. [DOI] [PubMed] [Google Scholar]
- 24.Vissa, V., and P. J. Brennan. 2001. The genome of Mycobacterium leprae: a minimal mycobacterial gene set. Genome Biol. 2:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, J., M. Blackman, J. Bill, J. Kappler, P. Marrack, D. Gold, and W. Born. 1989. Two better cell lines for making hybridomas expressing specific T cell receptors. J. Immunol. 143:1822-1825. [PubMed] [Google Scholar]
- 26.Wilkinson, R. J., K. A. Wilkinson, S. Jurcevic, A. Hills, S. Sinha, U. Sengupta, D. N. J. Lockwood, K. Katoch, D. Altman, and J. Ivanyi. 1999. Specificity and function of immunogenic peptides from the 35-kilodalton protein of Mycobacterium leprae. Infect. Immun. 67:1501-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 1998. Elimination of leprosy as a public health problem. Wkly. Epidemiol. Rec. 73:308-311. [PubMed] [Google Scholar]
- 28.World Health Organization. 1999. Global leprosy situation. Wkly. Epidemiol. Rec. 74:313-316. [PubMed] [Google Scholar]
- 29.Yokoyama, W. M. 1997. Production of monoclonal antibodies, p. 2.5.1-2.5.17. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, unit 2.5. John Wiley & Sons, Inc., New York, N.Y.
- 30.Young, D., and B. Robertson. 2001. Genomics: leprosy—a degenerative disease of the genome. Curr. Biol. 11:R381-R383. [DOI] [PubMed] [Google Scholar]