Abstract
Antimicrobial peptides are highly conserved evolutionarily and are thought to play an important role in innate immunity at intestinal mucosal surfaces. To better understand the role of the antimicrobial peptide human cathelicidin LL-37/human cationic antimicrobial protein 18 (hCAP18) in intestinal mucosal defense, we characterized the regulated expression and production of this peptide by human intestinal epithelium. LL-37/hCAP18 is shown to be expressed within epithelial cells located at the surface and upper crypts of normal human colon. Little or no expression was seen within the deeper colon crypts or within epithelial cells of the small intestine. Paralleling its expression in more differentiated epithelial cells in vivo, LL-37/hCAP18 mRNA and protein expression was upregulated in spontaneously differentiating Caco-2 human colon epithelial cells and in HCA-7 human colon epithelial cells treated with the cell differentiation-inducing agent sodium butyrate. LL-37/hCAP18 expression by colon epithelium does not require commensal bacteria, since LL-37/hCAP18 is produced with a similar expression pattern by epithelial cells in human colon xenografts that lack a luminal microflora. LL-37/hCAP18 mRNA was not upregulated in response to tumor necrosis factor alpha, interleukin 1α (IL-1α), gamma interferon, lipopolysaccharide, or IL-6, nor did the expression patterns and levels of LL-37/hCAP18 in the epithelium of the normal and inflamed colon differ. On the other hand, infection of HCA-7 cells with Salmonella enterica serovar Dublin or enteroinvasive Escherichia coli modestly upregulated LL-37/hCAP18 mRNA expression. We conclude that differentiated human colon epithelium expresses LL-37/hCAP18 as part of its repertoire of innate defense molecules and that the distribution and regulated expression of LL-37/hCAP18 in the colon differs markedly from that of other enteric antimicrobial peptides, such as defensins.
Antimicrobial peptides and proteins play an important role in innate host defense, and this may be particularly important at mucosal surfaces that form the initial barrier between the host and the external environment (3, 18). Cathelicidins are a family of antimicrobial peptides found in many mammalian species (20). The cathelicidins contain a conserved cathelin domain and a C-terminal domain that varies among species, yielding multiple different antimicrobial peptides (22). LL-37 is the only cathelicidin described thus far in humans. Like other cathelicidins, LL-37 is synthesized as a precursor, termed human cationic antimicrobial protein 18 (hCAP18). The precursor is cleaved to the mature peptide LL-37 by a serine protease, protease-3, in neutrophils after exocytosis (45), although it is not known whether the same processing mechanism operates in other cells.
LL-37, alone and in synergy with other antimicrobial proteins (e.g., lactoferrin and lysozyme), has a broad range of bactericidal activities against gram-positive and gram-negative bacteria (2, 4, 47). In addition, LL-37 can bind to and neutralize bacterial lipopolysaccharide (LPS) (31, 32) and is chemotactic for human peripheral monocytes, neutrophils, and CD4 T lymphocytes (1, 49). LL-37 has a linear amphipathic α-helical structure that is important for its antimicrobial activity (26, 40), and in this respect it differs markedly from human α- and β-defensins, which have three disulfide bonds and a β-sheet structure. LL-37/hCAP18 was initially found in specific neutrophil granules (9, 21, 44) but is now known to also be expressed by other leukocytes (1), as well as keratinocytes (15) and epithelial cells in the respiratory tract, urogenital tract, and gastrointestinal tract (4, 16).
Emerging evidence suggests that the distribution and regulation of different antimicrobial peptides differ in the gastrointestinal tract (17), suggesting that they occupy distinct functional niches in mucosal host defense. For example, α-defensins in human intestinal epithelium are expressed strictly by Paneth cells in the crypt region of the small intestine (27, 41) and can be aberrantly produced in metaplastic Paneth cells in the colons of patients with ulcerative colitis (10). In contrast, human β-defensins (hBD) are ubiquitously expressed by intestinal epithelium throughout the large and small intestine, with hBD-1 being constitutively expressed and hBD-2 being inducibly expressed in response to proinflammatory stimuli that activate the transcription factor nuclear factor κB (NF-κB) (38, 39).
Little is known about the distribution and regulated production of LL-37/hCAP18 within the intestinal mucosa, although earlier studies noted LL-37/hCAP18 mRNA and protein expression in the human colon and LL-37/hCAP18 mRNA in small intestinal epithelium (4, 25). The gene encoding LL-37/hCAP18, termed CAMP, contains potential binding sites for transcription factors activated by interleukin 6 (IL-6). Consistent with this, LL-37/hCAP18 expression appears to be regulated by IL-6 in some epithelial tissues (16). Moreover, LL-37/hCAP18 is upregulated in keratinocytes in response to inflammatory stimuli (15). However, a recent study reported that infection of intestinal epithelial cells with Shigella spp., which upregulates the epithelial cell production of proinflammatory mediators (14, 29), may actually downregulate epithelial cell LL-37/hCAP18 expression (25).
To better understand the role of LL-37/hCAP18 in intestinal mucosal defense, in the present study we characterized the regulated expression and production of this peptide by human intestinal epithelium. We report here that the expression of LL-37/hCAP18 by human colon epithelial cells coincides with epithelial cell differentiation and is not regulated by proinflammatory signals. These data suggest that cell differentiation is a key determinant of cathelicidin LL-37/hCAP18 expression by human colon epithelium.
MATERIALS AND METHODS
Cytokines, bacteria, and antibodies.
Recombinant human IL-1α, IL-6, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) were obtained from Peprotech, Rocky Hill, N.J. Sodium butyrate and bacterial LPS (Escherichia coli O111:B4) were obtained from Sigma Chemical Co., St. Louis, Mo. Phorbol 12-myristate 13-acetate (PMA) was obtained from Calbiochem, La Jolla, Calif. The following bacteria were used in these studies: Salmonella enterica serovar Dublin strain Lane (28), enteroinvasive E. coli (serotype O29:NM; ATCC 43892), Shigella dysenteriae (14), and Shigella flexneri M90T (kindly provided by D. Guiney). Rabbit immunoglobulin G (IgG) anti-LL-37 polyclonal antibody was obtained from B. Agerberth and G. H. Gudmundsson. This antibody recognizes both free LL-37 and its proform, hCAP18 (1, 25).
Human colon epithelial cell lines.
The human colon adenocarcinoma cell lines HCA-7 colony 29 (a kind gift from S. C. Kirkland), HT-29 (ATCC HTB 38), and Caco-2 (ATCC HTB 37) were grown in Dulbecco's modified Eagle medium supplemented with 10 or 15% (Caco-2) heat-inactivated fetal calf serum and 2 mM l-glutamine. The cells were maintained in 95% air-5% CO2 at 37°C. Cells in six-well tissue culture plates were stimulated with IL-1α (20 ng/ml), IL-6 (20 ng/ml), TNF-α (20 ng/ml), IFN-γ (40 ng/ml), PMA (100 ng/ml), or LPS (10 μg/ml) for 6 h. For bacterial infections, cells were cultured in serum-free Dulbecco's modified Eagle medium with 1 × 108 CFU of Salmonella serovar Dublin cells, 5 × 108 CFU of enteroinvasive E. coli cells, or 4 × 108 CFU of S. flexneri or S. dysenteriae cells for 1 h, after which the bacteria were removed by washing and 50 μg of gentamicin/ml was added to kill any remaining extracellular bacteria (28).
To induce cell differentiation, HCA-7, HT-29, or Caco-2 cells were treated with 2 mM n-butyrate for up to 7 days (24). Alternatively, Caco-2 cells were cultured for 21 days as confluent monolayers to induce spontaneous differentiation (6, 48). Alkaline phosphatase (AP) activity was determined with a commercial kit (Sigma Chemical Co.). Briefly, lysis buffer (0.1% Triton X-100, 1 mM EDTA, 150 mM NaCl, and 20 mM Tris-HCl [pH 7.5]) was added to the confluent plates, after which cells were removed, transferred to a centrifuge tube, and disrupted by ultrasonication. AP activity in the cell lysates was measured using p-nitrophenylphosphate as a substrate. The results were calculated as milliunits per milligram of protein and expressed as changes in activity relative to levels at day zero. AP activity on day zero was 0.05 ± 0.01 and 2.75 ± 0.52 mU/mg of protein for HCA-7 and Caco-2 cells, respectively. One unit was defined as the enzymatic activity that hydrolyzed 1 μmol of substrate per min at 37°C. Protein in the cell lysates was assayed by the Bradford method (Bio-Rad [Hercules, Calif.] protein kit). The results are expressed as means ± standard deviations (SD).
RNA extraction and reverse transcription (RT)-PCR analysis.
Total cellular RNA was extracted from cells by an acid guanidinium-phenol-chloroform method (TRIzol reagent; GIBCO-BRL Life Technologies, Grand Island, N.Y.). Five micrograms of total cellular RNA was reverse transcribed at 37°C for 70 min in a 20-μl volume containing 2.5 U of Superscript-II reverse transcriptase (GIBCO-BRL); 10 mM dithiothreitol; 1 mM (each) dATP, dTTP, dCTP, and dGTP; and 5 μg of oligo-dT primer (Pharmacia, Piscataway, N.J.)/ml. Reactions were stopped by heat inactivation at 85°C for 10 min. Two microliters of each cDNA sample from the reverse transcription reactions was amplified by PCR in 50 μl containing 1.5 mM MgCl2; 0.2 mM (each) dATP, dTTP, dCTP, and dGTP; 1.25 U of Taq polymerase (GIBCO-BRL); and 12.5 pmol of each of the following primers: 5"-AGG ATT GTG ACT TCA AGA AGG ACG-3" (sense) and 5"-GTT TAT TTC TCA GAG CCC AGA AGC-3" (antisense) for LL-37/hCAP18. After a hot start, the amplification profile was 35 cycles of 1 min of denaturation at 94°C and 2.5 min of annealing and extension at 66°C for LL-37/hCAP18. The size of the correct PCR products was 276 bp. Primers and PCR conditions for hBD-2, CCL9/Mig, and β-actin were described previously (13, 28, 39).
Real-time PCR.
Real-time PCR was performed using the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, Calif.). Each reaction mixture contained 12.5 μl of 2× SYBR Green Master Mix (containing 200 μM [each] dATP, dGTP, and dCTP; 400 μM dUTP; 2 mM MgCl2; 0.125 U of uracil N-glycosylase; and 0.313 U of Amplitaq Gold DNA polymerase) (PE Biosystems), 6.25 pmol of each sense and antisense primer, and 1 μl of cDNA in a final volume of 25 μl. The LL-37/hCAP18 primers were those described above. The β-actin primers were as follows: sense primer, 5"-CAA AGA CCT GTA CGC CAA CAC-3"; antisense primer 5"-CAT ACT CCT GCT TGC TGA TCC-3". The reaction mixtures were incubated at 50°C for 2 min followed by 95°C for 10 min to activate the Amplitaq Gold DNA polymerase. The amplification profile was 15 s of denaturation at 95°C followed by 1 min of annealing at 66°C for LL-37/hCAP18 and 60°C for β-actin, for a total of 40 cycles. The size of the correct PCR products was 218 bp for β-actin. Amplification of the expected single products was confirmed on 1% agarose gels and by staining with ethidium bromide. Data analysis was done with Sequence Detection System software (PE Biosystems) provided by the manufacturer. ΔRn was calculated using the equation ΔRn = Rn+ − Rn−, where Rn+ is the fluorescence signal of the product and Rn− is the fluorescence signal of the baseline emission. Ct is the cycle number at which ΔRn crosses the threshold. Fold changes in LL-37/hCAP18 mRNA expression were determined as 2ΔCt, where ΔCt = (CtLL-37/hCAP18 control − CtLL-37/hCAP18 stimulated) − (Ctβ-actin control−Ctβ-actin stimulated). The results are expressed as means ± SD.
Human fetal colon xenografts.
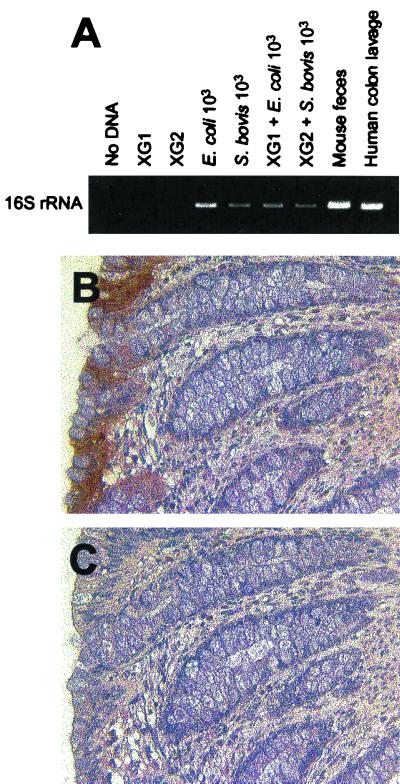
The human intestinal xenograft model was as described previously (33, 43). Briefly, human fetal colon (obtained from Advanced Biosciences Resources, Alameda, Calif.), gestational age of 16 to 20 weeks, was transplanted subcutaneously onto the backs of C57BL/6 mice with severe-combined immunodeficiency (SCID). The human fetal colon xenografts were allowed to develop for 10 weeks after implantation, at which time the epithelium, which is strictly of human origin, is fully differentiated (43). Xenograft tissue was removed and used for immunohistochemical analysis of LL-37/hCAP18. To analyze the presence of luminal bacteria, total DNA was extracted from the xenograft luminal contents (≈25 mg of a total of ≈400 mg of luminal contents per xenograft), mouse feces (10 mg), or human colon lavage fluid (10 μl) using a commercial kit (QIAamp DNA kit; Qiagen) according to the manufacturer's instructions and resuspended in a 100-μl volume. Five microliters of DNA from xenograft luminal contents or 2 μl from mouse feces and human colon lavage fluid were amplified by PCR using the following universal primers targeting the bacterial 16S rRNA gene: 5"-AAC TGG AGG AAG GTG GGG AT-3" (sense) and 5"-AGG AGG TGA TCC AAC CGC A-3" (antisense) (19). After a hot start, the amplification profile was 35 cycles of 1 min of denaturation at 94°C and 2.5 min of annealing and extension at 65°C. The size of the correct PCR products was 371 bp. As positive controls, known numbers (101 to 103 CFU) of E. coli O29:NM or Streptococcus bovis were added to sterile phosphate-buffered saline (PBS) or xenograft luminal contents and then submitted to DNA isolation and PCR analysis as described above.
Immunohistochemistry.
Confluent HCA-7 cells on coverslips were cultured with or without 2 mM butyrate for 5 days. The cells were fixed for 10 min in 1% zinc sulfate-10% formaldehyde and then blocked for 45 min with 1% bovine serum albumin (BSA), 0.2% nonfat dry milk, and 0.1% Tween 20 in PBS. The cells were incubated overnight at 4°C with 1 μg of rabbit IgG anti-LL-37/ml or an identical concentration of control rabbit IgG (Jackson Immunoresearch, West Grove, Pa.). The antibodies were diluted in PBS containing 1% BSA and 0.1% Tween 20. The cells were washed twice in PBS and then incubated for 1 h at room temperature with Cy3-conjugated goat anti-rabbit IgG in the same dilution buffer. After further washing, the nuclei were counterstained with 2 μg of Hoechst 33258 dye/ml.
Endoscopic-biopsy specimens were obtained from normal human colon and duodenum. Additional endoscopic-biopsy specimens were obtained from both normal-appearing and inflamed human colon from patients with ulcerative colitis. Surgical specimens of human colon were obtained from patients undergoing partial colectomy for colon cancer. The tissue specimens were fixed in 1% zinc sulfate-10% formaldehyde and embedded in paraffin. Sections (5 μm thick) were deparaffinized, rehydrated, and treated with 0.3% H2O2 in PBS for 20 min at room temperature to block endogenous peroxidase activity. The sections were incubated with 2% goat serum-2% IgG-free and protease-free BSA for 30 min at room temperature and then incubated overnight at 4°C with 1 μg of rabbit anti-LL-37 IgG/ml or an identical concentration of control rabbit IgG. Specific binding of primary antibody was detected with biotin-labeled goat anti-rabbit IgG (1:100 dilution; Jackson Immunoresearch) followed by streptavidin-horseradish peroxidase (DAKO, Carpenteria, Calif.), visualized with 3,3"-diaminobenzidine (Sigma), and counterstained with hematoxylin. Preabsorption of rabbit IgG anti-LL-37 with LL-37 removed all immunostaining activity (15, 25). The studies were approved by the University of California, San Diego, Committee on Human Subjects.
Bactericidal assays.
Aliquots (2,500 CFU) of exponentially growing Salmonella serovar Dublin or enteroinvasive E. coli O29 were resuspended in 25 μl of HEPES (25 mM; pH 7.5)-isotonic sucrose (9%) or PBS containing 0 to 32 μM synthetic LL-37 peptide (a gift from G. H. Gudmundsson). After incubation at 37°C for 2 h, the suspensions were spread on Luria-Bertani agar plates and incubated overnight at 37°C before the CFU were counted. The 50% effective concentration (EC50) was defined as the concentration of LL-37 that decreased CFU counts by 50% relative to the peptide-free control.
RESULTS
LL-37/hCAP18 expression by normal human colon epithelium.
To characterize the expression of LL-37/hCAP18 in human intestinal epithelium in vivo, sections from normal human colon were immunostained using an LL-37/hCAP18-specific antibody. Specimens from four different individuals were immunostained, and representative results, which were similar in all individuals, are shown in Fig. 1. LL-37/hCAP18 was predominantly expressed in the surface epithelium and adjacent upper crypts in normal human colon (Fig. 1A). In contrast, LL-37/hCAP18 staining was much weaker in the deeper crypt epithelium. Furthermore, little or no expression of LL-37/hCAP18 was detected in the villus or crypt epithelium in the proximal small intestine (Fig. 1C), although LL-37/hCAP18 staining was seen in some samples in duodenal Brunner's glands (Fig. 1E). Similar results were obtained in three additional colon and three proximal small intestinal mucosal specimens from different individuals. These data suggested that LL-37/hCAP18 expression is predominantly localized in the more highly differentiated colon epithelial cells.
FIG. 1.
Immunohistochemical detection of LL-37/hCAP18 in normal human colon and small intestine. Biopsy specimens from normal human colon (A and B) and proximal small intestine (C to F) were analyzed by indirect immunoperoxidase staining for LL-37/hCAP18 expression. The sections were immunostained with an LL-37/hCAP18-specific antibody (A, C, and E) or control rabbit IgG (B, D, and F). Magnification, ×400 (A to D) and ×100 (E and F).
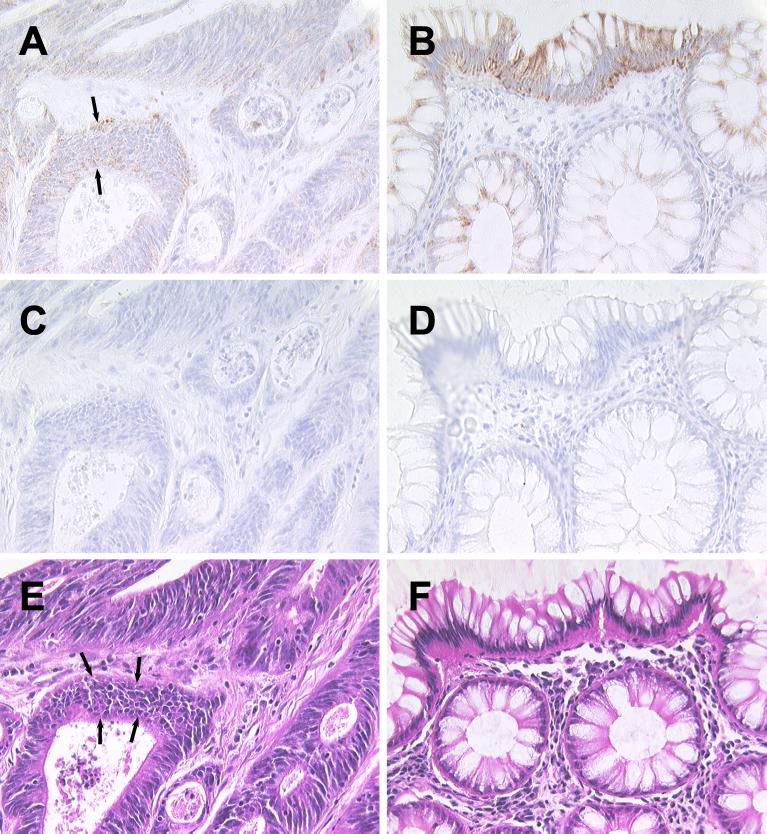
In contrast to normal colon, neoplastic colon epithelium, characterized by multiple layers of poorly differentiated epithelial cells (Fig. 2E, note area indicated by arrows), expressed only low levels of LL-37/hCAP18 (Fig. 2A, note area indicated by arrows), much lower than that seen in normal epithelium from the same surgical specimens (Fig. 2B). Similar results were obtained by immunostaining neoplastic and normal areas of colon epithelium from two additional individuals with adenocarcinoma of the colon. These data support the notion that LL-37/hCAP18 is predominantly expressed by the more highly differentiated colon epithelial cells.
FIG. 2.
Immunohistochemical detection of LL-37/hCAP18 in human colon cancer tissue. Colon tissue obtained at surgery from a patient with adenocarcinoma of the colon was analyzed by immunostaining for LL-37/hCAP18 expression. Sections were stained with LL-37/hCAP18-specific antibody (A and B), control rabbit IgG (C and D), or hematoxylin and eosin (E and F). The sections in panels A, C, and E are from the tumor, whereas the sections in panels B, D, and F are from normal mucosa from the same surgical specimen. Magnification, ×400.
Cell differentiation is important for upregulation of LL-37/hCAP18 expression in cultured human colon epithelial cells.
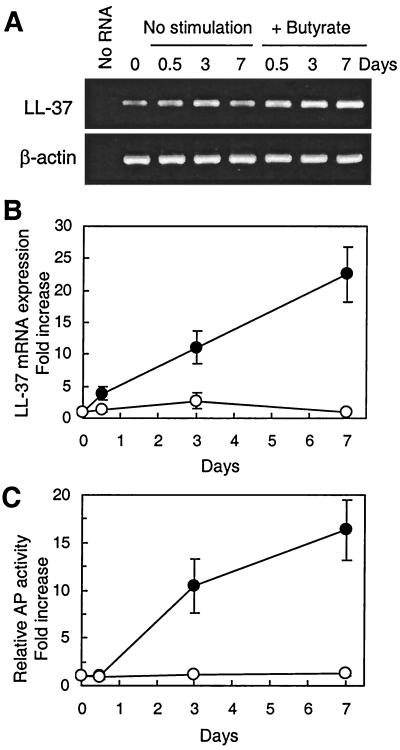
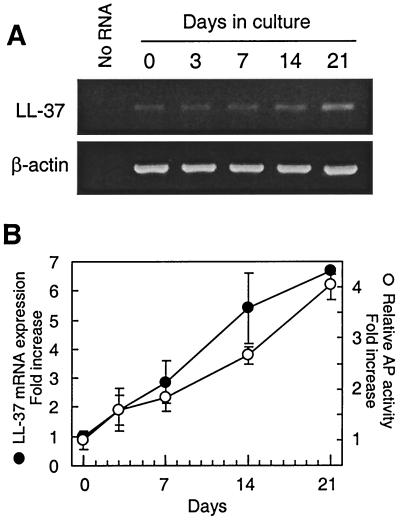
The in vivo staining pattern of LL-37/hCAP18 suggested that epithelial cell differentiation is important for inducing LL-37/hCAP18 expression. To confirm and extend the in vivo findings, we evaluated LL-37/hCAP18 mRNA expression during epithelial cell differentiation in vitro using two different models of colon epithelial cell differentiation. In the first model, the human colon epithelial cell line HCA-7 was treated with a differentiation-inducing agent, butyrate, for up to 7 days (24). Levels of LL-37/hCAP18 mRNA markedly increased at 3 and 7 days of butyrate treatment (Fig. 3A and B). The activity of AP, a brush border enzyme that has been used as a marker of epithelial cell differentiation (6, 23, 24), increased with a similar time course and relative extent (Fig. 3C). Similar results were obtained with two additional human colon epithelial cell lines, HT-29 and Caco-2, after treatment with butyrate for up to 7 days (data not shown). In a second model of epithelial cell differentiation, we evaluated LL-37/hCAP18 mRNA levels in the human colon epithelial cell line Caco-2, which was incubated for up to 21 days after reaching confluence to induce spontaneous differentiation (6, 48). LL-37/hCAP18 mRNA levels increased with time in confluent Caco-2 cultures (Fig. 4), and this was paralleled by an increase in AP activity (Fig. 4B).
FIG. 3.
LL-37/hCAP18 mRNA expression is upregulated by butyrate treatment of HCA-7 cells. HCA-7 cells were left unstimulated or were stimulated with 2 mM butyrate (+ butyrate) for up to 7 days, and total cellular RNA and cell lysates were prepared at the indicated time points. (A) Total cellular RNA was amplified by RT-PCR for LL-37/hCAP18 and β-actin, and the products were analyzed on a 1% agarose gel. As a negative control, RNA was omitted from RT and PCR amplification (No RNA). (B) LL-37/hCAP18 mRNA expression was analyzed by real-time PCR. The levels were normalized to those of β-actin, which were constant in both groups throughout the experiment. The data are expressed as the fold changes (± SD) in mRNA levels relative to the levels at day zero for unstimulated (○) and butyrate-treated (•) cells. (C) AP activity in cell lysates is expressed as changes (± SD) relative to levels at day zero.
FIG. 4.
LL-37/hCAP18 mRNA expression increases in spontaneously differentiating Caco-2 cells. Caco-2 cells were incubated for up to 21 days after reaching confluence to induce spontaneous cell differentiation. LL-37/hCAP18 mRNA levels were analyzed by qualitative RT-PCR (A) and real-time PCR (B, •) as described in the legend to Fig. 3. AP activity (B, ○) was analyzed by enzymatic assay.
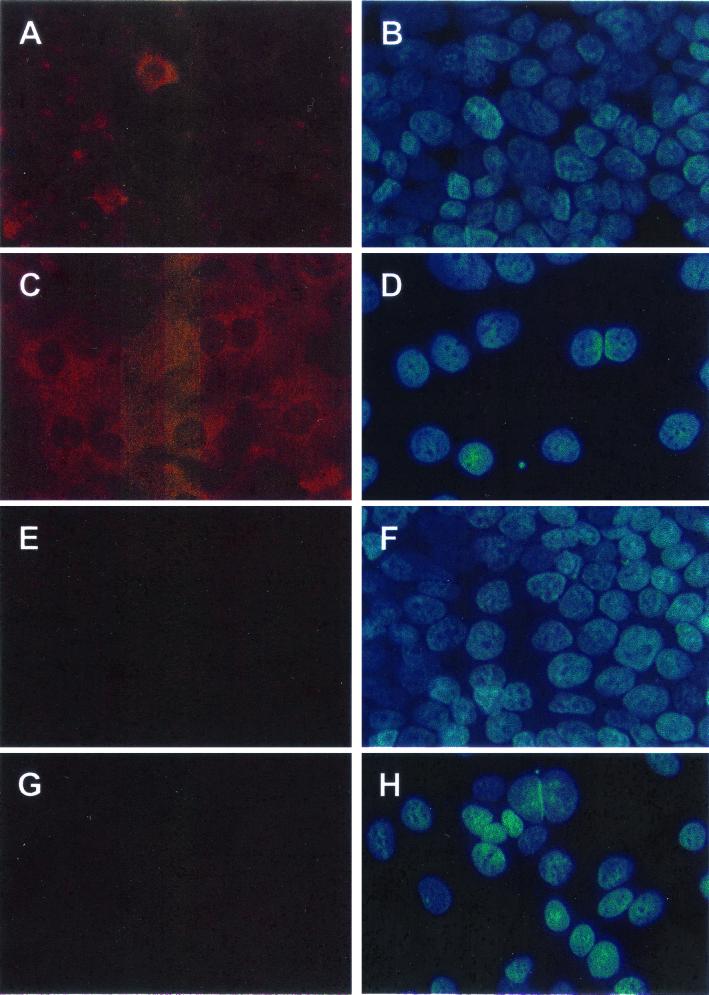
To determine if expression of LL-37/hCAP18 mRNA was paralleled by increased LL-37/hCAP18 production, butyrate-treated and untreated HCA-7 cells were immunostained with polyclonal anti-LL-37 IgG antibody, using normal rabbit IgG as a control. Only a small number of untreated control cells showed LL-37/hCAP18 staining (Fig. 5A), whereas butyrate-treated cells showed strong LL-37/hCAP18 staining in the vast majority of cells, with predominant cytoplasmic localization (Fig. 5C). Cell numbers in the butyrate-treated cultures were lower than those in untreated controls after incubation for 5 days (compare Fig. 5B and D), an observation consistent with previous reports that butyrate arrests cell proliferation in colonic epithelial cell lines (24). Taken together, these results indicate that differentiation of colonic epithelial cells in vitro increases LL-37/hCAP18 mRNA and protein expression and therefore further supports the in vivo observation that cell differentiation is a crucial determinant for upregulation of epithelial LL-37/hCAP18 expression.
FIG. 5.
Immunostaining of LL-37/hCAP18 in HCA-7 cells. Confluent HCA-7 cells were treated with butyrate for 5 days (C, D, G, and H) or were left unstimulated as controls (A, B, E, and F). LL-37/hCAP18 expression was analyzed by indirect immunofluorescence using an LL-37/hCAP18-specific antibody (A and C) or a control rabbit IgG (E and G). (B, D, F, and H) Nuclear counterstaining with Hoechst 33258 dye for each of the adjacent panels. Magnification, ×400.
Commensal bacteria are not required for constitutive epithelial LL-37/hCAP18 expression in the colon.
The studies described above showed that differentiated epithelial cells in the colon, but not in the proximal small intestine, express LL-37/hCAP18 constitutively. This might be explained by differences in bacterial exposure at those sites, since the epithelium in the colon, but not in the proximal small intestine, is normally exposed to large numbers of commensal bacteria. To evaluate the influence of bacterial exposure on epithelial LL-37/hCAP18 expression in vivo, we used a human fetal colon xenograft model. In this model, human fetal colon is transplanted subcutaneously onto the backs of SCID mice and allowed to develop for 10 weeks. At that time, the human colon xenografts contain a fully differentiated surface and crypt epithelium of strictly human origin (43). The xenograft tissues lack a commensal luminal microflora, since the fetal tissue was obtained and transplanted under sterile conditions, and bacteria were not detectable in xenograft luminal contents 10 weeks after transplantation, as determined by PCR analysis of bacterial 16S rRNA genes using universal primers (Fig. 6A). In contrast, strong signals were obtained by PCR of DNA samples isolated from mouse feces and human colon lavage fluid or xenograft luminal contents to which bacteria were added (Fig. 6A). Colon xenograft tissue stained positively for LL-37/hCAP18 in the surface epithelium (Fig. 6B), with a distribution comparable to that observed in normal human colon (Fig. 1A). Similar results were noted using two additional colon xenografts. These data suggest that the colon microflora is not required for constitutive expression of LL-37/hCAP18 by human colon epithelium.
FIG. 6.
Immunohistochemical detection of LL-37/hCAP18 in human colon xenografts. (A) Total DNA isolated from luminal contents of two different human fetal colon xenografts (XG1 and XG2) was amplified by PCR using universal primers targeting a highly conserved region of the bacterial 16S rRNA gene. In the lanes designated “XG1 + E. coli 103” and “XG2 + S. bovis 103,” the indicated bacteria (103 CFU) were mixed with xenograft luminal content before DNA extraction. DNA samples from mouse feces and human colon lavage fluid were analyzed as positive controls. (B and C) Human colon xenografts were immunostained with LL-37/hCAP18-specific antibody (B) or control rabbit IgG (C). Magnification for both panels, ×400.
Proinflammatory mediators do not upregulate LL-37/hCAP18 expression in vitro or in vivo.
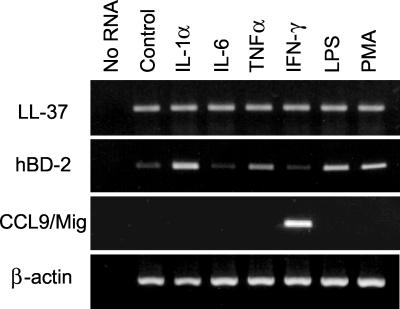
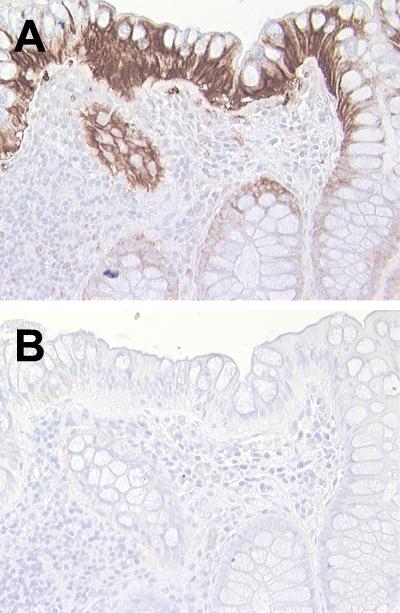
We next examined if expression of LL-37/hCAP18 mRNA in human colon epithelial cells is regulated under conditions other than cell differentiation, particularly stimulation with inflammatory mediators. HCA-7 cells were exposed to several proinflammatory cytokines (IL-1α, TNF-α, or IFN-γ), IL-6, LPS, or PMA for 6 h. None of these stimuli altered LL-37/hCAP18 mRNA levels in these cells (Fig. 7) or in two other colonic epithelial cell lines, HT-29 and Caco-2 (data not shown). However, these cell lines were responsive to the proinflammatory mediators tested here, since IL-1α, TNF-α, LPS, and PMA upregulated the expression of hBD-2 mRNA and IFN-γ upregulated the expression of the chemokine CCL9/Mig in the cells (Fig. 7). These data suggest that proinflammatory mediators do not upregulate epithelial LL-37/hCAP18 expression. Consistent with this conclusion, we also did not observe changes in epithelial LL-37/hCAP18 expression in inflamed human colon (Fig. 8A), since LL-37/hCAP18 was present in the surface epithelium at levels that appeared similar to those seen in normal human colon (compare Fig. 8A with Fig. 1A). Similar results were noted using three additional inflamed colon mucosal specimens from different individuals.
FIG. 7.
Epithelial LL-37/hCAP18 expression by cultured human colonic epithelial cells stimulated with inflammatory mediators. HCA-7 cells were left unstimulated (Control) or were stimulated with the indicated agonists. Total cellular RNA was isolated 6 h later and analyzed for LL-37/hCAP18, hBD-2, CCL9/Mig and β-actin mRNA expression by RT-PCR. As a negative control, RNA was omitted from RT and PCR amplification (No RNA).
FIG. 8.
Immunohistochemical detection of LL-37/hCAP18 in inflamed human colon. Biopsy specimens from inflamed human colon were analyzed by indirect immunoperoxidase staining for LL-37/hCAP18 expression. The sections were immunostained with an LL-37/hCAP18-specific antibody (A) or control rabbit IgG (B). Magnification, ×400.
Infection with Salmonella serovar Dublin and enteroinvasive E.coli upregulates epithelial LL-37/hCAP18 expression.
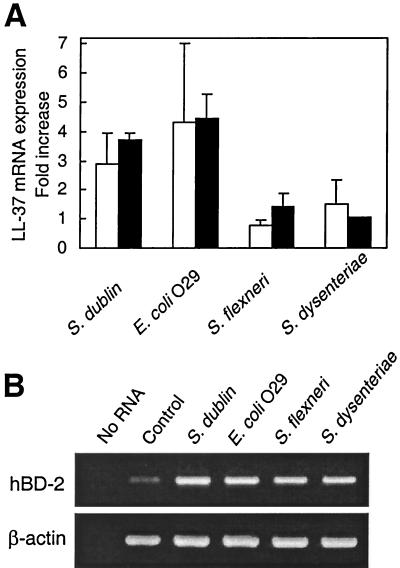
Infection of colon epithelial cells with Shigella spp. was recently reported to downregulate LL-37/hCAP18 mRNA expression (25). To evaluate the effect of infection with a variety of invasive bacteria on epithelial LL-37/hCAP18 mRNA expression, HCA-7 cells were cultured with invasive bacteria for 1 h, after which extracellular bacteria were removed by washing and the cells were further incubated with the non-membrane-permeant antibiotic gentamicin to kill any remaining extracellular bacteria. This protocol has been widely used to examine epithelial cell responses to bacterial infection (28, 37, 39), and in the case of Salmonella and enteroinvasive E. coli, it has little effect on epithelial viability for the first 24 h after infection (30). LL-37/hCAP18 mRNA levels increased by three- to fourfold at 6 and 24 h after Salmonella serovar Dublin and enteroinvasive E. coli infection (Fig. 9A). On the other hand, LL-37/hCAP18 mRNA levels remained essentially unchanged after infection with two different Shigella strains (Fig. 9A). Similarly, LL-37/hCAP18 mRNA levels in HT-29 colon epithelial cells remained unchanged in response to infections with these Shigella strains (data not shown). β-Actin mRNA levels remained constant over the same period. hBD-2 mRNA expression, which served as a positive control for these studies, was strongly induced by all four invasive bacteria (Fig. 9B).
FIG. 9.
LL-37/hCAP18 mRNA expression by human colonic epithelial cell lines infected with enteroinvasive bacteria. HCA-7 cells were left uninfected (Control) or were infected with the indicated bacteria as described in Materials and Methods. (A) LL-37/hCAP18 mRNA levels were analyzed by real-time RT-PCR at 6 (open bars) and 24 (solid bars) h after infection and are expressed as fold increases over controls (+ SD). (B) hBD-2 and β-actin mRNA expression was analyzed by qualitative RT-PCR 6 h after infection.
Since LL-37/hCAP18 mRNA was upregulated by infection with enteroinvasive E. coli O29 and Salmonella serovar Dublin, we examined whether LL-37 had bactericidal activity against these bacteria. LL-37 showed remarkable bactericidal activity, with EC50s of 2.8 (in HEPES-sucrose) and 6.0 (in PBS) μM against Salmonella serovar Dublin and 3.1 (in HEPES-sucrose) and 3.4 (in PBS) μM against E. coli O29. These data suggest that LL-37/hCAP18 upregulation in colonic epithelial cells after infection with enteroinvasive bacteria may contribute to host antimicrobial defense.
DISCUSSION
The epithelial localization and regulated expression of LL-37/hCAP18 in human intestine is shown to differ in several respects from that of other enteric antimicrobial peptides, such as the α- and β-defensins. LL-37/hCAP18 manifested a distinct crypt-to-surface cell gradient in human colon, with LL-37/hCAP18 protein being predominantly expressed in surface epithelial cells and adjacent epithelial cells in the upper crypts. In contrast, hBD-1 is expressed throughout the intestinal epithelium of the colon and small intestine with no apparent crypt-to-villus gradient in the small intestine or crypt-to-surface cell gradient in the colon (17, 39). Furthermore, little, if any, LL-37/hCAP18 was produced by epithelium in the upper small intestine, whereas the α-defensins HD-5 and HD-6, as well as the antimicrobial protein lysozyme, are produced by Paneth cells throughout the small intestine and can be aberrantly expressed in metaplastic Paneth cells in the colon in some patients with ulcerative colitis (10, 27, 35, 41). Nonetheless, mucus-producing cells in the Brunner's glands of some individuals, like mucus-producing glands in the submucosa of the respiratory tract (4), contained LL-37/hCAP18. In addition, Brunner's glands produce lysozyme (8), suggesting that these glands can contribute to small-intestinal host defense by releasing an array of antimicrobial products. Lysozyme is also expressed by colonic epithelial cells; however, in contrast to LL-37/hCAP18, expression was independent of cell differentiation (5). Taken together, these data demonstrate marked differences in the regional distribution of antimicrobial peptides in the epithelium of the intestinal mucosa. Although not studied here, such differences are likely to have arisen by natural selection and therefore predictably will be paralleled by differences in biological function.
The highest levels of epithelial LL-37/hCAP18 protein expression were observed in epithelial cells of the colon surface and upper crypts, although low levels of expression were also detected in the deeper crypt regions. Prior in situ hybridization studies had shown that LL-37/hCAP18 mRNA is expressed throughout the crypt and surface epithelium of the colon (4). These data, together with our findings, suggest that LL-37/hCAP18 protein may accumulate during cell differentiation and migration from the lower crypts to the surface. In addition, our studies using two different in vitro models of colon epithelial cell differentiation demonstrated that LL-37/hCAP18 mRNA expression paralleled increased protein levels during cell differentiation. This observation suggests that the constitutive expression of LL-37/hCAP-18 by colon epithelial cells is under the control of signals that govern epithelial cell differentiation. The underlying mechanisms are only beginning to be understood (7), but recent studies have revealed several signaling molecules and transcription factors that play pivotal roles in inducing intestinal epithelial cell differentiation, e.g., a cyclin-dependent kinase inhibitor p27kip1 (11, 42) and the homeobox gene products Cdx-1 and Cdx-2 (34, 46). Since the hCAP18 promoter contains a potential binding site for Cdx-2 located 270 bp upstream of the translation initiation site, it should be possible in future studies to address whether such molecules regulate LL-37/hCAP18 expression in colon epithelial cells.
LL-37/hCAP18 was not upregulated in human colon epithelial cells stimulated with cytokines that play a role in intestinal inflammatory responses, including TNF-α, IL-1α, IFN-γ, and IL-6. Furthermore, epithelial LL-37/hCAP18 expression did not appear to be increased in inflamed colon in vivo. In contrast to colon epithelium, LL-37/hCAP18 expression is upregulated in keratinocytes in skin disorders characterized by inflammation, although the specific inflammatory stimuli are not known (12, 15). The LL-37/hCAP18 gene has potential binding sites for several transcription factors, including NF-κB, NF-IL-6, and acute-phase response factor, which are important for cellular responses to inflammatory stimuli. The lack of increased LL-37/hCAP18 expression in human colon epithelial cell lines was not due to a lack of response of those cells to proinflammatory stimuli, as the same stimuli upregulated an array of chemokine and other proinflammatory genes (13, 14, 29), as well as the inducible antimicrobial peptide hBD-2, in those cells. Thus, LL-37/hCAP18 appears to be differentially regulated among different cell types, including different epithelial cell types in the skin and intestine. We further note that constitutive expression of LL-37/hCAP18 has also been observed in several other cell types, such as neutrophils and lymphocytes (1, 21, 44), as well as epithelial cells in the oral cavity, cervix, epididymis, and proximal airways (4, 16, 36).
Infection with Shigella spp. was recently reported to downregulate LL-37/hCAP18 mRNA expression in HT-29 human colon epithelial cells and in the human monocyte cell line U937 (25). In those studies, Shigella infections were conducted over extended periods (up to 9 h), which was associated with increased epithelial cell death, a finding we confirmed in our studies (data not shown). To minimize problems associated with cell death, we used an infection protocol that maximized epithelial cell viability (i.e., 1 h of infection followed by the addition of gentamicin). Under these conditions, epithelial LL-37/hCAP18 mRNA expression remained unchanged after infection with two different Shigella strains. The differences between our findings and those in the previous study could suggest that cell death, directly or indirectly, contributes to downregulation of LL-37/hCAP18 expression. In addition, it is possible that loss of differentiated colon epithelial cells in response to Shigella infection in patients may have contributed to the apparent downregulation of LL-37/hCAP18 expression in vivo associated with Shigella infection in those studies (25).
The biological activities of LL-37 are substantially more diverse than initially recognized. LL-37 has a wide range of bactericidal activities against gram-negative and gram-positive bacteria, including Pseudomonas aeruginosa and Staphylococcus aureus (2, 4, 47), and may play a key role in nonoxidative killing of bacterial pathogens (20). Consistent with this, we found that LL-37 also killed the enteroinvasive bacteria used in the present study with an EC50 comparable to those found for other pathogens (4). In addition, LL-37 can bind to and neutralize bacterial LPS (31, 32) and chemoattract different populations of leukocytes, including monocytes, neutrophils, and T cells (1, 49). It remains to be determined if the functional importance of LL-37 in colon epithelium lies in its antimicrobial properties or in its ability to neutralize bacterial LPS or to chemoattract cells important in host mucosal defense.
Acknowledgments
This work was supported by NIH grants DK35108 and DK58960.
We thank Jennifer R. Smith and Michael B. Dwinell for technical advice and Richard L. Gallo for helpful advice and discussions. We are grateful to Gudmundur H. Gudmundsson and Birgitta Agerberth for generously providing anti-LL-37 antibody and LL-37 peptide.
Editor: J. D. Clements
REFERENCES
- 1.Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jörnvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. [PubMed] [Google Scholar]
- 2.Agerberth, B., H. Gunne, J. Odeberg, P. Kogner, H. G. Boman, and G. H. Gudmundsson. 1995. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 92:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differentiation-associated antimicrobial functions in human colon adenocarcinoma cell lines. Exp. Cell Res. 226:80-89. [DOI] [PubMed] [Google Scholar]
- 6.Chantret, I., A. Barbat, E. Dussaulx, M. G. Brattain, and A. Zweibaum. 1988. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 48:1936-1942. [PubMed] [Google Scholar]
- 7.Clatworthy, J. P., and V. Subramanian. 2001. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech. Dev. 101:3-9. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho, H. B., T. I. Robalinho, V. B. Coutinho, A. M. Amorin, J. R. Almeida, J. T. Filho, E. Walker, G. King, H. F. Sewell, and D. Wakelin. 1996. Immunocytochemical demonstration that human duodenal Brunner's glands may participate in intestinal defense. J. Anat. 189:193-197. [PMC free article] [PubMed] [Google Scholar]
- 9.Cowland, J. B., A. H. Johnsen, and N. Borregaard. 1995. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 368:173-176. [DOI] [PubMed] [Google Scholar]
- 10.Cunliffe, R. N., F. R. A. J. Rose, J. Keyte, L. Abberley, W. C. Chan, and Y. R. Mahida. 2001. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 48:176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschenes, C., A. Vezina, J. F. Beaulieu, and N. Rivard. 2001. Role of p27Kip1 in human intestinal cell differentiation. Gastroenterology 120:423-438. [DOI] [PubMed] [Google Scholar]
- 12.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 13.Dwinell, M. B., N. Lugering, L. Eckmann, and M. F. Kagnoff. 2001. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 120:49-59. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohm, M., B. Agerberth, G. Ahangari, M. Ståhle-Bäckdahl, S. Lidén, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 16.Frohm Nilsson, M., B. Sandstedt, O. Sörensen, G. Weber, N. Borregaard, and M. Ståhle-Bäckdahl. 1999. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 67:2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye, M., J. Bargon, B. Lembcke, T. O. Wagner, and R. Gropp. 2000. Differential expression of human α- and β-defensins mRNA in gastrointestinal epithelia. Eur. J. Clin. Investig. 30:695-701. [DOI] [PubMed] [Google Scholar]
- 18.Ganz, T. 1999. Defensins and host defense. Science 286:420-421. [DOI] [PubMed] [Google Scholar]
- 19.Greisen, K., M. Loeffelholz, A. Purohit, and D. Leong. 1994. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 32:335-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudmundsson, G. H., and B. Agerberth. 1999. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J. Immunol. Methods 232:45-54. [DOI] [PubMed] [Google Scholar]
- 21.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 22.Gudmundsson, G. H., K. P. Magnusson, B. P. Chowdhary, M. Johansson, L. Andersson, and H. G. Boman. 1995. Structure of the gene for porcine peptide antibiotic PR-39, a cathelin gene family member: comparative mapping of the locus for the human peptide antibiotic FALL-39. Proc. Natl. Acad. Sci. USA 92:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gum, J. R., W. K. Kam, J. C. Byrd, J. W. Hicks, M. H. Sleisenger, and Y. S. Kim. 1987. Effects of sodium butyrate on human colonic adenocarcinoma cells. Induction of placental-like alkaline phosphatase. J. Biol. Chem. 262:1092-1097. [PubMed] [Google Scholar]
- 24.Heerdt, B. G., M. A. Houston, and L. H. Augenlicht. 1994. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 54:3288-3294. [PubMed] [Google Scholar]
- 25.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. H. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7:180-185. [DOI] [PubMed] [Google Scholar]
- 26.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 27.Jones, D. E., and C. L. Bevins. 1992. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267:23216-23225. [PubMed] [Google Scholar]
- 28.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirikae, T., M. Hirata, H. Yamasu, F. Kirikae, H. Tamura, F. Kayama, K. Nakatsuka, T. Yokochi, and M. Nakano. 1998. Protective effects of a human 18-kilodalton cationic antimicrobial protein (CAP18)-derived peptide against murine endotoxemia. Infect. Immun. 66:1861-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larrick, J. W., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent, F., L. Eckmann, T. C. Savidge, G. Morgan, C. Theodos, M. Naciri, and M. F. Kagnoff. 1997. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect. Immun. 65:5067-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorentz, O., I. Duluc, A. D. Arcangelis, P. Simon-Assmann, M. Kedinger, and J. N. Freund. 1997. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J. Cell Biol. 139:1553-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallow, E. B., A. Harris, N. Salzman, J. P. Russell, R. J. DeBerardinis, E. Ruchelli, and C. L. Bevins. 1996. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 271:4038-4045. [DOI] [PubMed] [Google Scholar]
- 36.Malm, J., O. Sörensen, T. Persson, M. Frohm-Nilsson, B. Johansson, A. Bjartell, H. Lilja, M. Ståhle-Bäckdahl, N. Borregaard, and A. Egesten. 2000. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 68:4297-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Neil, D. A., S. P. Cole, E. Martin-Porter, M. P. Housley, L. Liu, T. Ganz, and M. F. Kagnoff. 2000. Regulation of human β-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect. Immun. 68:5412-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 40.Oren, Z., J. C. Lerman, G. H. Gudmundsson, B. Agerberth, and Y. Shai. 1999. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 341:501-513. [PMC free article] [PubMed] [Google Scholar]
- 41.Ouellette, A. J. 1997. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology 113:1779-1784. [DOI] [PubMed] [Google Scholar]
- 42.Quaroni, A., J. Q. Tian, P. Seth, and C. Ap Rhys. 2000. p27kip1 is an inducer of intestinal epithelial cell differentiation. Am. J. Physiol. Cell Physiol. 279:C1045-C1057. [DOI] [PubMed]
- 43.Savidge, T. C., A. L. Morey, D. J. P. Ferguson, K. A. Fleming, A. N. Shmakov, and A. D. Phillips. 1995. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation 58:361-371. [DOI] [PubMed] [Google Scholar]
- 44.Sörensen, O., K. Arnljots, J. B. Cowland, D. F. Bainton, and N. Borregaard. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 45.Sörensen, O. E., P. Follin, A. H. Johnsen, J. Calafat, G. S. Tjabringa, P. S. Hiemstra, and N. Borregaard. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951-3959. [DOI] [PubMed] [Google Scholar]
- 46.Suh, E., and P. G. Traber. 1996. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol. Cell. Biol. 16:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Travis, S. M., N. N. Anderson, W. R. Forsyth, C. Espiritu, B. D. Conway, E. P. Greenberg, P. B. McCray, Jr., R. I. Lehrer, M. J. Welsh, and B. F. Tack. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vachon, P. H., and J.-F. Beaulieu. 1992. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 103:414-423. [DOI] [PubMed] [Google Scholar]
- 49.Yang, D., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]