Abstract
The avidity maturation and immunoglobulin G (IgG) isotype distribution of antibodies after vaccination with a meningococcal B outer membrane vesicle (OMV) vaccine were evaluated as indicators of protective immunity. Pre- and postvaccination sera from 134 healthy toddlers (ages, 2 to 3 years) immunized with a monovalent meningococcal B OMV (serosubtype P1.7-2,4) vaccine adsorbed with AlPO4 or Al(OH)3 were analyzed by enzyme-linked immunosorbent assay (ELISA) methods. The children were vaccinated three times with intervals of 3 to 6 weeks between vaccinations or twice with an interval of 6 to 10 weeks between vaccinations. A booster was given after 20 to 40 weeks. The avidity index (AI) of antibodies increased significantly during the primary series of vaccinations and after the booster was given. No differences in AIs were found when the results obtained with the two vaccination schedules or with the two adjuvants were compared. After vaccination, IgG1 was the predominant IgG isotype, followed by IgG3. No IgG2 or IgG4 was detected. There was a strong correlation between serum bactericidal activity (SBA) and ELISA titers (r = 0.85 [P < 0.0001] for total IgG, r = 0.83 for IgG1 [P < 0.0001], r = 0.82 for IgG3 [P < 0.0001], and r = 0.84 [P < 0.0001] for the avidity titer). When two subgroups with similar anti-OMV IgG levels were compared before and after the booster vaccination, the higher AI after the booster vaccination was associated with significantly increased SBA. We concluded that avidity maturation occurs after vaccination with a monovalent meningococcal B OMV vaccine, especially after boosting, as indicated by a significant increase in the AI. Vaccination with the monovalent OMV vaccine induced mainly IgG1 and IgG3 isotypes, which are considered to be most important for protection against meningococcal disease. An increase in the AI of antibodies is associated with increased SBA, independent of the level of specific IgG and the IgG isotype distribution. Measuring the AI and IgG isotype distribution of antibodies after vaccination can be a supplementary method for predicting protective immunity for evaluation in future phase III trials with meningococcal serogroup B vaccines.
Neisseria meningitidis is an important cause of meningitis and septicemia worldwide. In many countries in Western Europe, N. meningitidis serogroup B is most frequently isolated from seriously ill patients. In the struggle against meningococcal disease caused by this serogroup, great efforts have been made to develop a protective vaccine. The group B capsular polysaccharide is poorly immunogenic since it shows strong antigenic resemblance to structures expressed on human fetal neural cells (12). As a consequence, a serogroup B polysaccharide vaccine may induce antibodies that cross-react with human tissues. Therefore, vaccines containing outer membrane proteins have been developed and have been shown to induce protective immune responses (3, 11). At the National Institute for Public Health and the Environment (RIVM), workers developed a vaccine consisting of two outer membrane vesicle (OMV) preparations, each expressing three different PorA proteins representing the majority of circulating serosubtypes in The Netherlands and other countries in Europe (7). This vaccine has been tested in several phase I and II trials and has been proven to be safe and immunogenic (6, 8). Since serosubtype P1.7-2,4 is the cause of a current epidemic in New Zealand and is the most prevalent serosubtype in The Netherlands as well, a monovalent vaccine with double expression of this PorA was also constructed at the RIVM. This vaccine appeared to be safe and immunogenic in toddlers; more than 90% of vaccinated children showed a fourfold increase in serum bactericidal activity (SBA) (9).
There is a great need for well-defined markers for immunity induced by vaccination. These markers could serve as surrogates of vaccine protective efficacy and would be helpful for quick introduction of new or improved vaccines in the future. Measurement of total immunoglobulin G (IgG) titers by specific enzyme-linked immunosorbent assays (ELISA) does not provide any information concerning the functionality of antibodies. A fourfold increase in SBA after vaccination has been widely used to evaluate the immunogenicities and efficacies of various meningococcal B vaccines. However, Perkins et al. (23) showed that a fourfold increase in SBA appeared to underestimate clinical efficacy. In addition, SBA titers and IgG ELISA titers in sera obtained after vaccination with the RIVM hexavalent OMV vaccine correlated poorly (10). One possible explanation for a poor correlation between SBA and ELISA results is that only high-avidity antibodies are bactericidal. For vaccination with meningococcal C conjugate vaccines, the functional importance of antibody avidity maturation after vaccination has recently been demonstrated by Richmond et al. (27). Several studies of conjugate vaccines against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) have also shown that vaccination induces an increase in antibody avidity (2, 14, 28) and that low concentrations of passively administered high-avidity antibody can protect experimentally infected animals from disease (20, 31). Most investigators use an ELISA method in which sodium thiocyanate (NaSCN) is used as an agent to discriminate weak binding between antibody and antigen from high-affinity binding (25). By calculating an avidity index (AI), the relative avidities of specific antibodies in serum can be compared during a vaccination trial.
The functional activity of antibodies also depends on IgG isotypes (19). IgG1 and IgG3 are most effective in complement binding and activation (5), and IgG2 may also contribute to protection against meningococcal disease (1). Furthermore, affinity differences have been found in antibodies with similar antigen-binding specificities but different IgG isotypes (24). IgG1 and IgG3 are mainly directed at protein antigens, whereas IgG2 is predominantly found after vaccination with polysaccharide antigens in adults (15, 16, 29). The aim of this study was to compare SBA with the AI and IgG isotype distribution and to evaluate antibody avidity maturation as an indicator of protective immunity after vaccination with the monovalent P1.7-2,4 OMV vaccine. The AI was determined by an ELISA technique using NaSCN, and the IgG isotype distribution was determined by an isotype-specific ELISA (21, 26). The effects of different adjuvants and immunization schedules on avidity maturation and IgG isotype distribution were also examined.
(Parts of this study were presented at the 19th Annual Meeting of the European Society for Pediatric Infectious Diseases [ESPID], Istanbul, Turkey, 2001.)
MATERIALS AND METHODS
Vaccine and subjects.
In this study we used serum samples from a randomized, blind, comparative clinical phase II trial previously performed with toddlers and a PorA-based meningococcal OMV vaccine against serosubtype P1.7-2,4 (9). This vaccine is made from strain F91, which contains duplicate copies of the PorA gene and does not express class 3 and 4 proteins. The vaccine was adsorbed with either AlPO4 or Al(OH)3, and two vaccination schedules consisting of two or three vaccinations in the primary series and one booster vaccination were evaluated. In the 2+1 schedule two vaccinations were given with an interval of 6 to 10 weeks between vaccinations, whereas in the 3+1 schedule three vaccinations were given with intervals of 3 to 6 weeks between vaccinations. In both schedules a booster vaccination was given 20 to 40 weeks after the primary series. Blood samples were drawn by venipuncture before each vaccination and 4 to 6 weeks after the last vaccination of the primary series and after the booster. Thus, five and six blood samples were obtained from children immunized with the 2+1 and 3+1 schedules, respectively. A total of 134 toddlers participated in this study. The trial was designed so that we could detect a 40% difference between the immune responses of the different groups, with an alpha value of 0.05 (two sided) and 80% power with 30 children per group.
SBA.
SBA was measured as reported previously (9). Briefly, the serosubtype P1.7-2,4 isogenic variant of N. meningitidis serogroup B strain H44/76 was grown on a GC agar plate containing 1% IsoVitaleX (SVM, Bilthoven, The Netherlands) at 37°C for 18 to 20 h in the presence of 5% CO2. Single colonies were picked and suspended in 2 ml of Mueller-Hinton broth (SVM). A 20-ml flask containing Mueller-Hinton broth was inoculated with each suspension so that the optical density at 620 nm was 0.07 to 0.08. The bacteria were grown until the optical density at 620 nm was 0.22 to 0.24 (∼109 CFU/ml). Each culture was diluted in Gey’s balanced salt solution (GBSS) (Sigma Chemical Co., St. Louis, Mo.) with 0.5% bovine serum albumin (BSA) (ICN, Irvine, Calif.) to a concentration of ∼105 CFU/ml. In wells of a 96-well microtiter plate 6-μl portions of this dilution were added to 12-μl portions of twofold dilutions of heat-inactivated sera in GBSS-BSA. After 10 to 15 min 6 μl of complement (40% [vol/vol] in GBSS-BSA; final concentration, 10% [vol/vol]) from a negative human donor was added to each well. Time-zero plates were incubated overnight in triplicate as follows. Seven microliters from a well containing only bacteria, complement, and GBSS-BSA was spread on a GC agar plate with 1% IsoVitaleX. The microtiter plate was then incubated at 37°C in the presence of 5% CO2 for 60 min. Subsequently, 7 μl of the suspension in each well was spotted onto a GC agar plate with 1% IsoVitaleX. After 18 to 20 h of incubation at 37°C in the presence of 5% CO2 the numbers of CFU on time-zero plates were determined. The average number of CFU was defined as 100%. Then the CFU on the plates containing the serum dilutions were counted, and the serum bactericidal titer was determined by determining the reciprocal of the lowest serum dilution that resulted in ≥90% killing.
Avidity ELISA.
The AI was determined by an ELISA method described by Anttila et al. (2), with minor modifications. Briefly, each well of Immulon 2 (Dynex Technologies, Inc.) 96-well plates was coated overnight with 100 μl of a 4-μg/ml P1.7-2,4 monovalent OMV suspension. Sera were diluted 1:100 in phosphate-buffered saline (PBS) containing 0.1% Tween 80, and threefold serial dilutions of serum samples were incubated for 90 min at 37°C. One plate contained all samples from a single child in duplicate. As a positive control, a serum sample from a volunteer who had been vaccinated with the hexavalent OMV vaccine in a phase I trial and had a high antibody titer against P1.7-2,4 was included on every plate. Sodium thiocyanate was used to dissociate low-avidity antigen-antibody binding. To determine the optimal assay conditions for measuring the AI, different concentrations of NaSCN (ranging from 0 to 3 M) were tested first with a subset of serum samples obtained at different times. A 1.5 M solution resulted in strong reductions in the ELISA titers of some samples, whereas the ELISA titers of other samples were not affected. Therefore, we considered 1.5 M a good discriminating concentration. After three washes, 100 μl of a 1.5 M NaSCN solution in PBS was added to one half of each plate, and plain PBS was added to the other half. After incubation for 15 min at room temperature, all wells were washed three times, and for detection of antibody binding the plates were incubated with rabbit anti-human IgG conjugated 1:5,000 with horseradish peroxidase conjugate for 90 min at 37°C. After washing, 3,3′,5,5′-tetramethylbenzidine substrate was added, and the reaction was allowed to proceed for 10 min and was stopped by adding 100 μl of 2 M H2SO4 per well. The absorbance at 450 nm was read with an EL312e Bio-Kinetics reader. IgG antibody titers were determined by determining the dilutions that yielded 50% of the maximal optical density. Samples with antibody titers below the assay’s detection limit were assigned a value of 50. The titers obtained after treatment with NaSCN were called avidity titers. The AI was the percentage of antibodies that remained bound at the antigen coat after treatment with sodium thiocyanate and was calculated as follows: AI = (titer with NaSCN)/(titer without NaSCN) × 100 (2). As a control for antibody specificity, prevaccination, post-primary-series, and postbooster serum samples from 24 toddlers were also used in an OMV ELISA measuring the total IgG titer against OMVs of PorA-negative mutant strain H1.5 (30).
IgG isotype ELISA.
IgG isotype-specific antibody titers were determined by an OMV ELISA by using isotype-specific conjugates as described previously (10). Briefly, plates were coated and sera were incubated as described above for the avidity ELISA. After incubation of the sera, the plates were washed three times and incubated with mouse anti-human conjugate specific for each of the various IgG isotypes (clone HP6188 for IgG1, clone HP6014 for IgG2, clone HP6095 for IgG3, and clone HP6196 for IgG4) (CLB, Amsterdam, The Netherlands) (17, 18). After incubation for 90 min at 37°C, the plates were washed, TMB substrate was added, and after 15 min the reaction was stopped by adding 100 μl of 2 M H2SO4 per well. The plates were read with an EL312e Bio-Kinetics reader at 450 nm, and IgG antibody titers were determined by determining the dilution that yielded 50% of the maximal optical density.
Statistical analysis.
Antibody titers were log transformed before statistical analysis. To determine which immunization schedule and vaccine adjuvant induced the highest overall SBA and antibody titers in the course of vaccination, the values for total IgG, IgG1, IgG3, avidity, and SBA titers from sera obtained at four time points were added for every child. The means were calculated for each group, and a variance analysis was performed with SAS software. Before the primary series titers were generally undetectable, and therefore these titers were not included in the analysis; in addition, the extra titers obtained during the primary series of the 3+1 schedule were not included since such titers were not obtained for all trial participants. Spearman’s rho correlation coefficient was used to calculate correlations between the different IgG antibody titers and AI and SBA results by using SPSS. Parametric tests were used to calculate differences in AIs between time points and groups, and nonparametric tests were used to calculate differences in IgG and SBA titers between groups.
RESULTS
Total anti-OMV IgG titer.
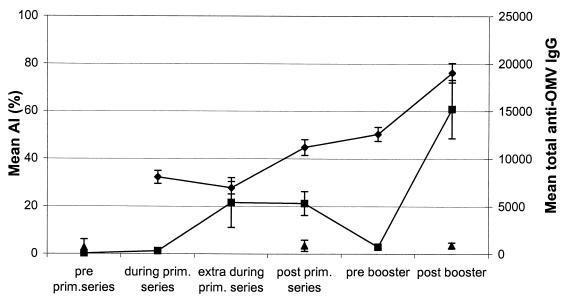
The total anti-OMV IgG titer increased during the primary series from 308 after one vaccination to 5,318 after the primary series (Fig. 1). In the prebooster samples, the total anti-OMV IgG titer dropped significantly, to 741. After the booster vaccination, the mean total anti-OMV IgG titer was 15,230. Variance analysis showed that the total anti-OMV IgG levels were highest following the 3+1 schedule (P < 0.0001) when AlPO4 was the adjuvant (P = 0.0002) (data not shown). The total IgG titers of serum samples obtained before the vaccination trial, after the primary series, and after the booster for 24 toddlers were measured against PorA-negative OMVs as a control for antibody specificity. The mean total IgG titers against these OMVs were 677 before vaccination, 881 after the primary series, and 911 after the booster (Fig. 1), indicating that the IgG titer against P1.7-2,4 OMVs was PorA specific.
FIG. 1.
Mean AI (⧫), mean level of total IgG against P1.7-2,4 OMVs (▪), and mean level of anti-OMV IgG against PorA-negative OMVs (▴) in sera of 134 toddlers after immunization with a monovalent meningococcal B OMV vaccine. The error bars indicate 95% CIs. prim., primary.
Avidity.
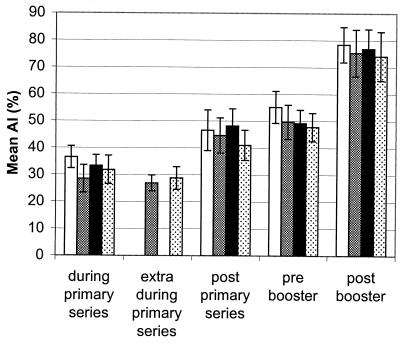
As found for the total anti-OMV IgG titer, variance analysis showed that the avidity titer was highest in the group that was vaccinated with the 3+1 schedule when AlPO4 was the adjuvant, indicating that this schedule and adjuvant resulted in significantly more avid antibodies (P = 0.0005 and P = 0.0243, respectively).
Mean AIs for all children are shown in Fig. 1. Only values for serum samples that contained measurable specific IgG antibodies are shown in Fig. 1, since it is not possible to calculate an AI when anti-OMV IgG titers are undetectable. Only 4 of 134 toddlers had detectable anti-OMV IgG titers before the first vaccination (AIs, 34, 40, 50, and 53%), suggesting that there was previous natural exposure to meningococcal antigens. The AIs of these four sera are not plotted in Fig. 1. All other serum samples obtained during the trial had detectable antibodies and were included in the analysis. The mean AI after the primary vaccination series was 44.8% (confidence interval [CI], 41.5 to 48.0%). Before the booster, the AI increased significantly, to 50.0% (CI, 47.5 to 53.2%) (paired sample t test, P = 0.015). After the booster vaccination the mean AI increased further, to 76.2% (CI, 72.2 to 80.3%) (P < 0.0001). Although IgG antibody titers decreased after the last vaccination of the primary series, the AIs of the antibodies increased (Fig. 1), whereas the AIs increased over time for the four groups that received different adjuvants and were subjected to different immunization schedules (Fig. 2). The differences between the AIs of the groups were not significant after the booster vaccination (analysis of variance, P = 0.746).
FIG. 2.
Mean AI for each group. The four kinds of bars show the mean AIs obtained with the two different vaccine adjuvants and the two different vaccination schedules. Open bars and solid bars, two primary vaccinations followed by a booster vaccination (2+1 schedule) with Al(OH)3 and AlPO4, respectively; mesh bars and dotted bars, three primary vaccinations followed by a booster vaccination (3+1 schedule) with Al(OH)3 and AlPO4, respectively. The error bars indicate 95% CIs.
To investigate the importance of the number of vaccinations in producing a high AI, two subgroups with similar mean total anti-OMV IgG titers were studied. One group of children (n = 86) had a mean total anti-OMV IgG titer of 3.4 (95% CI, 3.3 to 3.5) after the primary series, whereas the other group (n = 21) had a mean total anti-OMV IgG titer of 3.4 (95% CI, 3.3 to 3.6) after the booster (Mann-Whitney U test, P = 0.215). The AIs and SBA titers of these two groups were compared. Both the AI and the SBA were significantly higher in the postbooster group than in the post-primary-series group (73.6 versus 45.3% [P < 0.0001] and 1.0 versus 0.64 [P = 0.009], respectively), indicating that avidity maturation after boosting was independent of the IgG titer.
IgG isotype distribution.
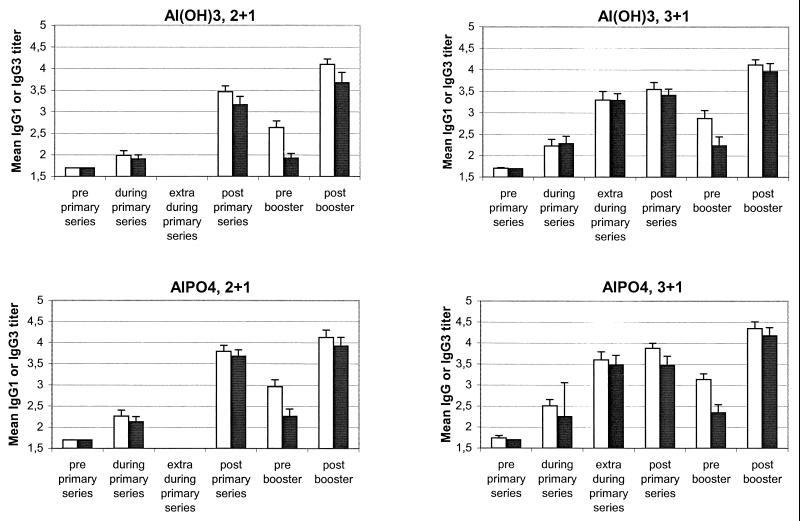
Figure 3 shows the distribution of IgG isotypes in the four groups. In every sample, IgG1 was the predominant isotype. High levels of IgG3 were also found, whereas no IgG2 or IgG4 was detected.
FIG. 3.
Serum log10 IgG isotype concentrations against P1.7-2,4 OMVs for each group. No IgG2 or IgG4 was found. 2+1, two primary vaccinations followed by a booster vaccination; 3+1, three primary vaccinations followed by a booster vaccination. Open bars, IgG1; solid bars, IgG3. The error bars indicate 95% CIs.
Variance analysis showed that the levels of IgG1 and the levels of IgG3 were both higher when the 3+1 schedule was used than when the 2+1 schedule was used (P = 0.0007 and P = 0.0008, respectively). The adjuvant AlPO4 induced significantly higher IgG1 titers than Al(OH)3 induced (P = 0.0002 and P = 0.0033). The specific IgG3 titers obtained with the two adjuvants were not different (P = 0.07).
Correlations between SBA, AI, and ELISA titers.
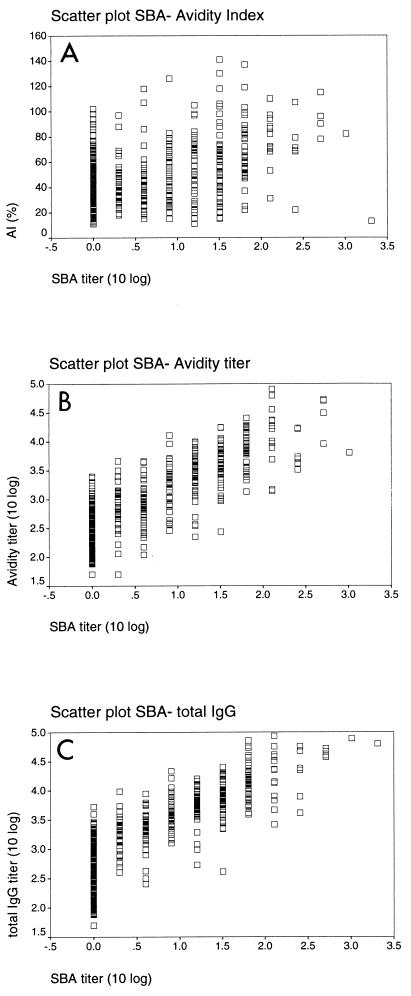
Levels of SBA for the sera have been reported previously (9). Briefly, 66% of all toddlers exhibited a fourfold or greater increase in SBA after the primary series, and the percentage increased to 97% after the booster. We used these results to correlate ELISA and AI results with SBA for each serum sample (Fig. 4). The SBA titers and ELISA titers correlated strongly (r = 0.85 for total IgG, r = 0.83 for IgG1, r = 0.82 for IgG3, and r = 0.84 for the avidity titer; P < 0.0001). The correlation between SBA and AI was markedly lower but still significant (r = 0.36; P < 0.0001).
FIG. 4.
Scatter plots of log10 SBA titer versus AI (A) (r = 0.36), log10 avidity titer (B) (r = 0.84), and log10 total IgG titer (C) (r = 0.85).
DISCUSSION
This study showed clearly that the AI of PorA-specific antibodies increased during the course of vaccination with a monovalent meningococcal OMV vaccine, even when IgG titers fell after the primary series. To our knowledge, this is the first study showing avidity maturation of antibodies elicited by a meningococcal group B vaccine in children.
To measure the AI, we used NaSCN in an ELISA at a concentration of 1.5 M. For antibodies against polysaccharides, Anttila et al. (2) used 0.5 M NaSCN in their assay to measure the AIs of antibodies elicited by various pneumococcal vaccines, while Goldblatt et al. (14) used a mean NaSCN concentration of up to 0.6 M in their elution ELISA. We obtained the best resolution between high- and low-avidity antibodies when we used a concentration of 1.5 M, which was comparable to the 1.6 M NaSCN that Pollard et al. (25) used to measure the AI after infection with serogroup B meningococci. The difference in concentration shows that the avidity of antibodies against proteins such as PorA is in general higher than the avidity of antibodies against (conjugated) polysaccharides. The pH of the high-concentration solution was reduced to 6.75, compared to pH 7.4 for PBS alone. However, Goldblatt et al. (13) showed that pH has no effect on the thiocyanate ion.
Although antibody avidity is an important surrogate of protective efficacy for several vaccine types, vaccines differ in the ability to evoke avidity maturation. Anttila et al. (2) showed that boosting with a pneumococcal polysaccharide vaccine did not result in an increase in the AI. This was in contrast to the response seen after a booster with pneumococcal conjugate vaccine, which induced a strong increase in AI, indicating that maturation of antibodies was induced by this vaccine. Usinger and Lucas (31) also reported that AI is a determinant of protective efficacy, and these authors showed that there was an inverse correlation between the magnitude of avidity and the amount of antibody required to protect mice against lethal pneumococcal bacteremia. Furthermore, Goldblatt et al. (14) showed that the AI could be used as a surrogate marker of successful priming by Hib conjugate vaccines. Children with anti-Hib IgG titers below the protective level (1.0 μg/ml) after primary immunization had antibodies with AIs after the booster that were significantly lower than the AIs of antibodies of children with high antibody titers after priming. After systemic infection with serogroup B meningococci the mean avidity of antibodies in infants was markedly lower than the mean avidity of antibodies in children who were more than 10 years old (25). This finding correlated with the absence of SBA in the serum samples from the infants and with the presence of SBA in the sera of children who were more than 10 years old (25). In our study, the booster vaccination induced an increase in the mean AI of anti-OMV IgG of up to 76%. By using OMVs of PorA-negative strain H1.5 as an antigen in the ELISA, the antibodies induced by the RIVM vaccine were shown to be mainly PorA specific (Fig. 1). Less than 10% of antibodies appeared to be directed against other antigens, such as lipopolysaccharides (LPS) or other outer membrane proteins. To differentiate an increase in antibody titer from an increase in AI upon boosting, the AIs of pre- and postbooster sera with similar anti-OMV IgG titers were compared. Although the IgG titers were equal, the postbooster group had a much higher AI. This was associated with a significant increase in the SBA titer. This finding indicates that the number of vaccinations which a child received was more important for avidity maturation than the level of IgG and that an increase in avidity after boosting had a strong effect on SBA. A booster vaccination seems to be necessary for optimal antibody maturation. Although the correlation between SBA and AI was rather low (r = 0.36), an increase in AI clearly coincided with an increase in SBA. However, in the prebooster samples AI increased significantly, while the anti-PorA antibody titers (Fig. 1), as well as SBA (9), decreased. This phenomenon was also seen in studies in which meningococcal C and Hib conjugate vaccines were used (4, 14). The authors of these studies suggested that avidity maturation after priming is associated with the establishment of immunological memory and that because of this memory, protection against disease at that time is possible. Analogously, the avidity maturation after a primary series of meningococcal B OMV vaccinations may predict immunological memory and long-term protection, but this can be proven only in a phase III efficacy trial. To confirm that the avidity maturation in our study was induced by vaccination and was not an effect of aging, we tested a selection of serum samples from a control group used in a previous study. The toddlers in the previous study were vaccinated with hepatitis B vaccine instead of a hexavalent OMV vaccine and received one dose of monovalent OMV vaccine 2.5 years later (ages, 5 to 6 years). The mean AI for this group was much lower than the mean AI after the booster vaccination in this study (29.9%; CI, 23.5 to 36.3%; n = 10).
Variation in the immunization schedules and different aluminum adjuvants had no effect on the postbooster AI. Variance analysis revealed that the difference between adjuvants was not significant (P = 0.13) during the course of vaccination. However, the 3+1 schedule resulted in higher anti-OMV IgG titers, which resulted in significantly higher SBA titers (P = 0.03), in accordance with previous results (9).
The isotype distribution of the immune response after immunization with an OMV vaccine was dominated by IgG1 and IgG3, in agreement with data obtained previously (22). As shown in Fig. 3, the distribution of IgG1 and IgG3 did not change during the vaccination trial, except for a significant decline in the IgG3 titer in the prebooster samples. This phenomenon was probably due to the shorter half-life of IgG3 (22). Thus, avidity maturation is not related to a shift in IgG isotype distribution during the course of vaccination. We did not detect specific IgG2 in any sample. A previous study performed in our laboratory revealed low IgG2 levels detected by a whole-cell ELISA after immunization with a hexavalent OMV vaccine (10). The difference between the two studies may be explained by differences in coating conditions, as we used OMVs for ELISA plate coating instead of whole cells. OMVs contain small amounts of galE LPS, whereas whole cells contain the wild-type LPS (immunotype L3). Perhaps the IgG2 antibodies were directed against LPS or other proteins or polysaccharides present in whole-cell preparations.
Previously, we could not find a good correlation between SBA and specific IgG or IgG subclasses using whole-cell ELISA after vaccination with a hexavalent vaccine. The correlation values ranged from insignificant up to 0.64 for total IgG or IgG subclasses (10). However, in a study in which the Norwegian monovalent P1.7,16 OMV vaccine was used, significant correlations were found for total IgG, IgG1, IgG3, and SBA (22). In this study, in which a monovalent vaccine was also used, the correlations with SBA were high, varying from 0.82 for IgG3 and 0.83 for IgG1 to 0.85 for total IgG (P < 0.0001). The correlation between SBA and the avidity titer was similar (r = 0.84; P < 0.0001). Most antibodies induced by a monovalent vaccine are probably functionally active, whereas not all antibodies induced by the hexavalent vaccine are. Furthermore, the whole-cell ELISA method, which was used in the hexavalent study, may not be a very effective method for measuring the specific response to PorA compared with an OMV ELISA. We observed high background signals when we used a whole-cell ELISA, even with prevaccination samples. It seems that whole cells bind more nonbactericidal antibodies than OMVs bind.
We concluded that a meningococcal B monovalent OMV vaccine against serosubtype P1.7-2,4 induced high levels of functional, PorA-specific IgG antibodies, as indicated by a strong correlation between ELISA titers and SBA. Avidity maturation of PorA-specific antibodies occurred during the course of vaccination, especially after boosting. The AIs of antibodies after the booster vaccination were comparable for the 2+1 and 3+1 vaccination schedules and with different aluminum adjuvants. Avidity maturation was independent of the level of IgG and the age at the time of vaccination. Serum antibodies with high AIs were more effective in SBA than low-AI antibodies. This cannot be explained by differences in IgG isotypes, since IgG1 predominated during the course of vaccination, followed by IgG3, and a booster vaccination did not change this pattern. Our results indicated that the SBA of serum after vaccination depends on PorA-specific antibody concentrations in particular and that there is an additional effect of AI. AI should be measured in future efficacy trials with serogroup B meningococcal vaccines to investigate its potential as a predictor of immunological memory and long-term protection.
Acknowledgments
We thank N. J. D. Nagelkerke for help with statistics and B. A. Adler for critically reading the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Aase, A., G. Bjune, E. A. Hoiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614–1621. [DOI] [PubMed] [Google Scholar]
- 3.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093–1096. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, D. R., L. Gregory, and R. Jefferis. 1986. Aspects of the molecular structure of IgG subclasses. Monogr. Allergy 19:7–35. [PubMed] [Google Scholar]
- 6.Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 17:2612–2619. [DOI] [PubMed] [Google Scholar]
- 7.Connolly, M., and N. Noah. 1999. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993-6. European Meningitis Surveillance Group. Epidemiol. Infect. 122:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, D. P. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rümke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2–3 and 7–8 years of age. Vaccine 18:1456–1466. [DOI] [PubMed] [Google Scholar]
- 9.de Kleijn, E. D., R. de Groot, A. B. Lafeber, J. Labadie, K. C. van Limpt, J. Visser, G. A. Berbers, L. van Alphen, and H. C. Rümke. 2000. Immunogenicity and safety of monovalent p1.7(h),4 meningococcal outer membrane vesicle vaccine in toddlers: comparison of two vaccination schedules and two vaccine formulations. Vaccine 19:1141–1148. [DOI] [PubMed] [Google Scholar]
- 10.de Kleijn, E. D., L. van Eijndhoven, C. L. Vermont, B. Kuipers, H. van Dijken, H. Rümke, R. de Groot, L. van Alphen, L., and G. van den Dobbelsteen. 2001. Serum bactericidal activity and isotype distribution of antibodies in toddlers and school children after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 20:352–358. [DOI] [PubMed] [Google Scholar]
- 11.de Moraes, J. C., B. A. Perkins, M. C. Camargo, N. T. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. Landgraf, V. L. Gattas, H. Vasconcelos, and I. M. Gral. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074–1078. [DOI] [PubMed] [Google Scholar]
- 12.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357. [DOI] [PubMed] [Google Scholar]
- 13.Goldblatt, D., L. van Etten, F. J. van Milligen, R. C. Aalberse, and M. W. Turner. 1993. The role of pH in modified ELISA procedures used for the estimation of functional antibody affinity. J. Immunol. Methods 166:281–285. [DOI] [PubMed] [Google Scholar]
- 14.Goldblatt, D., A. R. Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112–1115. [DOI] [PubMed] [Google Scholar]
- 15.Hammarstrom, L., and C. I. Smith. 1986. IgG subclass changes in response to vaccination. Monogr. Allergy 19:241–252. [PubMed] [Google Scholar]
- 16.Hammarstrom, L., and C. I. Smith. 1986. IgG subclasses in bacterial infections. Monogr. Allergy 19:122–133. [PubMed] [Google Scholar]
- 17.Jefferis, R., C. B. Reimer, F. Skvaril, G. de Lange, N. R. Ling, J. Lowe, M. R. Walker, D. J. Philips, C. H. Aloisio, T. W. Wells, J. P. Vaerman, C. G. Magnusson, H. Kubagawa, M. Cooper, F. Vartdal, B. Vandvik, J. J. Haaijman, O. Makela, A. Sarnesto, Z. Lando, J. Gergely, E. Rajnavölgyi, G. László, J. Radl, and G. A. Molinaro. 1985. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/W. H. O. collaborative study. Immunol. Lett. 10:223–252. [DOI] [PubMed] [Google Scholar]
- 18.Jefferis, R., C. B. Reimer, F. Skvaril, G. de Lange, D. M. Goodall, T. L. Bentley, D. J. Philips, A. Vlug, S. Harada, J. Radl, E. Claassen, J. A. Boersma, and J. Coolen. 1992. Evaluation of monoclonal antibodies having specificity for human IgG subclasses: results of the 2nd IUIS/W. H. O. collaborative study. Immunol. Lett. 31:143–168. [DOI] [PubMed] [Google Scholar]
- 19.Lottenbach, K. R., C. M. Mink, S. J. Barenkamp, E. L. Anderson, S. M. Homan, and D. C. Powers. 1999. Age-associated differences in immunoglobulin G1 (IgG1) and IgG2 subclass antibodies to pneumococcal polysaccharides following vaccination. Infect. Immun. 67:4935–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas, A. H., and D. M. Granoff. 1995. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J. Immunol. 154:4195–4202. [PubMed] [Google Scholar]
- 21.Macdonald, R. A., C. S. Hosking, and C. L. Jones. 1988. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J. Immunol. Methods 106:191–194. [DOI] [PubMed] [Google Scholar]
- 22.Naess, L. M., T. Aarvak, A. Aase, F. Oftung, E. A. Hoiby, R. Sandin, and T. E. Michaelsen. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754–764. [DOI] [PubMed] [Google Scholar]
- 23.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683–691. [DOI] [PubMed] [Google Scholar]
- 24.Persson, M. A., S. E. Brown, M. W. Steward, L. Hammarstrom, C. I. Smith, C. R. Howard, M. Wahl, B. Rynnel-Dagoo, G. Lefranc, and A. O. Carbonara. 1988. IgG subclass-associated affinity differences of specific antibodies in humans. J. Immunol. 140:3875–3879. [PubMed] [Google Scholar]
- 25.Pollard, A. J., and M. Levin. 2000. Production of low-avidity antibody by infants after infection with serogroup B meningococci. Lancet 356:2065–2066. [DOI] [PubMed] [Google Scholar]
- 26.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83–87. [DOI] [PubMed] [Google Scholar]
- 27.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160–163. [DOI] [PubMed] [Google Scholar]
- 28.Schlesinger, Y., and D. M. Granoff. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA 267:1489–1494. [PubMed] [Google Scholar]
- 29.Scott, H., O. Fausa, J. Ek, K. Valnes, L. Blystad, and P. Brandtzaeg. 1990. Measurements of serum IgA and IgG activities to dietary antigens. A prospective study of the diagnostic usefulness in adult coeliac disease. Scand. J. Gastroenterol. 25:287–292. [PubMed] [Google Scholar]
- 30.Tommassen, J., P. Vermeij, M. Struyve, R. Benz, and J. T. Poolman. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (porA) and class 3 (porB) outer membrane proteins. Infect. Immun. 58:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]