Abstract
Group B streptococci (GBS) express various surface antigens designated c, R, and X antigens. A new R-like surface protein from Streptococcus agalactiae strain Compton R has been identified by using a polyclonal antiserum raised against the R protein fraction of this strain to screen a lambda Zap library. DNA sequence analysis of positive clones allowed the prediction of the primary structure of a 105-kDa protein designated BPS protein (group B protective surface protein) that exhibited typical features of streptococcal surface proteins such as a signal sequence and a membrane anchor region but did not show significant similarity with other known sequences. Immunogold electron microscopy using a BPS-specific antiserum confirmed the surface location of BPS protein on S. agalactiae strain Compton R. Anti-BPS antibodies did not cross-react with R1 and R4 proteins expressed by two variant type III GBS strains but reacted with the parental streptococcal strain in Western blot and immunoprecipitation analyses. Separate R3 and BPS immunoprecipitation bands were observed when a cell extract of strain Compton R was tested with an antiserum against Compton R previously cross-absorbed to remove R4 antibodies. Immunization of mice with recombinant BPS protein by the subcutaneous route produced an efficient antigen-specific response, and immunized animals survived challenge with a lethal dose of a virulent strain. Therefore, BPS protein represents a new R-like protective antigen of GBS.
Group B streptococci (GBS) have emerged as an important cause of infection in neonates that is characterized by a high mortality rate, even in developed countries (1). In the United States alone, more than 15,000 cases and 1,300 deaths due to GBS occur each year (46). The problem of GBS infection of neonates lies in the fast and dramatic course of infection, which can only be treated inadequately with antibiotics (13). Since many women of child-bearing age have vaginal GBS colonization, they are tested during pregnancy for carriage of GBS (31). Besides affecting neonates, GBS cause a number of maternal peripartum diseases and are responsible for serious illness in nonpregnant adults (6). Necrotizing fasciitis and toxic shock-like syndrome due to GBS have recently been reported in adults (12).
GBS strains comprise nine serotypes based on the presence of specific capsular polysaccharides. Of these, serotypes Ia, Ib, II, III, and V have been most prevalent. Approximately 40% of isolates in cases with invasive GBS disease express Ia polysaccharide, and 27% express type III polysaccharide (24). In certain geographic areas, serotype V is emerging as the predominant serotype (14, 19, 30). The occurrence of type V isolates, however, increases with age, whereas that of other serotypes decreases (14, 19, 30). Because of increasing antibiotic resistance and the restriction of antibiotic therapy during pregnancy (33), it is desirable to develop alternatives to antibiotic therapy. Clinical studies have shown that newborns whose mothers have high titers of anti-GBS antibodies are seldom infected (2). An attractive alternative to classic antibiotic therapy could be vaccination of women of child-bearing age to protect newborns against GBS infection (3). Since the GBS capsule plays an important role in virulence, attempts have been made to develop a vaccine based on capsular polysaccharides. However, these vaccination studies have been unsuccessful because of antigenic variation and the low immunogenicity of capsular polysaccharides. A potential solution to this problem can be the use of glycoconjugates as candidate vaccines (43).
Because of the suboptimal immunogenicity of capsule-based vaccines, interest has shifted toward the surface protein antigens of GBS as vaccine candidates or carrier proteins for specific GBS polysaccharides. These antigens include the α and β antigen of the c protein complex (17, 26), an α-like protein (20), the R proteins (10), and protein Rib (38). R proteins are cell surface proteins of GBS that are resistant to certain proteases (10). Four distinct species of R protein in GBS have been described; of these, R4 is the predominant species (10, 44). Most of the isolates of serotype III express R proteins on the surface (10). In animal models, c protein antigens are protective (40). The c protein antigens are expressed by 90% of isolates of types Ia and Ib and by 50% of type II invasive isolates; however, they are rarely expressed by type III GBS (8). Rib protein also confers protective immunity in mice (38). Other R proteins are also biologically important, and a correlation between low levels of maternal antibodies to R proteins and neonatal septicemia has been reported (25). Another surface protein from type V GBS which shares N-terminal sequence homology with the α antigen of c proteins has been shown to confer protection against homologous challenge (21). In this paper we describe a new R-like protein designated BPS (group B protective surface protein) that is expressed by a number of clinically relevant GBS serotypes and is a protective antigen in a mouse model.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids, and media.
GBS strains were from the culture collection of the University of Minnesota (UM), Minneapolis. R protein prototype strains were Compton R (nontypeable/R3, R4, BPS) (Compton 25/60, NCTC 09828; J. Jelinkova, Prague, Czech Republic), 71-735 (III/R1) (Lancefield D136C; R. Lancefield), and 76-043 (III/R4) (UM). Wild GBS strains were H4A-0126 (Ia/R1, BPS), H4A-0148 (Ia,/R1, BPS) and B176 (Ia, BPS). The Escherichia coli strains XL1-Blue MRF and XLOLR were obtained from a commercial source (Stratagene). GBS were grown in Todd-Hewitt broth (Oxoid), while E. coli was grown in either NZY medium, Luria-Bertani medium alone (34), or Luria-Bertani medium supplemented with 1 g of MgCl2/liter and 4 g of maltose/liter. Bacteria were grown at 37°C unless otherwise stated. Where appropriate, E. coli was grown in the presence of 100 μg of ampicillin/ml, 15 μg of tetracycline/ml, 50 μg of kanamycin/ml, and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
Antisera.
Rabbit antisera included a polyvalent serum recognizing R3, R4, and BPS (anti-Compton R from J. Jelinkova and UM), a divalent serum for R3 and BPS (UM anti-Compton R absorbed with strain 76-043 to remove anti-R4), monospecific anti-R3 (UM anti-Compton R absorbed with strains 76-043 and H4A-0148 to remove anti-1, anti-R4, and anti-BPS), monospecific anti-R4 (produced with strain 76-043 [UM]), monospecific anti-R1 (produced with strain 71-735 [UM]), and anti-BPS (produced against the purified recombinant BPS protein). Anti-R2 serum was not included in the studies because, to date, R2 protein has been expressed only by group A and C streptococci. This antiserum does not recognize any surface protein in GBS lysates (10). For screening of the gene library, a polyvalent serum raised against the purified surface R proteins of Compton R was used.
Protein purification.
For purification of cell surface R proteins from Streptococcus agalactiae Compton R, a 1-liter shaken overnight culture was centrifuged (at 10,000 × g for 10 min) and the pellet was washed twice in 1.8% saline and then resuspended to 0.33 g/ml (wet weight) in 50 mM glycine-NaOH (pH 11). The pH was adjusted to 12 with 1 M NaOH. Alkali extraction of cell surface R proteins was allowed to proceed for 2 h at 37°C with shaking. The suspension was centrifuged (at 15,000 × g for 20 min), and the supernatant was adjusted to pH 7 using 1 M HCl. The supernatant (15 ml) was concentrated to a 2-ml final volume, and the buffer was changed against 20 mM Tris-HCl (pH 7.4) by using a Centriprep-30 concentrator (Amicon) at 4°C. The preparation was applied to a MonoQ HBPS/5 column (Pharmacia) with a flow rate of 1 ml/min using the same buffer. R proteins were eluted from the column by using a 20-ml linear NaCl gradient (0 to 0.4 M NaCl in 20 mM Tris-HCl). Fractions containing R protein were detected by immunoblotting with an antiserum raised against S. agalactiae Compton R whole cells. R protein-containing fractions were dialyzed against 1/10-diluted phosphate-buffered saline (PBS), lyophilized, and then resuspended in an appropriate amount of 1/10-diluted PBS. Recombinant glutathione S-transferase (GST)-tagged BPS protein was purified using glutathione-agarose affinity chromatography according to the manufacturer's instructions (Pharmacia). A His-tagged BPS fusion protein was purified under native conditions according to Qiagen protocols. Protein concentrations were determined by the method of Bradford (4).
DNA manipulations.
Chromosomal DNA from S. agalactiae Compton R was isolated by the method of Talay et al. (39). Purified chromosomal DNA was partially digested with the restriction enzyme Sau3AI and then subjected to NaCl gradient centrifugation. Isolated 4- to 8-kb DNA fragments were cloned into the BamHI site of Lambda Zap-Express-arms (Stratagene) according to the manufacturer's instructions. The ligation mixture was packaged in vitro into lambda heads and tails (Gigapack Gold 11; Stratagene) and transfected into E. coli XL-1 Blue MRF′ according to the manufacturer's instructions. Positive clones were in vivo excised to form pBKCMV derivatives by using the helper strain E. coli XLOLR in accordance with the Stratagene manual. Plasmid DNA was prepared using the QIAwell plasmid extraction kit (Qiagen) and sequenced by the method of Sanger et al. (35). Reactions were carried out using dye terminator ready reaction mix (Perkin-Elmer), and products were processed in an ABI 373A DNA sequencer (Applied Biosystems). For complete sequencing of both strands of the DNA analyzed, universal and internal primers were generated and used to initiate sequencing of DNA. Sequence analysis was undertaken using GENMON 4.4 software (German Research Center for Biotechnology). The PCR using the 5"-BamHI primer 5"-TTACATCTGGATCCACTCCAACAGGTG-3" and the 3"-SmaI primer 5"TAGTTGGAACCCGGGATTTATTGGTTGG-3" was performed in a thermocycler (MWG Biotech); the resulting PCR fragment was cloned into the BamHI and SmaI sites of the pGEX2T expression vector (Pharmacia) and then induced with IPTG by standard procedures (34). For the His-tagged BPS fusion protein, the gene was cloned into pQE30 (Qiagen), overexpressed, and purified by a previously described method (29).
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (22), and the gel was then stained with Coomassie brilliant blue R250 (Sigma). Prestained high-molecular-weight markers were used to determine the apparent molecular weights of proteins (Sigma). Alternatively, Western blotting of proteins electroeluted onto Immobilon P membranes (Millipore) was performed essentially as described by Burnette (5).
Immunoelectron microscopy.
For preembedment labeling of the BPS protein, bacteria were grown in Todd-Hewitt broth overnight, centrifuged, and resuspended in 500 μl of PBS (50 mM (each) potassium phosphate and sodium phosphate and 0.15 M NaCl [pH 6.9]). Fifty microliters of protein A-purified anti-BPS antibodies (2.7 mg of immunoglobulin G [IgG] protein/ml) was added, and samples were incubated at 37°C for 1 h. Antibodies purified from preimmune serum as well as anti-GST antibodies served as controls. After three washing steps with PBS, samples were resuspended in 500 μl of PBS, and 20 μl of protein A colloidal gold (diameter, 10 nm; British BioCell, Cardiff, United Kingdom) was added. After incubation for 30 min at 37°C and several washing steps, samples were fixed with 3% glutaraldehyde and 5% formaldehyde in PBS for 1 h on ice. Subsequently, they were postfixed with 1% aqueous osmium tetroxide for 1 h at room temperature. After the samples were embedded in 1.5% agar, they were dehydrated in a graded series of acetone and embedded by use of Spurr resin (37). After polymerization at 70°C for 8 h, ultrathin sections were cut with a diamond knife, and sections were poststained with uranyl acetate and lead citrate before examination with a Zeiss transmission electron microscope (TEM910) at an acceleration voltage of 80 kV.
For postembedment labeling, bacteria were fixed with 0.2% glutaraldehyde and 0.5% formaldehyde in PBS for 1 h on ice. After a wash in PBS containing 0.01 M glycine, samples were dehydrated with a graded series of ethanol and embedded in LRWhite resin (London Resin Company, Berkshire, United Kingdom). After polymerization at 50°C for 24 h, ultrathin sections were cut and collected onto Formvar-coated 300-mesh nickel grids. Sections were then incubated on drops of the anti-BPS antibodies (100 μg of IgG protein/ml) for 12 h at 4°C. After a wash with PBS, sections were incubated on drops of 0.4% skim milk in water for 5 min, blotted dry on filter paper, and incubated on drops of a 1:100-diluted protein A colloidal gold stock solution (diameter of gold particles, 10 nm) for 30 min. After a wash in PBS containing 0.01% Tween 20, sections were counterstained with 4% aqueous uranyl acetate for 5 min before examination with the electron microscope.
Immunoprecipitation reactions in agarose.
R proteins from GBS strains were detected in agarose slides by Ouchterlony double-diffusion (DD) immunoprecipitation using Lancefield hot HCl or 0.1% trypsin cell extracts (11). To examine the susceptibilities of the various R proteins to trypsin or pepsin digestion, HCl or 0.1% trypsin cell extracts were treated (1 h at 37°C; pH 8.2) either with 5 or 0.2% trypsin or with 0.5 or 0.2% pepsin (pH 2.0 and 4.0, respectively) (11, 38, 44, 45). Enzyme-treated or control (buffer only) samples were then tested to determine the effects of such treatments on the immunoprecipitation reactions.
Immunization and protection studies.
Four-week-old female BALB/c (H-2d) mice (Harlan Winkelmann) were immunized with recombinant BPS protein on days 1, 3, 6, and 27 (30 μg/dose) by the subcutaneous route with aluminum phosphate (Adju-Phos; Axell Accurate Chemical & Scientific Corp.) as the adjuvant. Groups of immunized and control animals (n = 9) were challenged on day 37 with an inoculum of the streptococcal strain Compton R corresponding to the 80% lethal dose (LD80; 5 × 108 bacteria/ml) in nonimmunized mice, and mortality was recorded daily.
ELISA.
Serum samples were collected on day 36 and monitored for BPS-specific antibodies by an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well Immuno MaxiSorp assay plates (Nunc, Roskilde, Denmark) were coated with 50 μl of BPS/well (5 μg/ml) in coating buffer (bicarbonate, pH 8.2). After overnight incubation at 4°C, plates were blocked with 10% fetal calf serum in PBS for 1 h at 37°C. Serial twofold dilutions of serum in 10% fetal calf serum-PBS were added (100 μl/well), and plates were incubated for 2 h at 37°C. After four washes with PBS-0.05% Tween 20, secondary antibodies were added (biotinylated μ-chain-specific goat anti-mouse IgM and γ-chain-specific goat anti-mouse IgG [Sigma]) and incubated for a further 2 h at 37°C. After four washes, 100 μl of peroxidase-conjugated streptavidin (PharMingen) was added to each well and plates were incubated at room temperature for 1 h. After four washes, reactions were developed using ABTS [2,2"-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)] in 0.1 M citrate-phosphate buffer (pH 4.35) containing 0.01% H2O2. Results were expressed as end point titers and correspond to the last dilution which gave an optical density at 405 nm (OD405) of 0.1 U above the OD405 of negative controls after a 10-min incubation.
Nucleotide sequence accession number.
The sequence of the gene encoding the BPS protein of GBS has been assigned EMBL accession number AJ133114.
RESULTS
Purification of cell surface R proteins from S. agalactiae Compton R.
The purified product obtained after alkaline extraction and fast protein liquid chromatography anion-exchange chromatography gave four bands of 125, 120, 115, and 110 kDa in a silver-stained SDS-PAGE gel. Western blot analysis indicated that all bands were immunoreactive against polyclonal reference R antiserum, with the 125- and 120-kDa bands reacting most prominently. Since the antiserum used was generated against all surface R proteins, it is likely that the less prominent 115- and 110-kDa bands represented other R proteins; this, however, could not be determined, because the antisera used could not differentiate between R3 and R4. Alkaline extraction in the presence of protease inhibitors or further purification by gel filtration did not change the gel pattern. These purified proteins were used to raise a polyclonal antiserum for screening of the gene library.
Cloning and nucleotide sequence analysis of the gene encoding BPS protein.
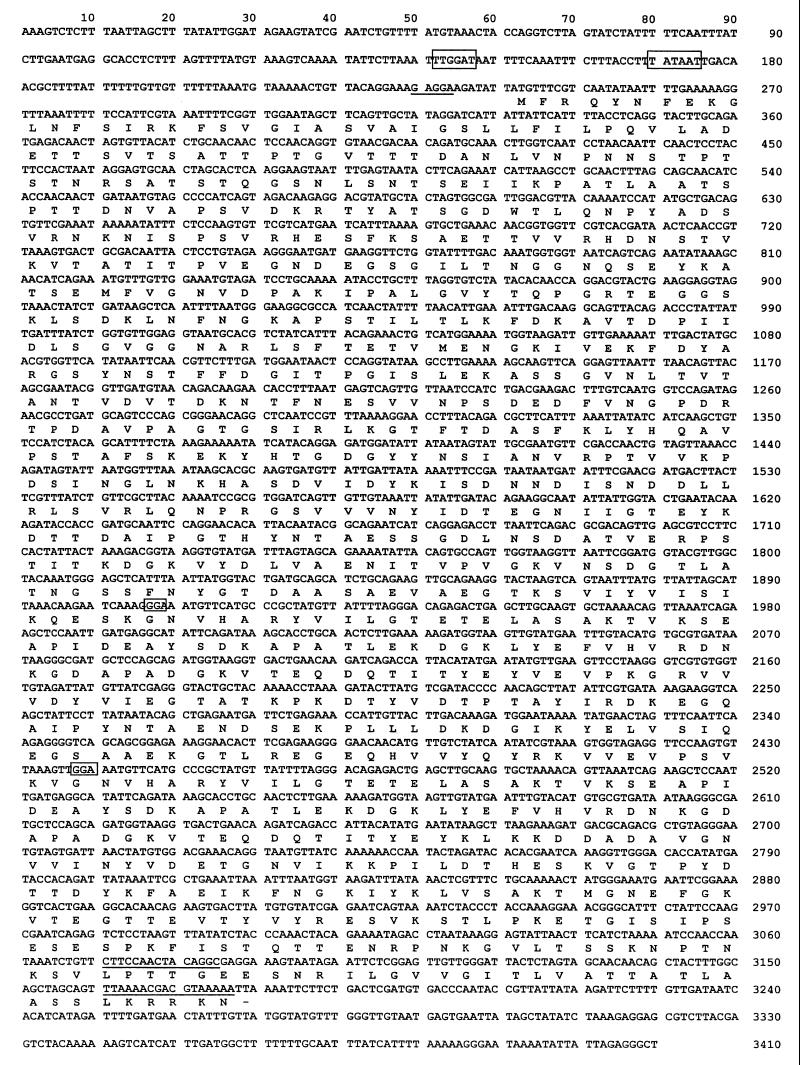
A lambda Zap gene library of S. agalactiae Compton R chromosomal DNA was screened using a polyclonal antiserum raised against purified cell surface R proteins isolated from the homologous strain. One clone, which expressed a protein with an apparent molecular size of 125 kDa that was reactive against the antiserum, was designated pSE3. The 5.0-kb DNA insert of the plasmid was subjected to DNA sequence analysis, which revealed a single open reading frame of 2,937 bp encoding a predicted 105-kDa protein (Fig. 1). A putative Shine-Dalgarno sequence (5"-GAGGAAG-3") was detected 5 bp upstream of the ATG start codon. Both −35 (5"-TTGGAT-3") and −10 (5"-TATAAT-3") consensus sequences at least typical for E. coli were detected 94 and 66 bp upstream of the open reading frame, respectively. A putative 39-amino-acid signal sequence was detected at the amino terminus of the putative protein and shared DNA similarity with other gram-positive cell surface protein signal sequences (41). The carboxy terminus of the protein contained an LPXTG consensus motif typical of membrane-anchored surface proteins of many streptococci and other gram-positive bacteria (9, 15). Within the carboxy-terminal half of the protein, two identical repeats of 76 amino acids each, separated by a 101-amino-acid spacer region, were found. After a search of the EMBL database, the protein was found to share limited DNA similarity only with the protein of unknown function encoded by the Streptococcus suis mrp gene (36). The R protein analyzed in this study, which was present in cell surface extracts of S. agalactiae Compton R, was immunologically distinct from R1, R3, and R4 (see below) and was thus designated BPS.
FIG. 1.
Nucleotide sequence and deduced amino acid sequence of the gene encoding the BPS protein of GBS (EMBL accession number: AJ133114). Putative −10 and −35 sequences are boxed, and the Shine-Dalgarno region is underlined. Within the BPS coding region, the initial codons of the two repeats are boxed and the regions coding for the LPXTG membrane anchor consensus sequence and the charged C-terminal tail are underlined. Amino acids are given in one-letter code.
Expression and purification of GST-tagged BPS.
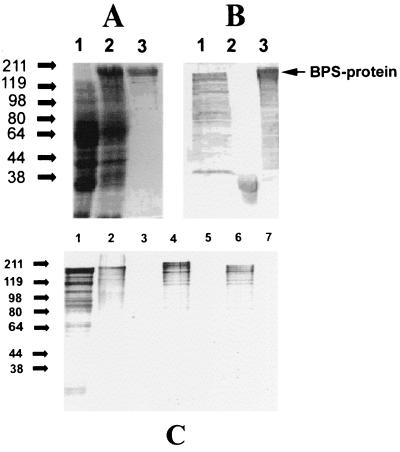
By use of the 5"-BamHI and 3"-SmaI primers, the bps gene was PCR amplified, excluding coding regions for the amino-terminal signal sequence and carboxy-terminal membrane anchor region, and ligated into the relevant enzyme restriction sites of the pGEX-2T expression vector (Pharmacia) to form pSE4. Induction of E. coli XL1-Blue MRF" (pSE4) resulted in the expression of GST-tagged BPS with an apparent molecular size of 158 kDa, which was subsequently purified using glutathione-agarose affinity chromatography (Fig. 2A). The purified recombinant GST-BPS fusion protein was used to raise a polyclonal rabbit antiserum which recognized the degraded BPS protein with an apparent molecular size of 125 kDa in S. agalactiae Compton R whole-cell extracts, the GST with an apparent molecular size of 32 kDa in E. coli XL1-Blue MRF" (pGEX-2T) whole-cell extracts, and the158-kDa degraded recombinant BPS fusion protein in E. coli XL1-Blue MRF" (pSE4) whole-cell extracts (Fig. 2B, lanes 1 to 3, respectively). A commercially acquired anti-GST monoclonal antibody reacted against GST and GST-tagged BPS, but not against native BPS, in S. agalactiae Compton R whole-cell extracts (data not shown).
FIG. 2.
(A) Recombinant expression of GST-tagged BPS in E. coli XL 1-Blue MRF" and purification by glutathione-agarose affinity chromatography. The Coomassie-stained gel shows E. coli whole-cell extracts prior to induction (lane 1) and after induction (lane 2) and purified recombinant BPS fusion protein (lane 3). (B) Western blot analysis of whole-cell lysates of GBS strain Compton R (lane 1), E. coli XL 1-Blue MRF" harboring plasmid pGEX2T (lane 2), and E. coli XL 1-Blue MRF" harboring plasmid pSE4 (lane 3) using a polyclonal antiserum raised against purified recombinant GST-BPS fusion protein. (C) Western blot analysis of purified recombinant BPS protein as well as whole-cell extracts of GBS and E. coli using BPS antiserum. Lane 1, purified recombinant BPS; lane 2, Compton R; lane 3, 71-735 (serotype III/R1); lane 4, H4A-0126 (type Ia/R1, BPS); lane 5, 76-043 (type III/R4); lane 6, E. coli XL1-Blue expressing BPS; lane 7, E. coli XL1-Blue control.
Western blot analysis using anti-BPS serum.
Western blot analysis using BPS antiserum revealed that BPS antigen produced by S. agalactiae Compton R, partially purified Compton R surface proteins, and purified recombinant GST-tagged BPS are degraded following sample preparation, resulting in detection of multiple bands (Fig. 2C, lanes 1, 2, 4, and 6). However, the lack of reactivity of the BPS antiserum against E. coli XL1-Blue MRF" confirms the lack of cross-reactive antibodies and the purity of the GST-tagged BPS preparation used to prepare the polyclonal antiserum (Fig. 2C, lane 1).
The BPS antiserum was also used to detect BPS in a number of different GBS serotypes. The lack of reactivity of the antiserum with S. agalactiae 71-735 (serotype III/R1; Fig. 2C, lane 3) and 76-043 (serotype III/R4; lane 5) revealed that BPS was not immunologically related to R1 and R4. S. agalactiae H4A-0126 (serotype Ia/R1), on the other hand, did express a protein of higher apparent molecular weight which was reactive with the BPS antiserum and produced a degradation profile similar to that of Compton R, suggesting that H4A-0126 also expressed BPS, that the BPS antigen displayed size variation, and that BPS was expressed in more than one serotype of S. agalactiae (Fig. 2C, lane 4). Further proof of the immunological distinction between R1, R3, R4, and BPS was the fact that the antiserum to R3 and BPS from Compton R did not react with proteins from strain 71-735 or 76-043 (Fig. 2C, lanes 3 and 5).
Cell surface expression of BPS in S. agalactiae Compton R.
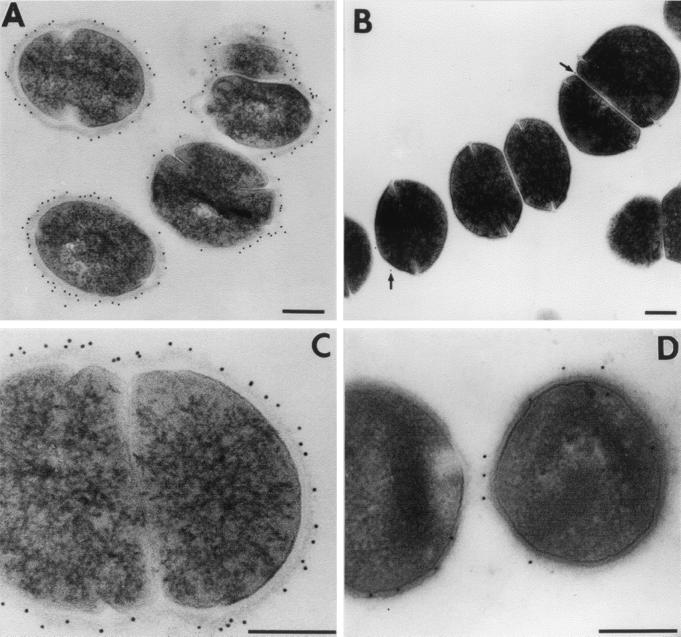
To confirm and characterize the location of BPS protein on the surfaces of S. agalactiae cells, immunoelectron microscopy was conducted by using a monospecific polyclonal anti-BPS antiserum. Immunogold particles were found to be evenly distributed on the cell surface of the parental S. agalactiae strain Compton R by pre- as well as postembedding techniques (Fig. 3A, C, and D). This indicates that BPS protein is homogeneously expressed on the cellular surface. No labeling was found in bacterial cytoplasm. A GBS isolate(76-043/III/R4) that does not express the BPS protein exhibited only a very few gold particles bound to the surface (Fig. 3B). Anti-GST antibodies as well as preimmune serum were used as controls and did not reveal any labeling (data not shown).
FIG. 3.
Immunoelectron microscopy of S. agalactiae Compton R using a monospecific BPS antiserum. Preembedment labeling studies (A and C) clearly demonstrate that BPS protein is located at the bacterial surface. A ring of gold-particles (black dots) can be found on the outer edge of the bacterial cell wall. The control strain (76-043/III/R4), which does not express the BPS protein, exhibits only a very few gold particles (B, arrows). Postembedding (D) also reveals that BPS protein is exclusively located on the bacterial surface despite the fact that less labeling could be detected due to loss of antigenicity during fixation, dehydration, and embedding. Almost no labeling was found in the bacterial cytoplasm. Bars, 250 nm.
Ouchterlony DD analysis of S. agalactiae Compton R proteins with different antisera.
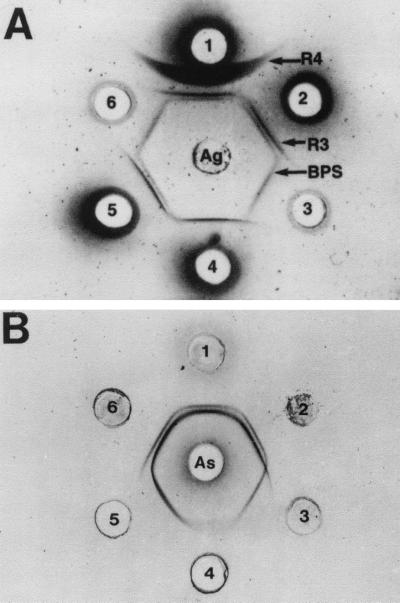
Identification of the cloned BPS protein as a R-like protein was based on DD studies of strain Compton R with reference antisera. Figure 4A shows that the HCl extract of strain Compton R (center well), tested with a UM antiserum to Compton R (well 1), gave three separate precipitin bands, with the diffuse R4 band closest to the antiserum well, the dense R3 band in the middle, and the lighter BPS band very close to the R3 band and farthest away from the antiserum well. Anti-Compton R sera from Prague and UM placed in adjacent wells showed the same three precipitin bands, but the BPS band was lighter and closer to R3 with the Prague antiserum than with the UM antiserum (data not shown). When the anti-Compton R was absorbed to remove anti-R4 antibodies (wells 2 and 5), only the R3 and BPS bands were seen. When the anti-Compton R serum was absorbed to remove anti-R4 and anti-BPS antibodies (well 4), only R3 was left. BPS antiserum produced with the recombinant protein (wells 3 and 6) gave one precipitin band, and this band gave a reaction of identity with the BPS band of the antiserum to Compton R produced with whole bacterial cells (wells 1, 2, and 5). In addition, when an antiserum to BPS protein was placed in the center well, a precipitin reaction of identity was observed with the purified recombinant protein and the HCl extract of strain Compton R, while the extracts of strains 71-735 (III/R1) and 76-043 (III/R4) did not react with this antiserum (data not shown).
FIG. 4.
(A) Identification of the surface proteins of S. agalactiae Compton R by immunodiffusion in agarose slides. Center well, HCl extract of the GBS Compton R strain; well 1, antiserum to the R3, R4, and BPS proteins of Compton R; wells 2 and 5:,antiserum to the R3 and BPS proteins of Compton R; wells 3 and 6, antiserum to the recombinant BPS protein (anti-BPS); well 4, antiserum to the R3 protein from Compton R. (B) Effect of enzyme treatment (1 h at 37°C) on the immunoprecipitation reactions of the R3 and BPS proteins from the HCl extract of S. agalactiae Compton R. Center well, antiserum to the R3 and BPS proteins of Compton R; well 1, untreated HCl extract; well 2, pH 4 buffer control; well 3, extract treated with 0.2% pepsin (pH 4); well 4, extract treated with 5% trypsin (pH 8); well 5, extract treated with 0.2% trypsin; well 6, pH 8 buffer control.
Enzyme susceptibility studies of the BPS from S. agalactiae Compton R.
To compare the enzyme susceptibility of BPS to that of R3, the HCl extract of Compton R was treated with trypsin or pepsin and then compared by DD to buffer-treated controls. Figure 4B shows the R3 (inside, denser) and BPS (outer, lighter) precipitin bands of the untreated extract (well 1) when the antiserum to R3 and BPS was placed in the center well. Treatment with 0.02% trypsin did not affect the BPS and R3 reactions. Treatment of the extract with 0.2% trypsin (well 5), however, eliminated BPS but did not affect the R3 reaction, while 5% trypsin greatly diminished the R3 reaction (well 4). By contrast, only the BPS precipitin band remained after treatment with 0.2% pepsin at pH 4 (well 3), and neither the R3 nor the BPS reaction was seen after treatment with 0.5% pepsin at pH 2, the optimum and conventional pH for pepsin activity (data not shown). Buffer only at pH 8, pH 4, or pH 2 (data not shown) did not affect either the R3 or the BPS precipitin band.
Vaccination with BPS protein protects mice against challenge with virulent strains of GBS.
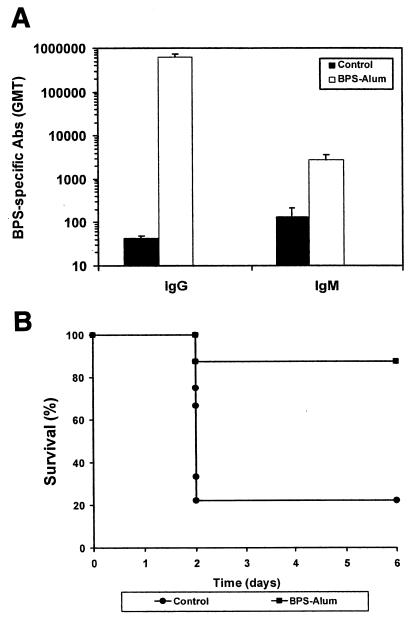
For immunization studies, His-tagged BPS was used to avoid the immune-modulatory effects of GST. The gene encoding the whole BPS protein was cloned into the pQE30 expression vector, and His-tagged protein was purified by affinity chromatography. Immunization of mice with recombinant BPS protein by the subcutaneous route triggered the elicitation of a very efficient antigen-specific response (Fig. 5A). When the immunized animals were challenged with strain Compton R, approximately 88% of the vaccinated animals survived (Fig. 5B), whereas mice in the control group were not protected (78% lethality).
FIG. 5.
Vaccination with the BPS protein triggers the elicitation of a protective response. (A) BPS-specific serum antibodies in mice as determined by ELISA after subcutaneous immunization with the BPS protein. Results are expressed as the geometric mean end point titers (GMT). Error bars, standard errors of the means.(B) Survival times of vaccinated and nonvaccinated mice after challenge with GBS. Animals were challenged with an inoculum of strain Compton R corresponding to the LD80 at day 37 after the primary immunization, and mortality was recorded daily. The differences in survival observed between immunized and control animals were statistically significant (P ≤ 0.05).
DISCUSSION
In spite of advances in diagnosis and treatment, GBS infections remain a major cause of neonatal mortality and morbidity. Development of an effective vaccine to prevent GBS disease through maternal immunization seems to be a promising strategy for the control of GBS infections. A prerequisite for the development of an effective vaccine is the identification and characterization of potential cell surface targets for therapeutic intervention. Because of the suboptimal potential of capsular polysaccharides, interest has shifted to protein antigens as components of vaccine candidates. A number of surface proteins such as β antigen (17, 18), α protein (27), protein Rib (38), and R proteins (10, 11) have been described. Most of these protein antigens have been shown to be protective either by contributing toward resistance to opsonization (32) or by eliciting protective immunity (21, 27). Elicitation of protection against encapsulated GBS strains (23) underlines the importance of GBS surface proteins as vaccine candidates. In this report we describe yet another GBS R-like surface protein that was cloned from strain Compton R; sequence analysis showed that it possesses all the typical features of gram-positive surface proteins such as the signal sequence (41) and the membrane anchor, which contains the sequence LPXTG (9). The surface location was confirmed by immunoelectron microscopy. Sequence analysis showed no similarity on a protein level with any known GBS proteins, indicating that this is a novel surface protein; therefore, it was designated BPS. The protein consists of 979 amino acids and contains two identical repeats of 76 amino acids separated by a 101-amino-acid spacer in the C-terminal region. BPS, therefore, belongs to a family of GBS surface proteins with repetitive structures (42). Two other members of this family, namely, protein Rib (38) and α protein (28), are also trypsin resistant and give a ladder-like pattern similar to the BPS pattern in SDS-PAGE and Western blot analysis. BPS, however, showed no sequence similarity to protein Rib or α protein. On the protein level, BPS shows some similarity (26% identity) to the mrp gene from S. suis (36). The function of the mrp gene product is not yet known.
To determine the prevalence of BPS, the protein was expressed as a fusion, purified, and used to raise a polyclonal antiserum. Immunoprecipitation in agarose was useful to determine that BPS was indeed an integral surface antigen of S. agalactiae Compton R and to identify it as a unique protein, separate from R3 and R4 proteins. In early (44) and subsequent (10) work on the R proteins of GBS, strain Compton R was identified as having only R3 and R4 proteins. When we tested our HCl extracts with Prague anti-Compton R serum, which was used in those studies, the BPS precipitin reaction was weak and practically fused with that of R3, making it difficult to recognize them as two distinct lines. However, separation and identification of three separate proteins in the HCl and trypsin extracts of Compton R were possible with our new polyclonal rabbit antiserum. Comparison of the reactions obtained with our new antiserum with those obtained with the antiserum from Prague showed that, although they were somewhat obscured, the Prague antiserum also contained antibodies to BPS (data not shown). This further confirmed that BPS is a new protein that is separate from R3 and R4, the previously recognized proteins of Compton R.
The results from the enzyme digestion studies provided additional support for the fact that BPS is a separate protein of Compton R. DD results indicated that mild trypsin treatment affected BPS more than R3. In contrast, the results from mild pepsin treatment of BPS at suboptimal conditions (pH 4) indicated that R3 was more pepsin susceptible than BPS. This reduced susceptibility of BPS to pepsin digestion is similar to that of R4 protein (11) and protein Rib, since treatment with pepsin at suboptimal pH conditions was used to characterize the latter and to compare it to the α component of the c protein (38), another trypsin-resistant protein of GBS (7).
Finding BPS in GBS strains such as H4A-0126 and H4A-0148 demonstrates that this protein of Compton R is found in wild GBS strains of other serotypes and protein profiles. Our results from examination of 1,627 human GBS isolates by double immunodiffusion and Southern hybridization indicated that BPS was found in 7.4% of serotype Ia/R1 isolates. 6.8% of serotype II isolates, and 5% of serotype V isolates (A. E. Flores, S. Erdogan, G. S. Chhatwal, C. J. Baker, S. Hillier, and P. Ferrieri, Abstr. XIVth Lancefield Int. Symp. Streptococci Streptococcal Dis., abstr. O2.8, 1999). The presence of both R1 and BPS in the serotype Ia isolates was analogous to the presence of both R1 and R4 in the majority of serotype V isolates (8). Furthermore, these results indicated that BPS was a marker found in recently isolated colonizing human isolates and that, as such, it may be useful in their characterization (7).
The results obtained demonstrated that the BPS protein is an antigen able to confer protective immunity against GBS. In order to evaluate whether the BPS protein can also confer protection against challenge with a heterologous strain, immunized animals were challenged with strain B176 (Ia, BPS). While 67% of the vaccinated animals survived, only 22% of the mice in the control group survived (data not shown). These results confirmed that immunization with the BPS protein can also protect against heterologous challenge (P ≤ 0.05).
Considering that the natural portal of entry for GBS is the mucosa from the respiratory and genitourinary tracts, it seems particularly attractive to stimulate not only an efficient systemic but also a local mucosal response following vaccination. This may lead to a more efficient protection of newborns by two different mechanisms, namely, (i) passive transfer of maternal antibodies leading to protection against disease and (ii) reduction of the risk of maternal colonization (i.e., infection) by the presence of vaginal antibodies. Of importance is the fact that BPS elicits a strong IgG response; this isotype can cross the placenta and therefore may provide passive protection. It has also been demonstrated that due to the existence of a mucosal network, coadministration of vaccine antigens and mucosal adjuvants by the intranasal route can lead to the elicitation of local responses in the urogenital tract (16). Preliminary studies from our group confirmed that protective immunity can also be achieved following vaccination by the intranasal route with the BPS protein in combination with the cholera toxin B subunit as a mucosal adjuvant.
Different serotypes of GBS express different surface proteins, which can become targets for the elicitation of a protective immune response. An effective vaccine, therefore, should contain more than a single antigen in order to confer protection against predominant circulating serotypes. Thus, BPS protein, which is expressed by isolates of serotypes Ia, II, and V, may represent an attractive candidate antigen for incorporation in anti-GBS vaccine formulations.
Acknowledgments
This work was supported in part by research contract NO1 AI25152 from the National Institutes of Health (to P.F. and A.E.F.). S.E. gratefully acknowledges a Ph.D. fellowship from the Boehringer-Ingelheim Fond. M.J.W. and P.K.F. thank the Alexander von Humboldt Foundation for fellowships. A travel grant from the University of Wollongong to M.J.W. for international collaboration is gratefully acknowledged.
We thank E. Moore for help in DNA sequencing and A. Müller for help in animal experiments. We acknowledge receiving two GBS strains from C. J. Baker, Baylor College of Medicine.
Editor: D. L. Burns
REFERENCES
- 1.Baker, C. J., and M. S. Edwards. 1995. Group B streptococcal infections, p. 980-1054. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. W. B. Saunders, Philadelphia, Pa.
- 2.Baker, C. J., and D. L. Kasper. 1976. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infections. N. Engl. J. Med. 294:753-756. [DOI] [PubMed] [Google Scholar]
- 3.Baker, C. J., M. A. Rench, M. S. Edwards, R. J. Carpenter, B. M. Hays, and D. L. Kasper. 1988. Immunization of pregnant women with a polysaccharide vaccine of group B Streptococcus. N. Engl. J. Med. 319:1180-1185. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1979. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radiolabelled protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 6.Farley, C., C. Harvey, and T. Stull. 1993. A population-based assessment of invasive disease due to group B streptococcus in non-pregnant adults. N. Engl. J. Med. 328:1807-1811. [DOI] [PubMed] [Google Scholar]
- 7.Ferrieri, P. 1988. Surface-localized protein antigens of group B streptococci. Rev. Infect. Dis. 10(Suppl. 2):363-366. [DOI] [PubMed] [Google Scholar]
- 8.Ferrieri, P., and A. E. Flores. 1997. Surface protein expression in group B streptococcal invasive isolates, p. 635-637. In T. Horaud, A. Bouvet, R. Leclercq, H. de Montclos, and M. Sicard, (ed.) Streptococci and the host. Proceedings of the XIIIth Lancefield International Symposium on Streptococci and Streptococcal Diseases. Plenum Press. New York, N.Y.
- 9.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 10.Flores, A. E., and P. Ferrieri. 1989. Molecular species of R-protein antigens produced by clinical isolates of group B streptococci. J. Clin. Microbiol. 27:1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores, A. E., and P. Ferrieri. 1996. Molecular diversity among the trypsin resistant surface proteins of group B streptococci. Zentbl. Bakteriol. 285:44-51. [DOI] [PubMed] [Google Scholar]
- 12.Gardam, M. A., D. E. Low, R. Saginur, and M. A. Miller. 1998. Group B streptococcal necrotizing fasciitis and streptococcal toxic shock-like syndrome in adults. Arch. Intern. Med. 158:1704-1708. [DOI] [PubMed]
- 13.Hall, R. T., W. Barnes, L. Krishnan, D. J. Harris, P. G. Rhodes, J. Fayez, and G. L. Miller. 1976. Antibiotic treatment of parturient women colonized with group B streptococci. Am. J. Obstet. Gynecol. 124:630-634. [DOI] [PubMed] [Google Scholar]
- 14.Hickman, M. E., M. A. Rench, P. Ferrieri, and C. J. Baker. 1999. Changing epidemiology of group B streptococcal colonization. Pediatrics 104:203-209. [DOI] [PubMed] [Google Scholar]
- 15.Hollingshead, S. K., V. A. Fischetti, and J. R. Scott. 1986. Complete nucleotide sequence of type 6 M protein of group A Streptococcus. Repetitive structure and membrane anchor. J. Biol. Chem. 261:1677-1686. [PubMed] [Google Scholar]
- 16.Holmgren, J., C. Czerkinsky, N. Lycke, and A. M. Svennerholm. 1992. Mucosal immunity: implication for vaccine development. Immunobiology 184:157-179. [DOI] [PubMed] [Google Scholar]
- 17.Jerlström, P. J., G. S. Chhatwal, and K. N. Timmis. 1991. IgA-binding β antigen of the c protein complex of group B streptococci: sequence determination of its gene and detection of two binding regions. Mol. Microbiol. 5:843-849. [DOI] [PubMed] [Google Scholar]
- 18.Jerlström, P. J., S. R. Talay, P. Valentin-Weigand, K. N. Timmis, and G. S. Chhatwal. 1996. Identification of an immunoglobulin A binding motif located in the β-antigen of the c protein complex of group B streptococci. Infect. Immun. 64:2787-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko, W. C., H. C. Lee, L. R. Wong, C. T. Lee, A. J. Liu, and J. J. Wu. 2001. Serotyping and antimicrobial susceptibility of group B streptococcus over an eight-year period in southern Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 20:334-339. [DOI] [PubMed] [Google Scholar]
- 20.Kvam, A. I., L. S. Bevanger, and O. J. Eversen. 1993. Characterization of a surface protein of group B streptococci resembling the α-antigen of C protein, p. 91-93. In A. Totolian, (ed.). Pathogenic streptococci: present and future. Lancer Publications, St. Petersburg, Ukraine.
- 21.Lachenauer, C. S., and L. C. Madoff. 1996. A protective surface protein from type V group B streptococci shares N-terminal sequence homology with the alpha c protein. Infect. Immun. 64:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Larsson, C., M. Stålhammar-Carlemalm, and G. Lindahl. 1996. Experimental vaccination against group B streptococcus, an encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and α. Infect. Immun. 64:3518-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, F.-Y. C., J. D. Clemens, P. H. Azimi, J. A. Regan, L. E. Weisman, J. B. Philips III, G. G. Rhoads, P. Clark, R. A. Brenner, and P. Ferrieri. 1998. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J. Infect. Dis. 177:790-792. [DOI] [PubMed] [Google Scholar]
- 25.Linden, V., K. K. Christensen, and P. Christensen. 1983. Correlation between low levels of maternal IgG antibodies to R protein and neonatal septicemia with group B streptococci carrying R protein. Int. Arch. Allergy Appl. Immunol. 71:168-172. [DOI] [PubMed] [Google Scholar]
- 26.Madoff, L. C., S. Hori, J. L. Michel, C. J. Baker, and D. L. Kasper. 1991. Phenotypic diversity in the alpha C protein of group B streptococci. Infect. Immun. 59:2638-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel, J. L., L. W. Madoff, D. E. Kling, D. L. Kasper, and F. M. Ausubel. 1991. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect. Immun. 59:2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel, J. L., L. Madoff, K. Olson, D. E. Kling, D. L. Kasper, and F. M. Ausubel. 1992. Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc. Natl. Acad. Sci. USA 89:10060-10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molinari, G., S. R. Talay, P. Valentin-Weigand, M. Rohde, and G. S. Chhatwal. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect. Immun. 65:1357-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyo, S. R., J. Mudzori, S. A. Tswana, and J. A. Maeland. 2000. Prevalence, capsular type distribution, anthropometric and obstetric factors of group B Streptococcus (Streptococcus agalactiae) colonization in pregnancy. Cent. Afr. J. Med. 46:115-120. [DOI] [PubMed] [Google Scholar]
- 31.Pass, M. A., B. M. Gray, S. Khare, and H. C. Dillon. 1979. Prospective studies of group B streptococcal infections in infants. J. Pediatr. 95:437-443. [DOI] [PubMed] [Google Scholar]
- 32.Payne, N. R., and P. Ferrieri. 1985. The relation of the Ibc protein antigen to the opsonization differences between strains of type II group B streptococci. J. Infect. Dis. 151:672-681. [DOI] [PubMed] [Google Scholar]
- 33.Pearlman, M. D., C. L. Pierson, and R. G. Faix. 1998. Frequent resistance of clinical group B streptococci to clindamycin and erythromycin. Obstet. Gynecol. 92:258-261. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, H. E., U. Vecht, L. J. Gielkens, and M. A. Smits. 1992. Cloning and nucleotide sequence of the gene encoding the 136-kilodalton surface proteins (muramidase-released protein) of Streptococcus suis type 2. Infect. Immun. 60:2361-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spurr, A. R. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-43. [DOI] [PubMed] [Google Scholar]
- 38.Stålhammar-Carlemalm, M., L. Stenberg, and G. Lindhal. 1993. Protein rib, a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 177:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talay, S. R., P. Valentin-Weigand, P. G. Jerlström, K. N. Timmis, and G. S. Chhatwal. 1992. Fibronectin binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 60:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valtonen, M. V., D. L. Kasper, and N. J. Levy. 1986. Isolation of a C (Ibc) protein from group B Streptococcus which elicits mouse protective antibody. Microb. Pathog. 1:191-204. [DOI] [PubMed] [Google Scholar]
- 41.Von Heijne, G. 1985. Signal sequences. The limits of variation. J. Mol. Biol. 184:99-105. [DOI] [PubMed] [Google Scholar]
- 42.Wästfelt, M., M. Stålhammar-Carlemalm, A. M. Delisse, T. Carbezon, and G. Lindahl. 1996. Identification of a family of streptococcal surface proteins with extremely repetitive structure. J. Biol. Chem. 271:18892-18897. [DOI] [PubMed] [Google Scholar]
- 43.Wessels, M. R., L. C. Paoletti, H. K. Guttormsen, F. Michon, A. J. D'Ambra, and D. L. Kasper. 1998. Structural properties of group B streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect. Immun. 66:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson, H. W. 1972. Comparison of streptococcal R antigens. Appl. Microbiol. 24:669-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson, H. W., and R. G. Eagon. 1971. Type-specific antigens of group B type Ic streptococcci. Infect. Immun. 4:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zangwill, K. M., A. Schuchat, and J. D. Wenger. 1992. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. Morb. Mortal. Wkly. Rep. 41:25-32. [PubMed] [Google Scholar]