Abstract
The mechanism whereby whole-cell pertussis vaccines (WCV) confer protection against Bordetella pertussis is still not fully understood. We have previously reported that macrophage activation produced by vaccination with WCV is associated with induction of NO synthesis by macrophages in response to in vitro stimulation with B. pertussis antigens. To determine whether NO production is an effector of protection or simply a marker of activation, the susceptibility of inducible nitric oxide synthase (type II, iNOS) knockout mice to infection with B. pertussis was examined. We showed that iNOS knockout mice were more susceptible to B. pertussis respiratory challenge than wild-type mice. iNOS-deficient mice also developed a less effective protective response than wild-type mice after the same immunization with WCV. This suggests that NO plays an important role in effecting protection against B. pertussis challenge.
Bordetella pertussis is a human pathogen which possesses tropism for the respiratory system, causing an acute and sometimes persistent disease. Although pertussis vaccines have been in use for mass vaccination in most countries for many years and have led to a major decrease in the incidence of pertussis, the mechanism by which they induce protection against pertussis in children is still unclear. Recent evidence indicates that B. pertussis is a facultatively intracellular organism and that clearance involves activated macrophages (4, 15, 20, 21). The mechanism whereby macrophage activation results in the killing of facultatively intracellular pathogens is still incompletely determined. However, it has become increasingly apparent in recent years that NO and reactive nitrogen intermediates (nitrite and peroxynitrite) are potentially important mediators of the immune system (1). Production of NO by activated murine macrophages has been implicated as an antimicrobial effector mechanism against several pathogens (2, 5, 9). We have reported previously that macrophage activation produced by vaccination with a whole-cell pertussis vaccine (WCV) is associated with induction of NO synthesis by macrophages in response to in vitro stimulation with B. pertussis antigens (20). The presence of small quantities of active pertussis toxin seems to be important for this process (21). The relationship between NO induced in macrophages in response to in vitro culture with bacterial antigens and protection in vivo in the mouse intracerebral challenge model indicates that macrophage activation is involved in protective immunity (20). However, it is not clear from these studies whether NO is an effector of protection or simply a coincidental marker of activation.
To clarify further the role of NO in protection against B. pertussis challenge, the induction of NO synthesis by macrophages and protection in vivo against aerosol challenge induced by a conventional WCV and the new-generation acellular pertussis vaccine (ACV) was investigated in inducible nitric oxide synthase (iNOS)-deficient mice.
MATERIALS AND METHODS
Vaccines.
A WCV (National Institute for Biological Standards and Control [NIBSC] reagent 88/522, 3rd British Reference Preparation; potency, 50 IU/ampoule) (14) and a commercially available three-component ACV containing 25 μg of pertussis toxoid (PT) chemically detoxified with formaldehyde and glutaraldehyde, 25 μg of filamentous hemagglutinin (FHA), and 8 μg of pertactin (PRN) per single human dose (SHD), in combination with diphtheria and tetanus toxoids (DTPa), was used for the immunization. All other reagents were of analytical grade.
Animals.
iNOS-deficient mice and their wild-type littermates were generated as described previously (17). The murine iNOS gene was disrupted by homologous recombination in 129sv embryonic stem (ES) cells. The recombinant allele was passed through the germ line following mating of ES cell chimeras with 129sv (Harlan UK Ltd., Oxford, United Kingdom). The homozygous, heterozygous, and wild type littermates of the 129sv strains were used at the ages of approximately 3 to 4 weeks.
Immunogenicity study.
Groups of five mice were immunized (intraperitoneally [i.p.]) with ACV at 0.25 SHD per dose and with WCV at 0.125 IU per dose (which is equivalent to approximately 0.03 SHD), and both vaccines were diluted in phosphate-buffered saline (PBS). Mice in the control group received PBS. Mice were terminally bled at 4 weeks postimmunization, and sera from individual animals were assayed for total immunoglobulin G (IgG) antibodies to the B. pertussis antigens PT, FHA, and PRN by a standard enzyme-linked immunosorbent assay (ELISA). The geometric mean ELISA units (EU) of the antibody to each antigen were calculated against the First World Health Organization (WHO) International Reference Anti-B. pertussis Serum (Mouse) (19). All the serum samples were always analyzed in parallel with the reference antiserum on the same plate. Relative concentrations of IgG1 and IgG2a specific for the B. pertussis antigens PT, FHA, and PRN were measured by using sheep anti-mouse IgG subclass-biotin and horseradish peroxidase-avidin conjugates (PharMingen) (11). Specific responses for each subclass were presented as the ratio of the optical density at 492 nm (OD492) of the test sample to the OD492 of the reference serum used in each plate.
Bacterial antigens.
Heat-killed B. pertussis 18.323 cells (HKC) were prepared by incubation of bacterial cells (5 × 109/ml) in PBS at 80°C for 30 min (20). Purified detoxified PT, FHA, and PRN were kindly provided by GlaxoSmithKline, Rixensart, Belgium.
Macrophages.
Mice were immunized with WCV or ACV at the indicated doses. Control mice received PBS. Macrophage cultures were prepared according to the method described by Torre et al. (16). In brief, mice were terminally bled on the indicated day postimmunization. The peritoneal cavity was then lavaged with sterile PBS to recover macrophages. Cells were pooled from groups of 6 to 10 mice and recovered by centrifugation. Cell pellets were resuspended in RPMI 1640 medium with l-glutamine supplemented with 10% fetal calf serum, 1% penicillin, and 1% streptomycin, placed in 24-well tissue culture plates at 2 × 106 cells per well, and incubated at 37°C under 5% CO2 in 90% humidity for 2 h. Cells were washed three times with RPMI 1640 medium to remove nonadherent cells.
Culture of macrophages and determination of nitrite production.
Macrophages were cultured in a total volume of 1 ml/well with 2 × 106 HKC/ml. Cultures were incubated at 37°C under 5% CO2 in 90% humidity for 24 h. Cell viability was checked by trypan blue exclusion before and after incubation. Nitrite determinations were made on 50 μl of sample mixed with 50 μl of the Griess reagent (16), and the A540 was measured using an Anthos ELISA Reader (Life Sciences International, Basingstoke, United Kingdom).
In vivo aerosol challenge.
Bacterial suspensions (B. pertussis 18.323), stored at −70°C in 5% glycerol, were spread on charcoal agar plates (charcoal agar base plus 10% defibrinated horse blood) and incubated at 37°C for 2 days. Two further subcultures were performed with incubation for 16 to 18 h under the same conditions. Bacterial cells were harvested and resuspended in 0.9% saline containing 1% casein and adjusted to an OD623 of 0.2 by using a spectrophotometer (MSE-Fisons, Loughborough, United Kingdom). The suspension was kept on ice until it was used for aerosol challenge. Aerosol challenge was performed on groups of five previously immunized mice that were exposed to B. pertussis 18.323 for 5 min by use of a custom-made aerosol apparatus (22). The lungs and tracheas were removed from each group at the indicated time point after challenge and were homogenized in 1 ml of 1% casein solution by means of a mini-bead beater using 2.5- to 3.5-mm-diameter glass balls. Viable counts were then performed on the homogenate by diluting across microtiter plates and plating onto charcoal agar plates. The mean viable count per lung from five mice was taken as the CFU per lung.
Statistical analysis.
Data obtained from immunized subjects were compared with those for other groups by the Student t test in order to determine the statistical significance of differences between two groups. P values below 0.05 were regarded as statistically significant.
RESULTS
The time course of B. pertussis infection in wild-type and mutant mice was investigated by aerosol challenge on naive mice, and CFU counts in their lungs were taken at indicated time points (Fig. 1). Replication of the organisms resulted in similar increases in the numbers of bacteria recoverable from the two groups of mice, approximately 2.5 to 3.0 log units by day 7 to 10. However, bacterial counts subsequently declined more rapidly in wild-type mice than in iNOS knockout mice. Thus, by day 18, bacterial counts in iNOS-deficient mice were at least 0.5 log unit higher than those in wild-type mice, and this difference (P < 0.05) was maintained until the end of the experiment.
FIG. 1.
Time course of infection. Groups of wild-type (•) and mutant (▴) mice were aerosol infected with B. pertussis 18.323. Lungs from five mice in each group were sampled, and viable counts were performed by four replicates. Each data point represents the mean CFU per lung. Error bars, standard deviations.
To assess the ability of murine peritoneal macrophages to generate NO in response to in vitro stimulation with HKC, mice were immunized with WCV or ACV. The peak time for production of NO in the macrophage cultures was approximately 10 to 15 days postimmunization (data not shown). Therefore, in this study all macrophages were isolated from mice on day 15 post immunization.
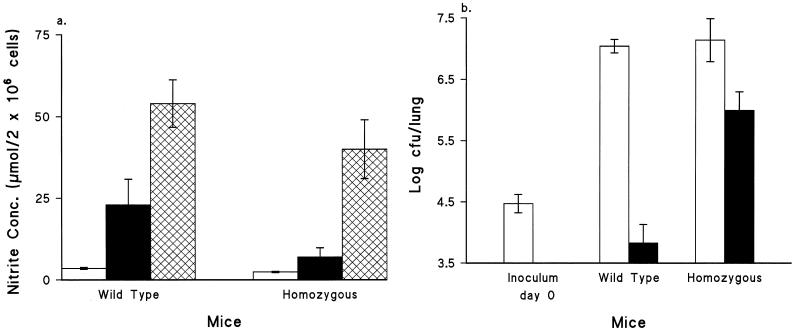
Peak NO concentrations in macrophage cultures from the wild type group were approximately threefold higher than those from the mutant group. Furthermore, macrophages from mice lacking iNOS produced lower NO concentrations in their cultures after in vitro restimulation with HKC than cells recovered from wild-type mice (Fig. 2a). This corresponded closely to the resistance to aerosol challenge observed in vivo in the two types of mice (Fig. 2b), where the CFU count per lung was approximately 2 log units lower in wild-type than in mutant mice.
FIG. 2.
(a) Ability of murine peritoneal macrophages to generate NO in response to HKC. Mice were immunized with WCV at 0.25 IU/dose, and macrophages were taken on day 15 postimmunization. Open bars, cells from control mice; solid bars, cells from immunized mice without stimulation in vitro; crosshatched bars, cells from immunized mice stimulated with HKC in vitro. Data are means ± standard deviations (n = 3). Cultures were incubated at 37°C for 24 h. (b) Response to aerosol challenge by groups of five mice immunized with PBS (open bars) or with WCV at 0.25 IU/dose (solid bars). Mice were aerosol challenged on day 15 after immunization. Mouse lungs were removed at day 7 after the challenge. Viable counts per lung were performed on four replicates. Bars represent mean CFU per lung; error bars, standard deviations.
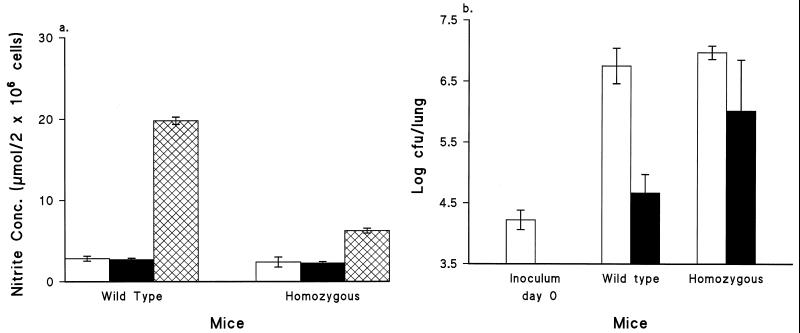
Macrophages from mice immunized with ACV were also assayed for NO production with and without the addition of HKC. Unlike those from mice immunized with WCV, macrophages from mice immunized with ACV did not produce NO concentrations higher than those for the control group before the stimulation in vitro (baseline) (Fig. 3a). However, these macrophages were able to produce NO in response to B. pertussis HKC stimulation, although the levels were lower than those for the WCV-immunized group. NO concentrations in cultures from the wild-type group were more than threefold higher than those in cultures from the homozygous group. This also closely paralleled the pattern of in vivo protection against aerosol challenge, where CFU counts in the lungs of the mutant group were approximately 1.5 log units higher (P < 0.05) than those in the lungs of the wild-type group (Fig. 3b).
FIG. 3.
(a) Ability of murine peritoneal macrophages to generate NO in response to B. pertussis (HKC). Mice were immunized with 0.25 SHD of ACV. Macrophages were collected on day 15 postimmunization. Open bars, cells from control mice stimulated with HKC in vitro; solid bars, cells from immunized mice in the absence of stimulant; crosshatched bars, cells from immunized mice stimulated with HKC in vitro. Data are means ± standard deviations (n = 3). Cultures were incubated at 37°C for 24 h. (b) Response to aerosol challenge by groups of five mice immunized with PBS (open bars) or ACV at 0.25 SHD (solid bars). Mice were aerosol challenged on day 15 after immunization. Mouse lungs were removed at day 7 after the challenge. Viable counts per lung were performed on four replicates. Bars represent mean CFU per lung; error bars, standard deviations.
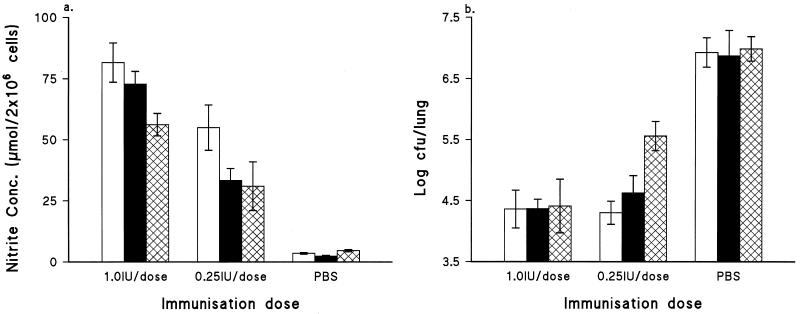
To investigate the relationship of the NO produced by activated macrophages to host defense against B. pertussis challenge, different immunization doses were used. Figure 4a shows that the synthesis of NO by macrophages in response to HKC restimulation was immunization dose dependent, and again, macrophages from the wild-type group had higher NO production than those from the mutant group. It was noted in the protection study that when mice were immunized with WCV at 1.0 IU/dose, similar reductions (of approximately 2.5 to 3.0 log units) in lung CFU counts occurred in both wild-type and mutant groups in comparison with the control group (Fig. 4b). This was in spite of the fact that cells from wild-type mice produced higher NO levels in their cultures than those from the mutant group. Reduction of the immunization dose from 1.0 to 0.25 IU resulted in different protection profiles for these two groups of mice, which corresponded with the NO concentrations achieved in their macrophage cultures. That is, the mutant mice showed a lower level of protection than the wild-type mice.
FIG. 4.
(a) Effect of immunization dose on NO induction by murine peritoneal macrophages taken from groups of five wild-type (open bars), heterozygous (solid bars), and mutant (crosshatched bars) mice at 2 weeks after immunization with WCV at the indicated dose and stimulated with HKC. All cultures were incubated at 37°C for 24 h. Bars represent means of triplicate cultures; error bars, standard deviations. (b) Effect of immunization dose on protection in groups of five wild-type (open bars), heterozygous (solid bars), and mutant (crosshatched bars) mice at 2 weeks after immunization with WCV at the indicated dose. All mice were challenged by aerosol exposure to B. pertussis.
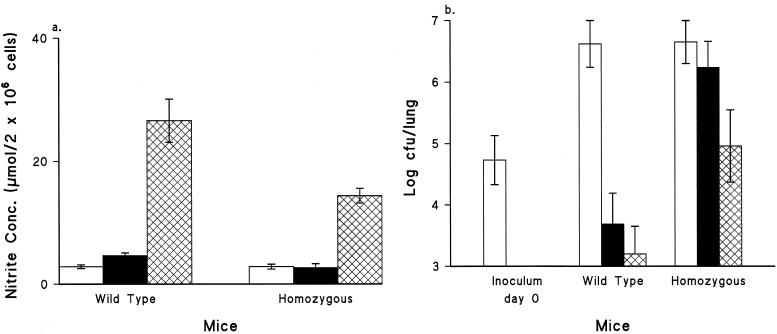
To investigate further the cellular and humoral immune responses induced by immunization of these two types of mice with WCV and ACV, pertussis-specific antibody production, macrophage activation, and in vivo protection were assessed 4 weeks after immunization. Macrophages from both wild-type and mutant mice immunized with WCV produced approximately 5.5-fold-higher NO concentrations in their cultures after stimulation with HKC than macrophages from the ACV-immunized group (P < 0.05) (Fig. 5a). The NO concentration in macrophage cultures from the mutant group immunized with WCV was approximately half that for the wild-type group. There was no difference (P > 0.05) in NO induction between the mutant group immunized with ACV and the control group.
FIG. 5.
(a) Ability of murine peritoneal macrophages to generate NO in response to HKC. Mice were immunized with PBS (open bars), 0.25 SHD of ACV (solid bars), or 0.125 IU of WCV (crosshatched bars). Four weeks later, macrophages were stimulated with HKC in vitro. Data are means ± standard deviations (n = 3). Cultures were incubated at 37°C for 24 h. (b) Response to aerosol challenge by groups of five mice immunized with PBS (open bars), ACV at 0.25 SHD (solid bars), or WCV at 0.125 IU (crosshatched bars). Mice were aerosol challenged at 4 weeks after immunization. Mouse lungs were removed at day 7 after the challenge. Viable counts per lung were performed on four replicates. Bars represent mean CFU per lung; error bars, standard deviations.
Titers of antibody to PT, FHA, and PRN (69 kDa) were much higher in the ACV group than in the WCV group (Table 1). The ratio of IgG1 to IgG2a showed that mice immunized with WCV gave a response shifted towards Th1, whereas those immunized with ACV gave a response biased towards Th2. No difference in antibody production was found between wild-type and mutant mice immunized with WCV. However, among mice immunized with ACV, lower levels of antibodies to FHA and PRN were observed in the mutant group than in the wild-type group. Aerosol challenge of mice at 4 weeks after immunization showed that mice immunized with WCV had developed better protection (P ≦ 0.05) than mice immunized with ACV despite higher antibody responses developing in the latter group (Fig. 5b). Comparison of wild-type mice and mutant mice showed that the former group were better protected from the challenge than the latter group (P < 0.05) when immunized with WCV or ACV.
TABLE 1.
Geometric means of total IgG antibody responses to PT, FHA, and PRN, and ratio of IgG1 to IgG2a at 4 weeks postimmunization
| Groupa | Vaccineb | Antibody responsec
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PT
|
FHA
|
PRN
|
|||||||
| Total IgG (EU/ml) | IgG1/IgG2a ratio | Total IgG (EU/ml) | IgG1/IgG2a ratio | Total IgG (EU/ml) | IgG1/IgG2a ratio | ||||
| WT | PBS | U | U | U | |||||
| Homo. | PBS | U | U | U | |||||
| WT | WCV | 0.01 | 0.64 | 5.77 | 0.18 | 4.53 | 0.12 | ||
| Homo. | WCV | 0.02 | 0.58 | 5.97 | 0.14 | 5.10 | 0.13 | ||
| WT | ACV | 658.77 | 1.91 | 211.76 | 2.93 | 54.93 | 1.93 | ||
| Hom | ACV | 628.07 | 2.53 | 150.67 | 3.41 | 24.08 | 3.19 | ||
WT, wild type; Homo., homozygous.
WCV was used at 0.125 IU/dose; ACV was used at 0.25 SHD.
U, undetectable.
DISCUSSION
Infection by B. pertussis, usually manifesting as pertussis (whooping cough), is still an important cause of morbidity and mortality among children in many parts of the world (18). It is also being recognized increasingly as a significant agent of respiratory disease in adults (Editorial, Lancet 339:526-527, 1992). The results of recent clinical trials have indicated that both established WCVs and the new generation of ACVs can stimulate protection in children (6, 7). We have previously reported that macrophage activation produced by vaccination with WCV is associated with induction of NO synthesis by macrophages in response to in vitro stimulation with B. pertussis antigens (20, 21). In the present study, iNOS knockout mice were used to further examine the role of NO in protection from B. pertussis respiratory challenge.
It has been reported previously that iNOS-deficient mice produced more gamma interferon (IFN-γ) and less interleukin 4 than similarly treated intact mice following infections and antigenic stimulation (10, 12, 13). In the present study, the results showed that elimination of B. pertussis from the lungs of infected iNOS knockout mice was slower than elimination from the lungs of wild-type mice after respiratory challenge. The iNOS-deficient mice also showed a lower level of protection than the wild-type mice following the same immunization with WCV or ACV in spite of producing a greater IFN-γ response to the bacterial antigens in vitro (data not shown). This provides a further indication that NO is an important effector molecule in protection against B. pertussis challenge.
Macrophages from wild-type and mutant mice immunized with the WCV all produced NO in response to in vitro stimulation with bacterial cells. This suggests that this type of vaccine is a very powerful inducer of NO synthase even in iNOS-deficient mice. That the latter still produced some NO in spite of the gene disruption may be attributable to the operation of compensatory mechanisms through other pathways. Constitutive NOS may also have contributed a background level of NO. It should be noted that the iNOS gene-targeting construct was produced by terminal extension and integration into the iNOS gene. This disrupts the gene and should prevent expression but may not completely eliminate it (3, 8). In the present study, these mice produced substantially less NO than wild-type mice. However, NO may not be the only effector of protection. It was notable that when mice were immunized with higher doses of the vaccine, there was no difference in protection between wild-type and mutant mice, even though the wild-type mice produced more NO in their macrophage cultures. This may have been because the high vaccine doses stimulated an adequate NO response in the knockout mice and the greater amount produced by the wild-type mice added nothing further to protection, or it may suggest that at high vaccine doses another mechanism comes into play which is not dependent on the bactericidal action of NO.
Macrophages isolated from mice immunized with WCV produced larger amounts of NO than those from the control group without additional stimulation. Our previous studies showed that this NO production was increased by adding HKC but not by IFN-γ and that NO induced by HKC was only partially blocked by concentrations of anti-IFN-γ which completely blocked NO production in control cell cultures (20). Taken together, these results suggested that these macrophages had already been activated in vivo. It is noteworthy that macrophages from mice immunized with ACV did not produce NO in vitro in the absence of stimulant. However, NO production was significantly increased by the addition of HKC, and this was clearly associated with protection in vivo. These results suggested that there might be a difference in the degree of macrophage activation produced in vivo by immunization with these two different types of vaccines. Furthermore, mice immunized with ACV produced lower IFN-γ levels (data not shown) in culture than those immunized with WCV after stimulation in vitro. This may indicate that mice immunized with WCV developed a stronger Th1 type response than those that received ACV.
Although pertussis vaccination is used throughout the world and has made a major contribution to decreasing morbidity and mortality from pertussis, its precise mode of action is still unclear. There is, however, increasing recognition of the importance of cell-mediated immunity in protection against B. pertussis. The present study using iNOS knockout mice has provided direct evidence that the reactive nitrogen intermediates play an important role in the immune response induced by both WCV and ACV and that this is associated with protective immunity in vivo . This adds further weight to the hypothesis that activation of the killing mechanisms of macrophages helps to eliminate intracellular B. pertussis and hence to clear infection.
Acknowledgments
We thank F. Y. Liew of the Department of Immunology and Bacteriology, University of Glasgow, Glasgow, United Kingdom, for suggesting the use of iNOS knockout mice and encouraging this study.
This work was supported in part by funding from a Home Office Animal Procedures Research Grant.
Editor: J. D. Clements
REFERENCES
- 1.Babior, B. M. 1984. The respiratory burst of phagocytes. J. Clin. Investig. 73:599-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. J. Immunol. 150:888-895. [PubMed] [Google Scholar]
- 3.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 4.Friedman, R. L., K. Nordensson, L. Wilson, E. T. Akporiaye, and D. E. Yocum. 1992. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect. Immun. 60:4578-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger, D. L., B. J. Hibbs, J. R. Perfect, and D. T. Durack. 1988. Specific amino acid (l-arginine) requirement for the microbiostatic activity of murine macrophages. J. Clin. Investig. 81:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco, D., S. Salmaso, P. Mastrantonio, and the Progetto Pertosase Working Group. 1996. A controlled trial of two acellular vaccines and one whole cell vaccine against pertussis. N. Engl. J. Med. 334:341-348. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. A. Storsaeter. 1996. Controlled trial of a two component acellular, a five component acellular, and a whole cell pertussis vaccine. N. Engl. J. Med. 334:349-355. [DOI] [PubMed] [Google Scholar]
- 8.Huang, F. P., W. Niedbala, X. Q. Wei, G. J. Feng, J. H. Robinson, C. Lam, and F. Y. Liew. 1998. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 28:4062-4070. [DOI] [PubMed] [Google Scholar]
- 9.James, S. L., and J. Glaven. 1989. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 143:4208-4212. [PubMed] [Google Scholar]
- 10.Maclean, A., X. Q. Wei, F. P. Huang, U. A. Al-Alem, W. L. Chan, and F. Y. Liew. 1998. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 79:825-830. [DOI] [PubMed] [Google Scholar]
- 11.Mawas, F., I. M. Feavers, and M. J. Corbel. 2000. Serotype of Streptococcus pneumoniae capsular polysaccharide can modify the Th1/Th2 cytokine profile and IgG subclass response to pneumococcal-CRM(197) conjugate vaccines in a murine model. Vaccine 19:1159-1166. [DOI] [PubMed] [Google Scholar]
- 12.Mclnnes, I. B., B. Leung, X. Q. Wei, C. C. Gemmell, and F. Y. Liew. 1998. Septic arthritis following Staphylococcus aureus infection in mice lacking inducible nitric oxide synthase. J. Immunol. 160:308-315. [PubMed] [Google Scholar]
- 13.Niedbala, W., X. Q. Wei, D. Piedrafita, D. Xu, and F. Y. Liew. 1999. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 29:2498-2505. [DOI] [PubMed] [Google Scholar]
- 14.Redhead, K., and R. E. G. Das. 1991. A collaborative assay of the proposed third British reference preparation for pertussis vaccine and of the relative potencies of the second international standard and the second British reference preparation for pertussis vaccine. Biologicals 19:107-111. [DOI] [PubMed] [Google Scholar]
- 15.Saukkonen, K., C. Cabellos, M. Burroughs, S. Prasad, and E. Tuomanen. 1991. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J. Exp. Med. 173:1143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torre, D., G. Ferrario, G. Bonetta, L. Perversi, and F. Speranza. 1996. In vitro and in vivo induction of nitric oxide by murine macrophages stimulated with Bordetella pertussis. FEMS Immunol. Med. Microbiol. 13:95-99. [DOI] [PubMed] [Google Scholar]
- 17.Wei, X. Q., I. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 1999. Informal consultation on the control of pertussis with whole cell and acellular vaccines. World Health Organization Report WHO/V&B/99.03. World Health Organization, Geneva, Switzerland.
- 19.Xing, D., P. Rigsby, P. Newland, and R. E. G. Das. 1999. International collaborative study evaluation of proposed international reference reagent of pertussis antiserum (mouse). World Health Organization Report WHO/BS/99.1901. World Health Organization, Geneva, Switzerland. [DOI] [PubMed]
- 20.Xing, D. K. L., C. Canthaboo, and M. J. Corbel. 1998. Nitric oxide induction in murine macrophages and spleen cells by whole cell Bordetella pertussis vaccine. Vaccine 16:16-23. [DOI] [PubMed] [Google Scholar]
- 21.Xing, D. K. L., C. Canthaboo, and M. J. Corbel. 2000. Effect of pertussis toxin on the induction of nitric oxide synthesis in murine macrophages and on protection in vivo. Vaccine 18:2110-2119. [DOI] [PubMed] [Google Scholar]
- 22.Xing, D. K. L., R. G. Das, L. Williams, C. Canthaboo, J. Tremmil, and M. J. Corbel. 1999. An aerosol challenge model of Bordetella pertussis infection as a potential bioassay for acellular pertussis vaccines. Vaccine 17:565-576. [DOI] [PubMed] [Google Scholar]