Abstract
The efficacy of Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine against pulmonary tuberculosis (TB) varies enormously in different populations. The prevailing hypothesis attributes this variation to interactions between the vaccine and mycobacteria common in the environment, but the precise mechanism has so far not been clarified. Our study demonstrates that prior exposure to live environmental mycobacteria can result in a broad immune response that is recalled rapidly after BCG vaccination and controls the multiplication of the vaccine. In these sensitized mice, BCG elicits only a transient immune response with a low frequency of mycobacterium-specific cells and no protective immunity against TB. In contrast, the efficacy of TB subunit vaccines was unaffected by prior exposure to environmental mycobacteria. Six different isolates from soil and sputum samples from Karonga district in Northern Malawi (a region in which BCG vaccination has no effect against pulmonary TB) were investigated in the mouse model, and two strains of the Mycobacterium avium complex were found to block BCG activity completely.
Tuberculosis (TB) is one of the most prevalent causes of death from infectious diseases in the world. As is the case for many intracellular pathogens, cell-mediated immunity plays an important role in host protection against TB (25, 29). In particular, gamma interferon (IFN-γ)-secreting T cells have been shown to be important for the protective immune response (17). The only vaccine currently available against TB is the attenuated Mycobacterium bovis strain bacillus Calmette-Guérin (BCG). The efficacy of this vaccine varies from 0 to 80% in different populations, with a consistently low efficacy in many tropical regions of the world where the vaccine is most needed (15, 16, 35, 38). The reason for the failure of BCG in some populations has been a subject of debate since the 1950s, and many different hypotheses have been suggested to explain the observed variation. Some investigators have suggested that differences in the strain of BCG (23), the age at vaccination (40), or methodological differences are important factors for the variation reported (8). The most widely accepted hypothesis relates the efficacy of BCG to geographic location, with low to nondetectable levels of protection against pulmonary TB seen in tropical regions such as Africa and India, where exposure to nontuberculous mycobacteria is common (15). One exception from this general rule is the consistent high efficacy when BCG is used to vaccinate newborns. Neonatal vaccination with BCG imparts protection against the childhood manifestations of TB (in particular, meningitis) (1, 9, 24), but the efficacy wanes over a period of 10 to 15 years, and therefore it does not prevent against the later breakdown with pulmonary TB in the adult population in the third world (37).
There is convincing evidence that exposure of laboratory animals to environmental mycobacteria can provide some protection against infection with M. tuberculosis (7, 14, 20, 30, 33). The influence of such cross protection on the efficacy of subsequent BCG vaccination is not yet clarified, but based on animal experiments, it has been suggested that the protection provided by environmental mycobacteria may partly mask the effect of a subsequent BCG vaccination (33, 42) or that environmental mycobacteria have a direct antagonistic influence on subsequent BCG vaccination (34, 36). Our study demonstrates that prior sensitization with environmental mycobacteria can inhibit BCG multiplication and thereby prevent the induction of an efficient BCG-mediated immune response and protection against TB challenge. Interestingly, different species isolated from soil and sputum in Karonga, Malawi, an area in which BCG has been shown to provide no protection against TB (22), differed in their ability to inhibit BCG multiplication. In contrast, a TB subunit vaccine had the same protective effect in naive and sensitized animals.
MATERIALS AND METHODS
Animals.
These studies were performed with pathogen-free 6- to 12-week-old CBA/J and C57BL/6J female mice, purchased from Bomholtegaard, Ry, Denmark, or, in some of the experiments, purchased from Harlan UK, Ltd., Belton, England, or Harlan Interfauna Ibérica, Barcelona, Spain.
Bacteria.
Mycobacterium avium (ATCC 15769), Mycobacterium scrofulaceum (ATCC 19275), and Mycobacterium vaccae (ATCC 15483) were grown in 7H9 broth until the mid-log phase of the bacterial growth. Mycobacterium tuberculosis (Edman) was grown at 37°C on Löwenstein-Jensen medium or in suspension in modified Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose. In preparation for immunization of mice, frozen aliquots of the bacterial strains were thawed and sonicated for 5 min with a Branson 2210 ultrasonifier, and the viability of each strain was enumerated on 7H11 plates. Mycobacterium fortuitum (S78/2) and M. fortuitum (S160/5) are soil isolates from the north and south of Karonga, Malawi, respectively. We used standard decontamination of samples with 4% sodium hydroxide and culture at 37°C on nutrient agar-based medium to isolate the organisms from soil. M. fortuitum (Sp2001), Mycobacterium chelonae (Sp2015), and two strains of the M. avium complex (Sp1891) and (Sp2011) were sputum isolates from donors with suspected TB in the Karonga district. The organisms were isolated with acidified Löwenstein-Jensen medium in Malawi, and their identity was confirmed at the Mycobacterium Reference Unit, Dulwich, United Kingdom, by standard biochemical identification tests for mycobacteria. Frozen aliquots of these strains were prepared for animal inoculation as described above.
Sensitization with environmental mycobacteria.
Mice were immunized subcutaneously (s.c.) in the back three times at 2-week intervals with 2 × 106 CFU of each of three ATCC strains of environmental mycobacteria (M. avium, M. scrofulaceum, and M. vaccae). To clear the remaining mycobacteria, sensitization was followed, 3 weeks after the last inoculation, by 1 month of treatment with rifampin (Sigma; 100 mg/liter), ethambutol (Sigma; 200 mg/liter), and clarithromycin (Abbott Laboratories, Solna, Sweden; 200 mg/liter) added to the drinking water.
To assess virulence of the strains isolated from Karonga, Malawi, mice were infected with 105 CFU of each environmental mycobacterial strain in a volume of 0.2 ml of phosphate-buffered saline by intravenous (i.v.) injection via a lateral tail vein. At the appropriate time points, mice were killed (four in each group), and the organs were removed for bacterial enumeration. Whole organs were homogenized in a 0.04% Tween 80 (Sigma) solution in distilled water, serial 10-fold dilutions were plated on Middlebrook 7H10 medium at 37°C, and the numbers of CFU were determined.
Vaccinations.
A single dose of BCG Danish 1331 (5 × 104 CFU) was injected s.c. at the base of the tail. There were no significant differences in the protection obtained with doses ranging from 5 × 104 to 107 BCG bacteria (results not shown). In one experiment, an i.v dose of 5 × 106 CFU of BCG was used to determine growth of BCG in naive versus sensitized mice. For subunit vaccination, the mice were immunized s.c. three times at 2-week intervals with 10 μg (per dose) of either ESAT-6 or the Ag85B-ESAT-6 fusion protein emulsified in dioctadecylammonium bromide (DDA; 250 μg/dose; Eastman Kodak, Inc., Rochester, N.Y.) plus 25 μg of monophosphoryl lipid A (MPL; Corixa, Hamilton, Mont.) as described recently (5).
M. tuberculosis infections.
Animals were infected with approximately 100 CFU of M. tuberculosis (Edman) per lung by the aerosol route in a Glas-Col inhalation exposure system. The mice were sacrificed 6 weeks after infection, and bacterial numbers in the lung and spleen were determined as described before (5).
The protective effect of BCG or subunit vaccination was expressed as the log10 reduction of the bacterial counts compared to that in the unvaccinated control mice. All results are based on five or six animals per group.
Mycobacterial antigens.
A crude BCG antigen preparation (BCG Ag) was produced as an ammonium sulfate-precipitated culture filtrate from cultures at week 6 as described in reference 2. In one of the experiments (see Fig. 4), the BCG responses to an ammonium sulfate-precipitated extract of the cell wall were measured as described elsewhere (31). These two preparations were found to give similar responses in vitro.
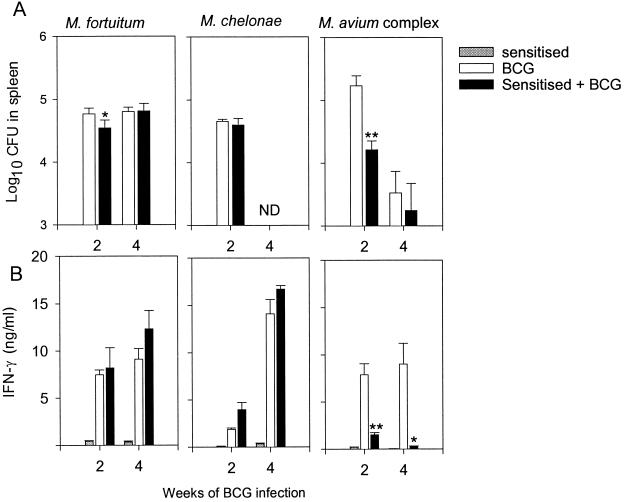
FIG. 4.
(A) Growth of BCG in sensitized and naive animals. Mice were infected s.c. with 2 × 106 CFU of either M. fortuitum, M. chelonae, or M. avium or were left untreated. After chemotherapy, mice were infected with BCG Pasteur. BCG CFU in the spleen are shown as means with standard errors (n = 4). Statistically significant differences between sensitized (solid bars) and naive (open bars) mice are indicated: ∗, P < 0.05; ∗∗, P < 0.01, according to Student's t test. ND, not done. (B) Effects of sensitization on the IFN-γ response to BCG. Spleen cells were pooled from four individual mice sensitized with the environmental strains (shaded bars), naive mice inoculated with BCG (open bars), or sensitized mice inoculated with BCG (solid bars). IFN-γ production was assessed in the supernatants of spleen cell cultures stimulated in vitro with BCG Ag and are given as means with standard errors. Statistically significant effects of sensitization on the response to BCG infection are labeled: ∗, P < 0.05; ∗∗, P < 0.01, according to Student's t test.
Protein concentration was quantified by the Micro bicinchoninic acid method (Pierce, Rockford, Ill.).
Recombinant ESAT-6 was produced as described previously (18). The LPS content was below 0.3 ng/μg of protein and had no influence on cellular activity. The fusion protein Ag85B-ESAT-6 was produced as described recently (26). The proteins were kept at −80°C until use.
Lymphocyte cultures.
Lymphocytes from spleens and blood were isolated and cultured as described previously (5). Briefly, cells from individual mice were cultured in microtiter wells (96 well; Nunc, Roskilde, Denmark) containing 2 × 105 cells in a volume of 200 μl of RPMI 1640 supplemented with 5 × 10−5 M 2-mercaptoethanol, penicillin-streptomycin, 1 mM glutamine, and 5% (vol/vol) fetal calf serum. Based on previous dose-response investigations, BCG Ag and ESAT-6 were each used at 5 μg/ml in the cultures. Phytohemagglutinin at a concentration of 1 μg/ml was used in all experiments as a positive control for cell viability. IFN-γ, interleukin 4 (IL-4), and IL-5 were detected in 72-h culture supernatants by duplicate enzyme-linked immunosorbent assay (ELISA).
Enzyme-linked immunospot (ELISPOT) analyses were conducted with cells from individual mice or, when blood was analyzed, with cells pooled from groups of mice, as described in reference 6. The detection level was 10 spots.
Statistical methods.
Because all of the data show a normal distribution, the assessment of experiments was carried out by analysis of variance. Differences between means were assessed by Dunnett's test (Tables 1 and 2) or Student's t test (see Fig. 2 and 4). A P value of <0.05 was considered significant.
TABLE 1.
Sensitization with environmental mycobacteria blocks the protective effect of BCG
| Group of micea | Result inb:
|
||||
|---|---|---|---|---|---|
| Spleen
|
Lung
|
||||
| CFUc | Log10 resistanced | CFU | Log10 resistance | ||
| Naive | 4.44 ± 0.13 | 6.34 ± 0.11 | |||
| BCG | 3.76 ± 0.16 | 0.68* | 5.21 ± 0.08 | 1.13* | |
| Sensitization | 4.36 ± 0.17 | 0.08 | 6.14 ± 0.11 | 0.20 | |
| Sensitization + BCG | 4.33 ± 0.17 | 0.11 | 6.25 ± 0.06 | 0.09 | |
Naive or sensitized mice were BCG vaccinated (5 × 104 CFU) followed by aerosol challenge with virulent M. tuberculosis.
The experiment was repeated twice with similar results.
Bacterial numbers determined by growth of individual whole-organ homogenates 6 weeks postinfection.
Protective effect expressed as the log10 reduction in bacterial loads compared to those of naive mice. Bacterial numbers significantly different (P < 0.05) from those seen in naive mice are indicated by an asterisk.
TABLE 2.
Bacterial numbers in organs of naive and sensitized mice after vaccination and aerosol challenge with virulent M. tuberculosis
| Vaccine groupa | Result in:
|
||||
|---|---|---|---|---|---|
| Lung
|
Spleen
|
||||
| Log10 CFUb | Log10 resistancec | Log10 CFU | Log10 resistance | ||
| Expt 1 | |||||
| Naive | |||||
| Control | 6.36 ± 0.08 | 4.71 ± 0.05 | |||
| BCG | 5.83 ± 0.06 | 0.53* | 4.12 ± 0.12 | 0.59* | |
| DDA-MPL | 6.34 ± 0.09 | 4.94 ± 0.12 | |||
| ESAT-6 | 5.76 ± 0.09 | 0.60* | 4.39 ± 0.09 | 0.32* | |
| Sensitized | |||||
| Control | 6.18 ± 0.08 | 4.82 ± 0.16 | |||
| BCG | 6.27 ± 0.07 | <0.05 | 4.79 ± 0.05 | <0.05 | |
| DDA-MPL | 6.39 ± 0.05 | 4.73 ± 0.11 | |||
| ESAT-6 | 5.74 ± 0.16 | 0.44* | 4.43 ± 0.05 | 0.39 | |
| Expt 2 | |||||
| Naive | |||||
| Control | 6.88 ± 0.12 | 5.10 ± 0.18 | |||
| DDA-MPL | 7.19 ± 0.05 | 5.48 ± 0.11 | |||
| Ag85B-ESAT-6 | 6.03 ± 0.12 | 0.85* | 4.40 ± 0.08 | 0.70* | |
| Sensitized | |||||
| Control | 6.30 ± 0.08 | 4.39 ± 0.09 | |||
| DDA-MPL | 6.49 ± 0.05 | 4.27 ± 0.08 | |||
| Ag85B-ESAT-6 | 5.37 ± 0.14 | 0.93* | 3.89 ± 0.07 | 0.50* | |
Naive or sensitized mice were immunized s.c. with BCG or injected three times with a subunit vaccine emulsified in DDA-MPL.
Bacterial numbers are given as log10 CFU of M. tuberculosis isolated from the lung and spleen 6 weeks after aerosol challenge with virulent M. tuberculosis.
Protective effects of the two vaccines are expressed as log10 reductions in bacterial numbers compared to those in unvaccinated control mice. Bacterial numbers significantly different from those seen in control mice are indicated by an asterisk.
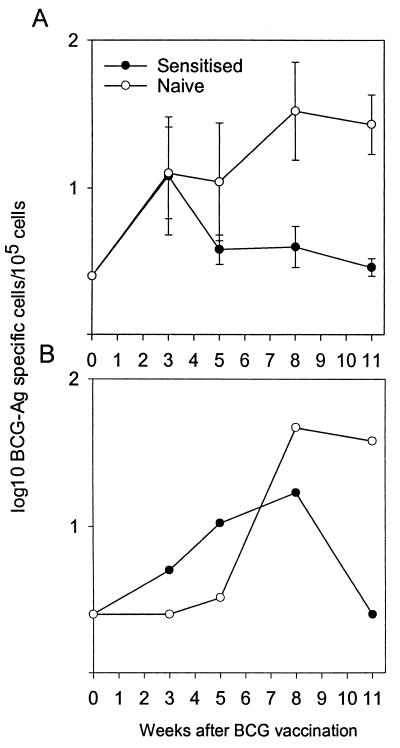
FIG. 2.
Influence of previous sensitization with environmental mycobacteria on the BCG-specific immune responses. BCG was administered s.c., and the frequencies of IFN-γ-producing cells isolated from the draining lymph nodes (A) and the blood (B) in naive mice (open symbols) and sensitized mice (solid symbols) were detected by the ELISPOT assay postvaccination after in vitro stimulation with BCG-Ag. The data presented here represent the logarithmic mean of results obtained from lymph node cells from three individual mice per group ± standard errors. The responses in the blood were analyzed on cells pooled from three animals for each time-point. A pilot experiment conducted on weeks 2, 4, and 6 supported the overall difference in the response profiles of the two groups of animals.
RESULTS
The multiplication of BCG is inhibited in mice sensitized with certain environmental mycobacteria.
We inoculated CBA/J mice s.c. three times at 2-week intervals with a mixture of the mycobacterial strains M. avium, M. scrofulaceum, and M. vaccae. These species have repeatedly been isolated from soil and water samples in tropical regions (21). Three weeks postinoculation, a low but significant mycobacterium-specific recall response was measured in the spleen, with detectable levels of IFN-γ release in response to BCG Ag. (1.26 ± 0.01 ng/ml) (data not shown). The BCG Ag preparation gave no IFN-γ release (<0.05 ng/ml) from splenocytes isolated from naive mice. No IL-4 or IL-5 was detected in any of the supernatants. Three weeks after the last inoculation with environmental mycobacteria, we subjected the mice to 4 weeks of chemotherapy to clear remaining live mycobacteria. After the end of chemotherapy treatment, no environmental mycobacteria were detected in any of the target organs (liver, spleen, and lymph nodes).
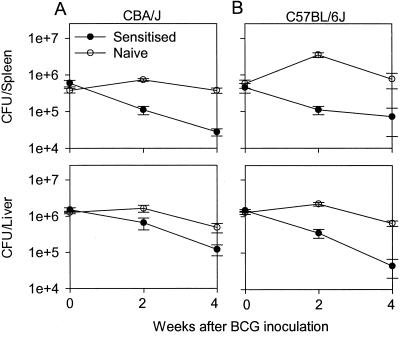
We inoculated groups of sensitized and age-matched naive CBA/J mice i.v. 1 week after the end of chemotherapy treatment with 5 × 106 BCG and monitored the growth in the spleen and liver over time. Sensitization with environmental mycobacteria resulted in inhibition of the initial multiplication of BCG in the spleen and liver (Fig. 1A). In naive mice, the initial multiplication of BCG resulted in 10- to 30-fold more bacteria in the spleen postinoculation than in sensitized mice. A difference was also seen after a conventional s.c. vaccination, although the bacterial numbers were at lower levels (data not shown). Similar data were obtained with C57BL/6J mice, which are more susceptible to BCG (11). In this strain, larger differences in BCG numbers were found between sensitized and nonsensitized mice (Fig. 1B).
FIG. 1.
BCG multiplication is inhibited in mice previously sensitized with environmental mycobacteria. (A) CBA/J mice. (B) C57BL/6J mice. The growth of BCG was compared in naive mice (open symbols) and in sensitized mice (solid symbols). The data shown are the means of BCG CFU ± standard errors. For both groups, five animals were sacrificed for each time point. The experiment was repeated twice with similar results.
Immune responses induced by BCG vaccination in sensitized and naive mice.
We continued by investigating the immune response induced by BCG in sensitized and age-matched naive control CBA/J mice. ELISPOT was used to monitor frequencies of BCG-specific T cells before and 3, 5, 8, and 11 weeks after the s.c. vaccination with BCG (Fig. 2). Before BCG vaccination, no mycobacterium-specific IFN-γ-producing T cells were detected in any of the mice. Three weeks after BCG inoculation, the number of BCG-specific IFN-γ-producing cells in the draining lymph nodes had increased and reached the same level in sensitized and in naive mice (Fig. 2A). The response in sensitized mice was, however, transient, and from 5 weeks after BCG inoculation and onwards, a higher frequency of mycobacterium-specific cells was found in naive vaccinated mice. At the termination of the experiment (week 11), a 10-times-higher frequency of BCG-specific T cells was found in the naive vaccinated group than in the sensitized vaccinated group (P = 0.032). A similar dynamic development of responses was found in the blood, although it was delayed so that higher frequencies of specific T cells were found from week 8 onwards in naive vaccinated mice (Fig. 2B). At no time point after vaccination was IL-4 or IL-5 detected in the supernatants of the stimulated cultures (results not shown).
Sensitization with environmental mycobacteria blocks the protective effect of BCG, but not a TB subunit vaccine.
We continued by vaccinating sensitized and naive age-matched control CBA/J mice 4 to 5 weeks after the end of chemotherapy-treatment, followed 2 months later by an aerosol challenge with M. tuberculosis. The mice were killed 6 weeks post-TB infection, and M. tuberculosis CFU were enumerated in the lungs and spleens. The BCG vaccine imparted appreciable protection to naive mice against the TB challenge, with significantly reduced bacterial numbers in the organs (0.68 to 1.13 log10 reduction; Table 1). Sensitization with environmental mycobacteria on its own, or followed by BCG vaccination, failed to induce a statistically significant level of protection against TB (Table 1).
We also asked if a previous sensitization with environmental mycobacteria would influence protection induced by a subunit vaccine. Groups of naive and sensitized CBA/J mice were vaccinated with BCG or injected (three times at 2-week intervals) with recently developed TB subunit vaccines based on the immunodominant antigens ESAT-6 and Ag85B mixed with a DDA-MPL adjuvant emulsion (5, 26). ESAT-6-vaccinated animals mounted a very strong recall immune response (5 to 7 ng of IFN-γ/ml) to the homologous preparation 1 week postvaccination in the blood (data not shown). The protection obtained by BCG in control mice was log 0.53, and as in the previous experiment, BCG did not protect presensitized mice (Table 2, experiment 1) The ESAT-6 subunit vaccine, in contrast, induced a similar degree of protection in both naive and sensitized mice. A subunit vaccine based on a fusion protein of Ag85B and ESAT-6 has recently been demonstrated to induce levels of protection similar to those of BCG in the mouse model (26), and this vaccine also protected against TB challenge at the same level in naive and sensitized mice (Table 2, experiment 2).
Mycobacterial species isolated in Karonga, Malawi, differ in their ability to block BCG activity.
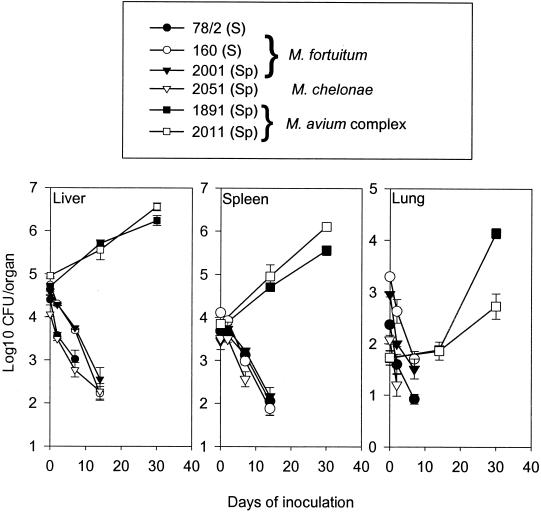
We investigated six different isolates from soil and sputum samples from Karonga District in Northern Malawi in the mouse model. Three of these isolates were typed as M. fortuitum, one was a strain of M. chelonae, and two were classified as belonging to the M. avium complex (Fig. 3). The growth of these isolates in spleen, liver, and lung was investigated with C57BL/6J mice over a period of 30 days. Most of the isolates were rapidly cleared to below the level of detection, but the strains from the M. avium complex multiplied and reached bacterial numbers 3 logs above those of M. chelonae and M. fortuitum after day 14 (Fig. 3). The mice were treated with chemotherapy, followed by an injection of BCG according to our standard protocol. BCG counts in the spleen of these mice were quantified at week 2 postinoculation (Fig. 4A). M. fortuitum and M. chelonae did not inhibit the growth of BCG, whereas bacteria from the M. avium complex reduced BCG numbers by 1 to 1.5 log (P < 0.01). This difference correlated with the immune responses induced by the BCG vaccine. There was no influence on the level of IFN-γ responses to BCG Ag by sensitization with M. chelonae or M. fortuitum, whereas the previous inoculation with bacteria from the M. avium complex completely ablated BCG immune responses (Fig. 4B). All strains, on the other hand, induced low and variable responses to antigens extracted from the homologous strain of environmental mycobacteria (results not shown).
FIG. 3.
Growth of isolates from Karonga, Malawi, in the mouse model. To evaluate the virulence of the isolates, mice were infected i.v. with M. fortuitum strains S160 (open circles), S78/2 (solid circles), and 2001 (solid triangles); M. chelonae strain 2015 (open triangles); and M. avium complex strains 1891 and 2011 (solid and open squares, respectively). The mycobacterial loads were determined in the liver, spleen, and lung at the time points indicated. M. chelonae and M. fortuitum were all below the level of detection from day 14 onwards. Data are given as means with standard errors (n = 4).
DISCUSSION
This study demonstrates that animals exposed to certain environmental mycobacteria raise an immune response that controls the multiplication of BCG, thereby curtailing the vaccine-induced immune response before it is fully developed. The finding is important for the long-held discussion on the failure of BCG vaccination against TB in some parts of the world (15, 16, 38). One hypothesis to explain the failure of BCG was presented in 1966 by Palmer and Long, based on large-scale guinea pig experiments. They argued that because contact with nontuberculous bacteria offers some level of protective immunity to TB, the protective effect of a superimposed BCG vaccine would be masked (33). The present study confirms the classical observation that priming with environmental mycobacteria promotes some levels of protective immunity to other mycobacteria (7, 10, 14, 33), in this case to BCG. However, this effect was not sufficient to significantly reduce the growth of M. tuberculosis, which multiplied at an almost unchanged rate in these sensitized animals. The difference from the partial protection imparted by environmental mycobacteria in the guinea pig model (14, 33) may be related to the fact that the earlier studies made no effort to clear the environmental mycobacteria by chemotherapy before challenge with M. tuberculosis, as well as the different genetic makeup and susceptibility of mice versus guinea pigs. The differences in these models and their relevance to human disease are the subject of an ongoing study.
That prior sensitization to environmental mycobacteria interferes in a similar way with human BCG vaccination is strongly suggested by a number of classical epidemiological observations: (i) the finding of strong efficacy of BCG in trials in which tuberculin skin test-positive (and therefore sensitized) donors have been vigorously excluded (19); (ii) the consistent success with BCG in neonates vaccinated before any significant sensitization from environmental mycobacteria occurs (1, 9, 24); and, (iii) finally, the observation of a lower rate of skin test conversion, much smaller average diameter, and rapidly waning responses after BCG vaccination in areas with environmental sensitization (India and Egypt), compared with those in areas with minimal environmental exposure (Denmark) (4, 32). This observation was recently confirmed and extended by the observation of only minimal in vitro IFN-γ responses to purified protein derivative (PPD) induced by BCG vaccination in donors from Karonga, Malawi, compared to those from the United Kingdom (P. E. Fine and H. Dockrell, personal communication). Taken together, these findings are in agreement with the low and transient immune response in the group of animals sensitized with environmental mycobacteria before vaccination, whereas the naive animals developed strong and sustained responses (Fig. 3). Our experimental model is therefore relevant to the many tropical regions where BCG is not protective against pulmonary TB and where the high incidence of TB indicates that any partial protection provided by exposure to environmental mycobacteria is insufficient for the prevention of TB.
Our main conclusion is that BCG, as a live vaccine, is particularly sensitive to the influence of preexisting immune responses to antigens shared with certain environmental strains. In this regard, a recent study has demonstrated the cross-recognition of a large number of antigens shared between M. avium and BCG (T. Pais and R. Appelberg, unpublished results). Multiplication is a precondition for the induction of immunity by BCG and killing of BCG by chemotherapy after administration has been demonstrated to abrogate subsequent immunity completely (13, 39). In the present study, this blocking is achieved by immunological control instead of chemotherapy, but the outcome in both cases is interference with the protective immune response, which would normally develop in response to the growing BCG. The requirement for BCG multiplication can be explained as a simple consequence of dosage, but more likely is due to the fact that only live BCG secretes many antigens of importance for the induction of a protective immune response (3, 28). Interestingly, our data from the animal model also suggest that only environmental strains, which are capable of an initial multiplication in the host, block the activity of BCG. A detailed evaluation of a large number of different soil isolates from Karonga, Malawi, and of their interactions with BCG is ongoing. In the future, information on the geographical distribution of such strains would be a valuable resource when trying to understand the huge variation in BCG efficacy in human trials.
This inhibitory effect of the environmental mycobacteria on the growth and activity of BCG provides an important argument in the ongoing discussion of live attenuated vaccines versus nonviable subunit vaccines against TB (12, 27, 44). In comparison with live attenuated vaccines, the present study suggests that subunit vaccines may be much less influenced by prior contact with environmental mycobacteria. As mentioned above, neonatal BCG vaccination consistently imparts protection against the childhood manifestations of TB (mostly extrapulmonary disease), but as its efficacy wanes over a period of 10 to 15 years (37), the adult pulmonary manifestations of TB are prevented neither by neonatal vaccination, by vaccination in adolescence after exposure to environmental mycobacteria (41), nor by a BCG revaccination strategy (22, 43). A TB subunit vaccine could therefore fulfill the criterion of having consistently high efficacy in different populations and may have a particularly important use for revaccination of third world children in adolescence.
Acknowledgments
This study has been supported by the Danish Research Council and The European Commission (contract no. 18CT970254). Lise Brandt is supported by the Faculty of Health Science, University of Copenhagen.
Environmental mycobacteria from Malawi were isolated within the context of the Karonga Prevention Study (KPS) with the assistance of H. Phiri, S. Chagulkuka, and G. Black and were classified by M. Yates at the U.K. Mycobacterium Reference laboratory in Dulwich. The KPS is coordinated by Paul Fine and supported by The Wellcome Trust.
Paul Fine is thanked for valuable discussion, advice, and helpful comments on the manuscript. We thank Vita Skov, Lene Rasmussen, and Tina Lerche for excellent technical assistance.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Al-Kassimi, F. A., M. S. Al-Hajjaj, I. O. Al-Orainey, and E. A. Bamgboye. 1995. Does the protective effect of neonatal BCG correlate with vaccine-induced tuberculin reaction? Am. J. Respir. Crit. Care Med. 152:1575-1578. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, A. B., Z.-L. Yuan, K. Hasløv, B. Vergmann, and J. Bennedsen. 1986. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J. Clin. Microbiol. 23:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, P. 1997. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand. J. Immunol. 45:115-131. [DOI] [PubMed] [Google Scholar]
- 4.Baily, G. V. 1980. Tuberculosis prevention trial, Madras. Indian J. Med. Res. 72:1-74. [PubMed] [Google Scholar]
- 5.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt, L., T. Oettinger, A. Holm, and P. Andersen. 1996. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J. Immunol. 157:3527-3533. [PubMed] [Google Scholar]
- 7.Brown, C. A., I. N. Brown, and S. Swinburne. 1985. The effect of oral Mycobacterium vaccae on subsequent responses of mice to BCG sensitization. Tubercle 66:251-260. [DOI] [PubMed] [Google Scholar]
- 8.Clemens, J. D., J. J. Chuong, and A. R. Feinstein. 1983. The BCG controversy. A methodological and statistical reappraisal. JAMA 249:2362-2369. [PubMed] [Google Scholar]
- 9.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 10.Collins, F. M. 1971. Immunogenicity of various mycobacteria and the corresponding levels of cross-protection developed between species. Infect. Immun. 4:688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis, M., A. Forget, M. Pelletier, R. Turcotte, and E. Skamene. 1986. Control of the Bcg gene of early resistance in mice to infections with BCG substrains and atypical mycobacteria. Clin. Exp. Immunol. 63:517-525. [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty, T. M., and P. Andersen. 2000. Tuberculosis vaccines: developmental work and the future. Curr. Opin. Pulm. Med. 6:203-208. [DOI] [PubMed] [Google Scholar]
- 13.Dworski, M. 1973. Efficacy of bacillus Calmette-Guérin and isoniazid-resistant bacillus Calmette-Guérin with and without isoniazid chemoprophylaxis from day of vaccination. Am. Rev. Respir. Dis. 108:294-300. [DOI] [PubMed] [Google Scholar]
- 14.Edwards, M. L., J. M. Goodrich, D. Muller, A. Pollack, J. E. Ziegler, and D. W. Smith. 1982. Infection with Mycobacterium avium-intracellulare and the protective effects of Bacille Calmette-Guerin. J. Infect. Dis. 145:733-741. [DOI] [PubMed] [Google Scholar]
- 15.Fine, P. E. 1989. The BCG story: lessons from the past and implications for the future. Rev. Infect. Dis. 11(Suppl. 2):S353-S359. [DOI] [PubMed] [Google Scholar]
- 16.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 17.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harboe, M., A. S. Malin, H. S. Dockrell, H. G. Wiker, G. Ulvund, A. Holm, M. C. Jørgensen, and P. Andersen. 1998. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect. Immun. 66:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart, P. D., and I. Sutherland. 1977. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br. Med. J. 2:293-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamala, T., C. N. Paramasivan, D. Herbert, P. Venkatesan, and R. Prabhakar. 1996. Immune response & modulation of immune response induced in the guinea-pigs by Mycobacterium avium complex (MAC) & M. fortuitum complex isolates from different sources in the South Indian BCG trial area. Indian J. Med. Res. 103:201-211. [PubMed] [Google Scholar]
- 21.Kamala, T., C. N. Paramasivan, D. Herbert, P. Venkatesan, and R. Prabhakar. 1994. Isolation and identification of environmental mycobacteria in the Mycobacterium bovis BCG trial area of South India. Appl. Environ. Microbiol. 60:2180-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karonga Prevention Trial Group. 1996. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 23.Lagranderie, M. R., A. M. Balazuc, E. Deriaud, C. D. Leclerc, and M. Gheorghiu. 1996. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect. Immun. 64:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miceli, I., I. N. de Kantor, D. Colaiacovo, G. Peluffo, I. Cutillo, R. Gorra, R. Botta, S. Hom, and H. G. ten Dam. 1988. Evaluation of the effectiveness of BCG vaccination using the case-control method in Buenos Aires, Argentina. Int. J. Epidemiol. 17:629-634. [DOI] [PubMed] [Google Scholar]
- 25.North, R. J. 1973. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 7:166-176. [DOI] [PubMed] [Google Scholar]
- 26.Olsen, A. W., L. A. H. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme, I. M. 1997. Progress in the development of new vaccines against tuberculosis. Int. J. Tuberc. Lung Dis. 1:95-100. [PubMed] [Google Scholar]
- 28.Orme, I. M., P. Andersen, and W. H. Bloom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167:1481-1497. [DOI] [PubMed] [Google Scholar]
- 29.Orme, I. M., E. S. Miller, A. D. Roberts, S. K. Furney, J. P. Griffin, K. M. Dobos, D. Chi, B. Rivoire, and P. J. Brennan. 1992. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J. Immunol. 148:189-196. [PubMed] [Google Scholar]
- 30.Orme, I. M., A. R. Roberts, and F. M. Collins. 1986. Lack of evidence for a reduction in the efficacy of subcutaneous BCG vaccination in mice infected with nontuberculous mycobacteria. Tubercle 67:41-46. [DOI] [PubMed] [Google Scholar]
- 31.Pais, T. F., R. A. Silva, B. Smedegaard, R. Appelberg, and P. Andersen. 1998. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunology 95:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer, C. E. 1952. BCG vaccination and tuberculin allergy. Lancet May 10:935-941. [DOI] [PubMed]
- 33.Palmer, C. E., and M. W. Long. 1966. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 94:553-568. [DOI] [PubMed] [Google Scholar]
- 34.Rook, G. A., G. M. Bahr, and J. L. Stanford. 1981. The effect of two distinct forms of cell-mediated response to mycobacteria on the protective efficacy of BCG. Tubercle 62:63-68. [DOI] [PubMed] [Google Scholar]
- 35.Smith, D. W., E. H. Wiegeshaus, and M. L. Edwards. 1988. The protective effects of BCG vaccination against tuberculosis, p. 341-370. In M. Bendinelli and H. Friedman (ed.), Mycobacterium tuberculosis. Plenum Publishing Corporation, New York, N.Y.
- 36.Stanford, J. L., M. J. Shield, and G. A. Rook. 1981. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle 62:55-62. [DOI] [PubMed] [Google Scholar]
- 37.Sterne, J. A., L. C. Rodrigues, and I. N. Guedes. 1998. Does the efficacy of BCG decline with time since vaccination? Int. J. Tuberc. Lung Dis. 2:200-207. [PubMed] [Google Scholar]
- 38.ten-Dam, H. G. 1984. Research on BCG vaccination. Adv. Tuberc. Res. 21:79-106. [PubMed] [Google Scholar]
- 39.Toyohara, M., S. Kudoh, and Y. Obayashi. 1959. Studies on the effect of isoniazid upon the antituberculous immunity induced by BCG vaccination. Tubercle 40:184-191. [DOI] [PubMed] [Google Scholar]
- 40.Tripathy, S. P. 1983. The case for BCG. Ann. N. Y. Acad. Med. Sci. 19:11-21. [PubMed] [Google Scholar]
- 41.Tuberculosis Research Centre (ICMR). 1999. Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention, Chennai. Indian J. Med. Res. 110:56-69. [PubMed] [Google Scholar]
- 42.Weiszfeiler, J. C., and V. Karasseva. 1981. Mixed mycobacterial infections. Rev. Infect. Dis. 3:1081-1083. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 1995. W.H.O. news and activities. W.H.O. Bull. OMS 73:805-807. [Google Scholar]
- 44.Young, D. B. 2000. Current tuberculosis vaccine development. Clin. Infect. Dis. 30:S254-S256. [DOI] [PubMed] [Google Scholar]