Abstract
The Streptococcus pneumoniae pore-forming toxin, pneumolysin, is an important virulence factor in pneumococcal pneumonia. The effect of pneumolysin on human lung epithelial and monocyte cell viability was compared. Pneumolysin caused a dose-dependent loss of viability of human lung epithelial (A549 and L132) and monocyte (U937 and THP-1) cell lines. Analysis of the dose-response curves revealed similar log 50% inhibitory concentration (pIC50) values for A549, L132, and THP-1 of 0.12± 0.1, 0.02± 0.04, and 0.12± 0.13 hemolytic units (HU), respectively, but U937 cells showed a significantly greater pIC50 of 0.42± 0.12 HU. Differentiation of A549 and L132 with phorbol ester or THP-1 with gamma interferon had no effect on their sensitivity to pneumolysin. However, a significant decrease in the potency of pneumolysin against U937 cells followed gamma interferon treatment. The Hill slopes of the inhibition curves were greater than unity, indicating that pneumolysin may act with positive cooperativity. Analysis of pneumolysin-treated THP-1 cells by electron microscopy revealed membrane lesions of between 100 and 200 nm in diameter.
Streptococcus pneumoniae is an important cause of human diseases such as pneumonia, meningitis, and otitis media. One of the major virulence determinants of S. pneumoniae is pneumolysin, a 53-kDa protein toxin (9). Reduced mortality and morbidity has been demonstrated in mice infected with pneumolysin—deficient pneumococci compared with wild-type bacteria (2). Furthermore, recombinant pneumolysin injected into the rat lung caused severe pneumonia that was indistinguishable from that caused by injection of S. pneumoniae (4).
Pneumolysin is a pore-forming toxin that causes cytolysis and complement activation. Pneumolysin has been shown to cause lysis of all eukaryotic cells tested if present at sufficiently high concentration (9). At lower, sublytic concentrations, the activity of a variety of cells will be altered (9). It is not clear how the sensitivity of cells to pneumolysin might vary or how maturation of cells may influence their sensitivity; no comparative study has been reported. A difference in sensitivity is worth considering, given that during respiratory infection with pneumococci, several types of cells, e.g., phagocytes and epithelial cells, are exposed to pneumococcal products, such as pneumolysin, and differentiation of cells will be occurring in response to immune factors.
An important factor in immunity is gamma interferon (IFN-γ). It has been shown, for example, that mice lacking IFN-γ are more sensitive to infection by S. pneumoniae (12). In addition, administration of IFN-γ has been shown to enhance survival following subsequent challenge with S. pneumoniae (17). The protection provided by IFN-γ against bacterial infections is believed to originate from its ability to activate the antimicrobial activity of phagocytes (8).
We hypothesised that IFN-γ may also function in immunity by increasing resistance of host cells to damage by bacterial products. In order to address this hypothesis, we compared the toxicity of pneumolysin to human cell lines (lung epithelial cells [A549 and L132] and monocyte lines U937 and L132). We also examined if there was any difference in the susceptibility of the cells to the toxin after differentiation with phorbol ester (epithelial cells) or IFN-γ (monocytes-macrophages). As part of these studies, the membrane lesions induced by pneumolysin were examined by electron microscopy. We found that sensitivity to pneumolysin differs between monocytes and epithelial cells, between differentiated and undifferentiated cells, and also between phenotypically similar cell lines.
Cells were grown in RPMI 1640 with Glutamax (Life Technologies, Paisley, Scotland) containing 10% (vol/vol) fetal calf serum, penicillin (100 IU/ml), streptomycin (100 μg/ml), and fungizone (2.5 μg/ml). Fresh medium was either added (up to 15 ml for 4 days for cells in suspension) or replaced (every 2 days up to a maximum of 7 days for adherent cells). Cultured epithelial cells were adherent, whereas the monocytes were cultured in suspension. Adherent cells were harvested by brief exposure to 25 mM HEPES-1 mM EDTA (pH 7.4), and cells in suspension were collected by centrifugation at 2,000 × g for 2 min. The pellets were resuspended in Krebs-HEPES buffer (pH 7.4).
Pneumolysin was purified as previously described (7) and diluted in Krebs-HEPES buffer. The specific activity of the purified recombinant pneumolysin was 150 hemolytic units (HU) μg of protein.
The human monocytic cell lines were differentiated using 100 IU of IFN-γ/ml. Cell suspensions from 2- to 3-day-old cultures were seeded into 24-well Nunclon tissue culture plates in 900 μl of tissue culture medium. The plates were then incubated (5% CO2 at 37°C) for 24 h. The cells were then resuspended in 1 ml of Krebs-HEPES buffer containing pneumolysin (0 to 100 HU) and incubated at 37°C for 20 min. The human lung epithelial cell lines were differentiated by incubation in 10 μM phorbol 12-myristate 13-acetate (PMA) in tissue culture medium. After 24 h of incubation with PMA (5% CO2 at 37°C), cell monolayers were used for cytotoxicity experiments.
Hemolytic activity was assayed in round-bottomed microtiter plates. Test solution (50 μl) was serially diluted into 50 μl of phosphate-buffered saline (PBS), and 50 μl of a 2% (vol/vol) suspension of compacted (4,000 × g for 2 min) sheep red blood cells was then added to each well. Plates were incubated for 30 min at 37°C. HU were calculated from the well at which 50% hemolysis had occurred, this well being the inverse of the number of dilutions made from the original solution.
For measurement of trypan blue dye exclusion, cells were resuspended in Hanks-HEPES buffer (pH 7.4) to 105 cells/ml. Cells were treated with 0 to 100 HU of pneumolysin/ml for 20 min at 37°C. Trypan blue (0.4%, wt/vol) was then added (125 μl to 50 μl of cell suspension) and left to stand for 15 min at 37°C before counting. Before measurement of lactate dehydrogenase (LDH) release, cells were treated with pneumolysin (0 to 100 HU/ml) at 37°C for 20 min. Following incubation with pneumolysin, the cell supernatant was assayed for LDH activity (Sigma Diagnostics) by following NAD+ formation at 340 nm.
For scanning electron microscopy, cells were fixed with 4% (wt/vol) glutaraldehyde in PBS. Cells were centrifuged for 5 min at 1,000 × g, and the pellet was resuspended in fresh buffer. The cells were postfixed in osmium tetroxide for 1 h, after which the sample was centrifuged (5 min, 1,000 × g). Fresh buffer was used to rinse and resuspend the cells prior to another round of centrifugation. The samples were then dehydrated through a graded ethanol series and immersed in hexamethyldisilazane (HMDS). The HMDS was evaporated, leaving the cells fixed to aluminum stubs. These were then sputter-coated with gold prior to examination.
For transmission electron microscopy (TEM), cells were fixed and postfixed as for scanning electron microscopy, except that after rinsing the osmium tetroxide, the cells were placed into a drop of 2% (wt/vol) liquid agar. This was centrifuged and allowed to solidify at room temperature prior to dehydration through a graded ethanol series and subsequent infiltration with liquid Araldite resin. Sections (0.5 μm) were stained with 1% (wt/vol) toludine blue in 1% (wt/vol) sodium tetraborate. Suitable areas were selected and resectioned (70 nm) on an ultramicrotome (Reichert-Jung Ultracut E) and stained with 0.5% (wt/vol) Reynolds lead citrate and uranyl acetate before TEM.
All data presented are mean ± standard error of the mean (SEM) for 4 to 12 independent experiments. Individual curves were analyzed using GraphPad Prizm V. 2 (nonlinear regression, variable slope) and tested by analysis of variance. In order to determine the potency of pneumolysin, log 50% inhibitory concentrations (pIC50) were derived from each inhibition curve, and to quantify the steepness of each inhibition curve, Hill slope factors were derived. Individual pIC50 and Hill slope values were compared using an unpaired Student t test.
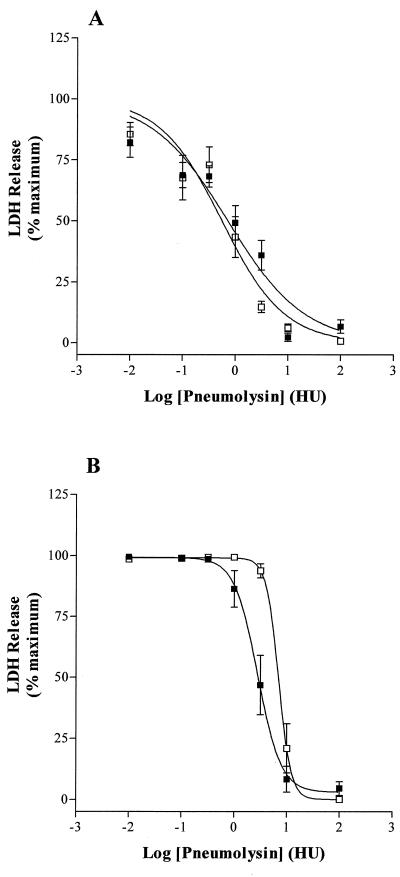
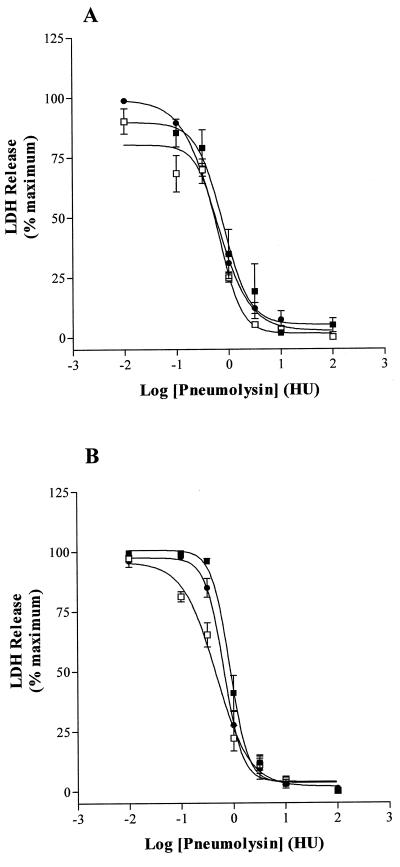
Pneumolysin induced a dose-dependent leakage of LDH from each type of cell tested, but the sensitivity to pneumolysin varied between cells. U937 cells were significantly more resistant to pneumolysin than THP-1 cells (P < 0.05) ( Table 1, Fig. 1A and B). In contrast, pneumolysin was equipotent for the two lung epithelial cell lines in terms of induction of LDH-leakage ( Table 1, Fig. 2A and B). There was no statistical difference in the potency of pneumolysin to THP-1 and the epithelial cells (P > 0.05). The Hill slope factors for the dose-response curves, using the LDH assay, revealed steep curves, indicating a positive cooperative action of pneumolysin.
TABLE 1.
Dose-dependent stimulation of LDH release from undifferentiated and differentiated human epithelial cells and monocytes by pneumolysina
| Cells and treatment | pIC50 (HU) | Hill slope |
|---|---|---|
| A549 | 0.12 ± 0.1 (0.76) | 1.4 ± 0.1 |
| A549 + DMSO | 0.35 ± 0.05 (0.44) | 1.1 ± 0.2 |
| A549 + DMSO + PMA | 0.23 ± 0.06 (0.58) | 1.3 ± 0.1 |
| L132 | 0.02 ± 0.04 (0.95) | 2.7 ± 0.5 |
| L132 + DMSO | 0.34 ± 0.06 (0.46) | 1.4 ± 0.2 |
| L132 + DMSO + PMA | 0.16 ± 0.06 (0.70) | 2.2 ± 0.2 |
| U937 | 0.42 ± 0.12 (2.63)* | 2.3 ± 0.3 |
| U937 + IFN-γ | 0.83 ± 0.06 (6.76)** | 4.4 ± 0.2 |
| THP-1 | 0.12 ± 0.13 (0.75) | 2.7 ± 0.1 |
| THP-1 + IFN-γ | 0.49 ± 0.2 (0.32) | 1.4 ± 0.3 |
pIC50 and Hill slopes were obtained using the curve-fitting program Graphpad-Prizm. Individual curves were fitted for each experiment using a variable slope (four-parameter logistic) equation. Data are means ± SEM for 4 to 12 experiments. *, significantly (P < 0.05) increased compared with THP-1. **, significantly (P < 0.05) increased compared with U937. DMSO was included to assess its effects on the action of pneumolysin.
FIG. 1.
Pneumolysin-induced LDH release from (A) undifferentiated THP-1 (▪) and IFN-γ-differentiated THP-1 (□) monocytes and from (B) undifferentiated (□) and IFN-γ-differentiated (□) U937 monocytes. Data are means ± SEM for five to six independent experiments.
FIG. 2.
(A) Dose-dependent LDH release with increasing pneumolysin concentration from (A) A549 (▪), A549 + DMSO (□), and A549 + DMSO + PMA (bM) lung epithelial cells and (B) L132 (B7), L132 + DMSO (□), and L132 + DMSO + PMA (○) lung epithelial cells. Data are means ± SEM for five experiments.
Differentiation of U937 cells with IFN-γ further increased their resistance to pneumolysin (P < 0.05 compared with undifferentiated U937). As can be seen from Table 1 and Fig. 1B, there was an approximate doubling (0.42 to 0.83 log10 HU) in the pIC50 of pneumolysin after treatment with IFN-γ. This effect of interferon on U937 cells was confirmed by experiments in which viability was assessed by trypan blue exclusion. After exposure to 1 HU of pneumolysin per ml, the percentage of undifferentiated cells that were viable was 6.7 ± 1.9% (n = 4), whereas IFN-γ-treated cells were 45 ± 3.7% viable (n = 4) (P < 0.05). U937 cells were distinct from the other cells in this regard also. There was no significant difference between the pneumolysin-induced LDH release dose-response curves of undifferentiated and IFN-γ-differentiated THP1 cells ( Table 1, Fig. 1A). Likewise, differentiation of A549 and L132 epithelial cells with PMA did not significantly (P > 0.05) increase their resistance to pneumolysin ( Table 1, Fig. 2A and B). Dimethyl sulfoxide (DMSO) was the vehicle for PMA and was included as a control to assess its action on the lytic effects of pneumolysin.
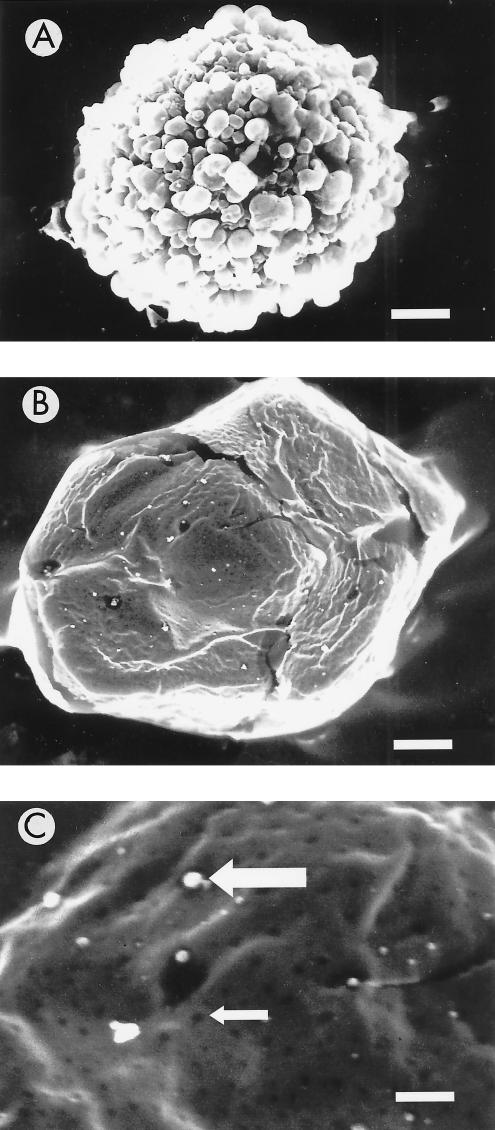
Previously, the appearance of pneumolysin-induced pores had only been described in erythrocytes and liposomes, but not in nucleated cells. Because of the relative ease of sectioning compared with the other cells tested, the THP-1 cell line was selected for the ultrastructural electron microscopy studies. Figure 3A shows a scanning electron micrograph of an untreated THP-1 cell. When pneumolysin was added ( Fig. 3B and C), pseudopodia disappeared and numerous membrane lesions were evident. Closer examination revealed that the membrane lesions had an internal diameter ranging from 100 to 200 nm ( Fig. 3C small arrow). The white areas ( Fig. 3C large arrow) are above the focal plane and may represent exuded cytoplasm.
FIG. 3.
Scanning electron micrographs of THP-1 cells in the absence (A) and presence (B and C; 100 HU for 30 min) of pneumolysin. The small arrow shows one of many membrane lesions, whereas the large arrow shows exuded cytoplasm from a membrane lesion. Bar, approximately 1,840 nm (A and B) and 550 nm (C).
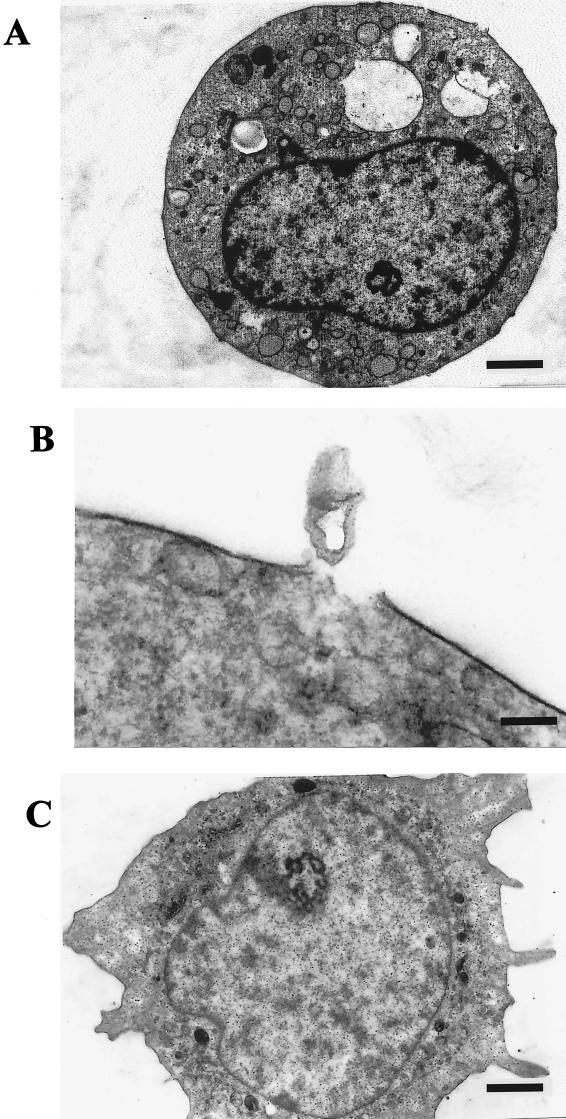
Transmission electron micrographs show distinct lesions in the membranes of THP-1 cells treated with pneumolysin ( Fig. 4A and B).In addition, there appears to have been heavy endocytotic activity, evident from the large number of cytoplasmic vacuoles present ( Fig. 4A). Figure 4B shows a clear break in the lipid membrane, and a plug of cell material has escaped from the cytoplasm of the cell. These features are not present in untreated THP-1 cells ( Fig. 4C).
FIG. 4.
Transmission electron micrographs of sections through THP-1 cells in the absence (C) and presence (A and B; 100 HU for 30 min) of pneumolysin. Following pneumolysin treatment, there is an increase in the number of exocytotic vesicles (A) and cytoplasmic material (B) associated with the membrane pores. Bar, approximately 1,300 nm (A and C) and 130 nm (B).
Although the toxic effects of pneumolysin on pulmonary epithelial cells and monocytes have been studied separately (5, 10, 11), there was no comparative investigation into differential cellular sensitivity to pneumolysin. Furthermore, the effect of differentiation of cell lineage on sensitivity to pneumolysin has not been investigated. However, it has been shown that another thiol-activated toxin, streptolysin-O, had differential effects on murine macrophages. Tanigawa and colleagues (13) showed that murine macrophage cell lines varied in susceptibility to streptolysin-O and that differentiation increased sensitivity. In part, our data agree in that IFN-γdifferentiates U937 cells to a phenotype that is much less sensitive to pneumolysin. However, we also found that U937 cells are inherently more resistant than other cell lines. In contrast to Tanigawa et al. (13), we found that differentiation of the monocyte cell line THP-1 had no effect on the sensitivity to pneumolysin. It has been shown that different macrophage cell lines exhibit distinct patterns of gene expression in response to IFN-γ stimulation (6). However, it is possible that IFN-γ stimulation may affect other macrophage cell lines differently, which may warrant a more inclusive investigation. In addition, the time -course for the effects of IFN-γ stimulation on pneumolysin sensitivity using other monocyte cell preparations would be worthy of further study.
The difference in pneumolysin sensitivity between cell types probably results from differences in the structure and dynamics of the plasma membrane. It has been suggested that in membranes the pore— forming toxins aerolysin from Aeromonas hydrophila and staphylococcal α-toxin associate with cholesterol-glycolipid rafts (1). Differences in such rafts have been proposed as the reason for the differential sensitivity of rabbit and human erythrocytes to α-toxin (14). If pneumolysin also associates with cholesterol-glycolipid rafts, then their abundance and composition in each cell type may influence the potency of pneumolysin. Alternatively, differentiation also may change the dynamic properties of the plasma membrane.
It has been suggested that cells may recover from low doses of pore-forming toxin by shedding of toxin (16) or by repair of lesions by fusion of cytoplasmic vesicles with the plasma membrane (15). Also, it has been demonstrated that polymorphonuclear leukocytes shed complement membrane attack complexes (3). It may be significant that TEM demonstrated numerous intracellular vesicles compared to control cells, which may be due to increased cell membrane turnover following treatment with pneumolysin.
REFERENCES
- 1.Abrami, L., M. Fivaz, P. E. Glauser, R. G. Parton, and F. G. van der Goot. 1998. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J. Cell Biol. 140:525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell, A. K., and B. P. Morgan. 1985. Monoclonal antibodies demonstrate protection of polymorphonuclear leukocytes against complement attack. Nature 317:164–166. [DOI] [PubMed] [Google Scholar]
- 4.Feldman, C., N. C. Munro, P. K. Jeffery, T. J. Mitchell, P. W. Andrew, G. J. Boulnois, D. Guerreiro, J. A. Rohde, H. C. Todd, and P. J. Cole. 1991. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am. J. Resp. Cell Mol. Biol. 5:416–423. [DOI] [PubMed] [Google Scholar]
- 5.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates the production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62:1501–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas, D. M., M. A. Lokuta, M. A. McDowell, J. E. Doan, and D. M. Paulnock. 1998. Analysis of the IFN-gamma-signalling pathway in macrophages at different stages of development. J. Immunol. 160:4337–4342. [PubMed] [Google Scholar]
- 7.Mitchell, T. J., J. A. Walker, F. K. Saunders, P. W. Andrew, and G. J. Boulnois. 1989. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim. Biophys. Acta 1007:67–72. [DOI] [PubMed] [Google Scholar]
- 8.Morris, A., and I. Zvetkova. 1997. Origins of cytokine research: the interferon paradigm. J. Pathol. 50:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paton, J. C., P. W. Andrew, G. J. Boulnois, and T. J. Mitchell. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 47:89–115. [DOI] [PubMed] [Google Scholar]
- 10.Rubins, J. B., P. G. Duane, D. Charboneau, and E. N. Janoff. 1992. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect. Immun. 60:1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubins, J. B., P. G. Duane, D. Clawson, D. Charbonea, J. Young, and J. Niewoehner. 1993. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect. Immun. 61:1332–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanigawa, T., J. Suzuki, T. Ueta, T. Katsumoto, and Y. Tanaka. 1996. Different sensitivity to streptolysin-O of cells in macrophage lineage. Microbiol. Immunol. 40:81–84. [DOI] [PubMed] [Google Scholar]
- 14.Valeva, A., I. Walev, and S. Bhakdi. 2000. The nature of the high-affinity binding site for staphylococcal alpha toxin: introducing the concept of a quasi receptor. Med. Microbiol. Immunol. 189:27–54.11195804 [Google Scholar]
- 15.Walev, I., S. C. Bhakdi, F. Hofmann, N. Djonder, A. Valeva, K. Aktories, and S. Bhakdi. 2001. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin O. Proc. Natl. Acad. Sci. USA 98:3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walev, I., M. Palmer, E. Martin, D. Jonas, U. Weller, H. Hohn-Bentz, M. Husmann, and S. Bhakdi. 1994. Recovery of human fibroblasts from attack by the pore-forming alpha-toxin of Staphylococcus aureus. Microb. Pathog. 17:187–201. [DOI] [PubMed] [Google Scholar]
- 17.Weigent, D. A., T. L. Huff, J. W. Peterson, G. J. Stanton, and S. Baron. 1986. Role of interferon in streptococcal infection in the mouse. Microb. Pathog. 1:399–407. [DOI] [PubMed] [Google Scholar]