Abstract
Vaccination of mice with yeast-secreted Plasmodium yoelii-derived 19-kilodalton merozoite surface protein 1 (yMSP119) has been shown to afford protection from challenge with a lethal strain of P. yoelii. Sterile immunity can be achieved when MSP119 is emulsified in Freund adjuvant but not when it is adsorbed to aluminum hydroxide gel (alum). Because complete Freund adjuvant is not an acceptable adjuvant for use in humans, alternative adjuvants must be identified for formulating MSP119 as a vaccine for use in humans. To determine whether oligodeoxynucleotides with CpG motifs (ODN), reported to be a powerful new class of adjuvants, could enhance the immunogenicity of yMSP119, C57BL/6 mice were vaccinated either with yMSP119 formulated with Freund adjuvant, with alum, or with ODN plus alum and challenged intravenously with P. yoelii 17XL asexual blood-stage parasites. Adsorption of immunogen and adjuvant to alum was optimized by adjusting buffer (phosphate versus acetate) and pH. We found that the adjuvant combination of ODN plus alum with yMSP119, injected intraperitoneally (i.p.), increased immunoglobulin G (IgG) yMSP119-specific antibody production 12-fold over Freund adjuvant given i.p., 3-fold over Freund adjuvant given subcutaneously (s.c.), 300-fold over alum given i.p., and 48-fold over alum given s.c. The predominant antibody isotype in the group receiving alum-ODN-yMSP119 was IgG1. Increased antibody levels correlated to protection from a challenge with P. yoelii 17XL. Supernatant cytokine levels of gamma interferon in yMSP119-stimulated splenocytes were dramatically elevated in the alum-ODN-yMSP119 group. Interleukin-10 (IL-10) levels were also elevated; however, no IL-5 was detected. The cytokine profile, as well as the predominant IgG1 antibody isotype, suggests the protective immune response was a mixed Th1/Th2 response.
Malaria, an infectious disease caused by species of the parasite genus Plasmodium, is a leading healthcare concern in the world today. In the estimated 300 to 500 million clinical cases of malaria each year (49), mortality rates are approximately one million per year. The majority of these deaths occur among young children at an estimated rate of one death every 30 s. The associated morbidity of this disease is incalculable because many of the children who survive are repeatedly infected, with resultant poor nutrition, impaired development and, perhaps, increased susceptibility to comorbid infections and associated disease.
Approaches to controlling malaria, including vector control measures and improvements in the development and use of drugs for prophylaxis and treatment of infection, have not proven effective in the long term. Development of vaccines directed against proteins expressed during various times of the complex Plasmodium life cycle (16) holds great promise for combatting malaria. In this study, we have used a murine model to analyze the immune correlates associated with immunogenicity and protection of a blood-stage vaccine candidate by using various adjuvants, including the recently described oligodeoxynucleotides with CpG motifs (ODN) (24).
The nineteen-kilodalton C terminus of merozoite surface protein 1 (MSP119), a leading blood-stage malaria vaccine candidate, was found in monkey challenge trials (28) to elicit a protective immunologic response only when Freund complete adjuvant (FCA) was used; however, FCA is not acceptable for prophylactic vaccine use in humans. In a murine study in which protective immunity was induced with MSP119 the effective adjuvant was again FCA (15). These studies and others conclude that MSP119 would be a good candidate for prevention of disease and/or prophylaxis from Plasmodium infection based on murine and nonhuman primate studies. In a human phase I clinical trial of MSP119 (21), fewer than half of the subjects immunized produced an antibody response when aluminum hydroxide (alum) was used as the adjuvant, and several apparent hypersensitivity reactions were reported. We report here a candidate adjuvant that may be both capable of adequate immunostimulation and suitable for human use.
Oligodeoxynucleotides with an increased frequency of unmethylated CpG dinucleotide motifs (CpG) have been found to be immunostimulatory and useful as adjuvants for peptide vaccines against a variety of pathogens (1, 7, 8, 44, 48). The immunostimulatory effects are determined by the sequence of the nucleotides and are species specific, implying a receptor-mediated mechanism. Hemmi et al. (14) reported a Toll-like receptor, TLR-9, that was required for immune activation with CpG. These immunostimulatory effects may have evolved as a nonspecific immune response linked to the presence of viral or bacterial DNA breakdown products released after infection (25, 26). Several groups using alum as a coadjuvant with CpG (1, 7, 8, 44, 48) found that specific immune stimulation to antigens was markedly increased over the use of alum and antigen alone and that the immune response, despite the presence of alum, was predominantly a Th1-type response.
We investigated whether the combination of ODN and alum with recombinant MSP119 improved the immunogenicity of this protein over other formulations and measured correlates of protection to elucidate possible mechanisms of immunity in a murine malaria model.
MATERIALS AND METHODS
Proteins for vaccine formulation.
Recombinant MSP119 of Plasmodium yoelii was produced as a His6-tagged protein in Saccharomyces cerevisiae (yMSP119) as previously described (47) and stored at −70°C in phosphate-buffered saline (PBS; pH 7.0). Lot number y980325Z was used in the first trial, and lot number y990309Z was used in the second trial. Recombinant epidermal growth factor 3 (EGF3), the third EGF-like domain of the Plasmodium falciparum antigen Pfs25, was prepared as described elsewhere (45). EGF3 is nonimmunogenic in mice and was therefore used as a control for protein in this study (lot number 981105zbimrum01/02) and was stored at −70°C in PBS (pH 7.0).
Adjuvants for vaccine formulation.
The oligodeoxynucleotide designated 1826, 5′-TCCATGACGTTCCTGACGTT-3′, with a phosphorothioate backbone (ODN) is an especially strong stimulator of B cells, monocyte-derived cells, and NK cells (32). ODN was purchased from Oligos, Etc. (Wilsonville, Oreg.) as a level I (90 to 95% purity as determined by high-pressure liquid chromatography) sodium salts preparation. Lyophilized ODN was diluted with 10 mM Tris-1 mM EDTA (pH 7.0) to an approximate concentration of 3 to 4 mg/ml and stored at −70°C. Complete and incomplete Freund adjuvant (Sigma ImmunoChemicals, St. Louis, Mo.) were used as control adjuvants. Aluminum hydroxide gel adjuvant, 2% (alum; Superfos Biosector, Vedbaek, Denmark), batch number 2179, was used as a control and coadjuvant for ODN.
Buffers for vaccine formulation.
PBS buffer was prepared at pH 6.0, 6.4, 6.8, 7.2, 7.6, and 8.0; the pH was adjusted with hydrochloric acid or sodium hydroxide. Sodium acetate buffer (0.1 M) was prepared at pH 6.0, 7.0, and 8.0. The pH was adjusted with acetic acid or sodium hydroxide.
Alum-binding assays to determine optimal pH and buffer conditions.
In preparation for vaccine formulation, optimal conditions for adsorption of proteins (yMSP119 and EGF3) and the ODN to alum were determined. ODN (300 μg/ml) and either yMSP119 (500 μg/ml) or EGF3 (250 μg/ml) (equimolar concentrations) were mixed with the various buffer preparations. The solution was divided in half; 500 μg of alum per ml was added to one aliquot, and to the other aliquot a volume of buffer equal to the volume of alum was added. The mixture was incubated at room temperature for 30 min and then centrifuged at 16,000 × g for 5 min in a microcentrifuge. DNA concentrations from the solutions with alum ([aDNA]) and without alum ([DNA]) were determined by measuring the optical density (OD) of the supernatant fluid at 260 nm (Ultrospec 2000; Pharmacia Biotech, Cambridge, United Kingdom). Protein concentrations from both the with-alum ([aProt]) and without-alum ([Prot]) samples were measured by using a bicinchoninic acid microtiter plate protocol (Pierce, Rockford, Ill.). The percentage of the DNA or protein bound in the samples was determined using the following formulae (Table 1):
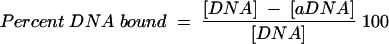 |
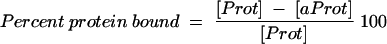 |
TABLE 1.
Percentage of ODN (DNA) or protein adsorbed to alum under different buffer conditionsa
| Buffer | pH | % DNA bound
|
% Protein bound
|
% DNA bound (ODN + EGF3) | % Protein bound
|
|||
|---|---|---|---|---|---|---|---|---|
| ODN | ODN + MSP119 | MSP119 | ODN + MSP119 | EGF3 | ODN + EGF3 | |||
| PBS | 6.0 | 0.4 | 2.0 | 7.1 | 17.2 | 8.6 | 26.5 | 41.8 |
| 6.4 | 0.0 | 1.0 | 1.8 | 6.0 | 0.2 | 18.9 | 20.6 | |
| 6.8 | 3.2 | 0.3 | 7.0 | 9.3 | 12.2 | 9.2 | 8.2 | |
| 7.2 | 6.9 | 0.1 | 3.1 | 9.1 | 24.3 | 6.6 | 5.3 | |
| 7.6 | 1.6 | 0.1 | 3.8 | 2.9 | 5.9 | 2.8 | 9.8 | |
| 8.0 | 0.0 | 2.0 | 3.7 | 5.9 | 0.0 | 12.8 | 13.8 | |
| Sodium acetate | 6.0 | 62.0 | 72.1 | 24.8 | 27.0 | 35.4 | 13.4 | 57.1 |
| 7.0 | 85.9 | 41.8 | 17.2 | 13.7 | 33.6 | 11.5 | 41.5 | |
| 8.0 | 76.6 | 53.7 | 16.3 | 20.8 | 16.0 | 12.9 | 46.9 | |
The percentage of DNA or protein adsorbed to 500 μg of alum per ml at differing pH values, with either PBS or sodium acetate buffer, is indicated. The concentration of ODN was 300 μg/ml; the concentrations of yMSP119 and EGF3 were 500 and 250 μg/ml, respectively. The highest values are indicated in boldface.
To determine whether changes in the buffer or pH had an effect on the protein already adsorbed to alum, with or without ODN, the alum pellet was resuspended in a volume of PBS buffer (pH 7.4) equal to the volume of sodium acetate buffer that was removed. The resuspended alum-PBS buffer mixture was incubated at room temperature for 30 min and then centrifuged at 16,000 × g for 5 min. Protein concentrations in the supernatant fluid of the PBS-treated alum (pProt) were determined, and the percentage of protein released was determined by using the following formula:
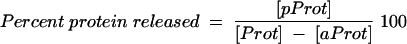 |
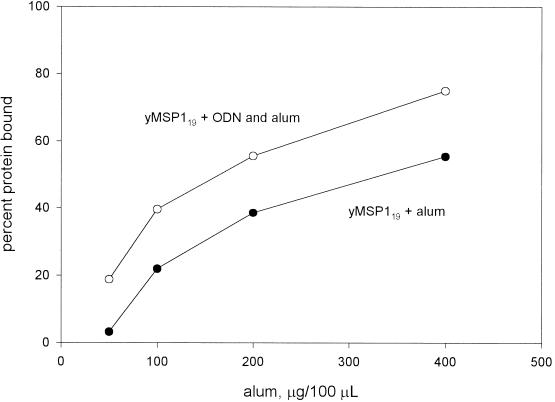
The optimal concentration of alum was determined as follows: mixtures of alum and yMSP119 were made in sodium acetate buffer with increasing concentrations of alum. The percentage of the protein bound was determined, as described above, and a graph of the concentration of alum versus the percentage of protein bound was made (see Fig. 1).
FIG. 1.
Binding of yMSP119 to alum with or without ODN.
The optimal adsorption condition for the vaccine material was 0.1 M sodium acetate buffer (pH 6.0). The concentrations of the materials in this buffer for immunization were as follows: ODN, 300 μg/ml; yMSP119, 500 μg/ml; EGF3, 250 μg/ml; and alum, 4 mg/ml.
Vaccination schedule, parasite challenge material, and description of groups.
All in vivo experiments were performed under the auspices of the NIH Animal Research Advisory Committee and its guidelines. C57BL/6 mice, 9 to 12 weeks old (Harlan-Sprague-Dawley, Indianapolis, Ind.) were immunized four times at 3-week intervals. Then, 100 μl of the material was injected either subcutaneously (s.c.) or intraperitoneally (i.p.), as described below. The amounts of materials in each 100-μl dose were as follows: ODN, 30 μg; yMSP119, 50 μg; EGF3, 25 μg; and alum, 400 μg (all in 0.1 M sodium acetate buffer at pH 6.0). The parasites used for challenge were from a frozen stock of P. yoelii 17XL, originally obtained from the laboratory of Carole A. Long. Five days prior to challenge, the frozen parasite stock was thawed and 0.5 ml was immediately injected i.p. into each donor mouse. The percent parasitemia was monitored over the next several days; when the percent parasitemia was between 2 and 10%, blood from the donor mouse was obtained by cardiac puncture in the presence of the anticoagulant, citrate phosphate dextrose (Baxter Healthcare Corp., Deerfield, Ill.). The parasites were then diluted to either 104 or 107 parasites per 100 μl with normal saline and immediately injected into the mice intravenously, via the tail vein, for challenge.
Trial 1.
Six groups of mice, eight animals in each group, were immunized as in the schedule above; all immunizations were i.p. The formulations for each group are as follows: alum plus ODN and yMSP119, Freund adjuvant plus yMSP119, alum plus yMSP119, ODN plus alum alone, EGF3 plus ODN and alum, and sodium acetate buffer with no antigen (see Table 2 for descriptions of the groups). The Freund adjuvant group received four i.p. immunizations as follows: for the first immunization, an equal volume of FCA was emulsified with 1,000 μg of yMSP119 per ml, with a final concentration of yMSP119 of 500 μg/ml; the second and third immunizations were similarly prepared, except that Freund incomplete adjuvant was used; for the fourth immunization, yMSP119 in sodium acetate buffer without adjuvant was injected i.p. At 10 days after the final immunization, three mice from each group were sacrificed, and their spleens were removed and processed for cytokine determinations. At 14 days after the last immunization, five mice in each group were challenged with 107 P. yoelii 17XL blood-stage parasites via tail vein injection.
TABLE 2.
Description of immunization groups and numbers of survivorsa
| Trial and formulation | Route | Challenge dose (no. of parasites) | No. of survivors/ total no. |
|---|---|---|---|
| Trial 1 | |||
| yMSP119-alum-ODN | i.p. | 107 | 4/5 |
| yMSP119-Freund | i.p. | 107 | 0/5 |
| yMSP119-alum | i.p. | 107 | 0/5 |
| Alum-ODN | i.p. | 107 | 0/5 |
| EGF3-alum-ODN | i.p. | 107 | 0/5 |
| Sodium acetate buffer, pH 6.0 | i.p. | 107 | 0/5 |
| Trial 2 | |||
| yMSP119-alum-ODN | i.p. | 107 | 4/5 |
| yMSP119-alum-ODN | s.c. | 107 | 0/5 |
| yMSP119-alum-ODN | s.c. | 104 | 2/5 |
| yMSP119-Freund | i.p. | 107 | 0/3 |
| yMSP119-Freund | s.c. | 107 | 2/2 |
| yMSP119-Freund | s.c. | 104 | 1/3 |
| yMSP119-ODN | s.c. | 104 | 0/5 |
| yMSP119-alum | s.c. | 104 | 1/5 |
| Alum-ODN | i.p. | 107 | 0/5 |
| Alum-ODN | s.c. | 107 | 0/5 |
| Alum-ODN | s.c. | 104 | 0/5 |
| No immunization | 104 | 0/5 |
Immunization formulae, routes of injection, numbers of parasites in the challenge dose, and the numbers of mice surviving 35 days after challenge are as indicated.
Trial 2.
Twelve groups of mice, with five mice in each group, were immunized as described in the schedule above. The formulations and routes of immunizations for the groups were as follows: alum plus ODN and yMSP119 given i.p. or s.c., Freund adjuvant plus yMSP119 given i.p. or s.c., alum plus yMSP119 given s.c., ODN plus yMSP119 given s.c., ODN plus alum alone given s.c., and no immunizations. The immunization material was prepared as described above for the Freund groups. The s.c. injections were given as one or two injections in the nape of the neck, and the fourth immunization, without adjuvant, was injected i.p. In addition to varying the route of immunization, some groups of mice received a challenge of 107 parasites, while others received a challenge of 104 parasites (Table 2). At 19 days after the last immunization the mice were challenged with P. yoelii 17XL with either the 104 or 107 parasites via the tail vein. In addition, 2 days prior to the challenge, a group of six mice that had not received any immunizations was injected with ODN alone at 30 μg/ml in sodium acetate buffer i.p. and then challenged with either 104 or 107 P. yoelii 17XL parasites, via the tail vein, with the same schedule as the rest of the groups.
Cytokine secretion.
Ten days after the fourth immunization, spleens from three mice in each of the six groups in trial 1 were collected, mechanically disrupted to single-cell suspensions, and subjected to osmotic lysis with ammonium chloride in potassium bicarbonate buffer (ACK; BioWhittaker, Walkersville, Md.) to deplete them of red blood cells (RBC). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 μg of penicillin per ml, 100 μg of streptomycin per ml, 25 mM HEPES, 1 mM sodium pyruvate, nonessential amino acids, and 50 μM 2-β mercaptoethanol at 37°C in 5% CO2 (18). All culture medium material was purchased from Gibco-BRL (Grand Island, N.Y.). Spleen cells from three mice in each of the groups in trial 1 were pooled, and 1.0 ml of a 106-cell/ml suspension per well was cultured in 24-well plates (Costar, Corning, N.Y.). The cells were then stimulated with either concanavalin A (ConA; 5 μg/well) or yMSP119 (10 or 30 μg/well) for 72 h, and then the supernatant fluids from these cultures were collected and frozen at −70°C. Gamma interferon (IFN-γ), interleukin-5 (IL-5), and IL-10 were measured by specific sandwich enzyme-linked immunosorbent assay (ELISA) (33, 41). Cytokine concentrations were calculated by reference to standard curves prepared with known amounts of recombinant cytokine.
ELISA for antibody levels.
The wells of Immulon-4 microtiter plates (Dynatech Laboratories, Chantilly, Va.) were coated with yMSP119 at 1 μg/ml in sodium bicarbonate buffer (pH 9.5) and incubated overnight at 4°C. The wells were washed three times with PBS containing 0.05% (vol/vol) Tween 20 (PBS-Tween). The uncoated sites were blocked with 3% (wt/vol) milk powder in PBS-Tween at room temperature for 6 h. The plates were washed again with PBS-Tween. Threefold dilutions of the sera were made by using PBS-Tween with 1% (wt/vol) milk powder; 100 μl of the diluted sera was pipetted into duplicate wells, and the plates incubated at 4°C overnight. After washing, horseradish peroxidase-conjugated rabbit anti-mouse sera, the second antibody, was added and incubated at room temperature for 3 to 4 h. The rabbit anti-mouse sera were rabbit anti-mouse immunoglobulin G (IgG; H+L), IgG1, IgG2a, IgG2b, and IgG3 (Zymed, South San Francisco, Calif). The dilution of the second antibody to total IgG was 1:2,000; for the remaining secondary antisera, the dilution was 1:1,000. The wells were washed again, and bound antibody was detected by adding 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for 5 min. The reaction was stopped by adding 10 μl of 10% (wt/vol) sodium dodecyl sulfate, and the absorbance (OD) was measured at 410 nm by using an ELISA plate reader (Dynatech Laboratories, Guernsey, Channel Islands, United Kingdom). The average OD of the duplicates versus the inverse of the dilution was graphed. Antibody levels were determined by choosing two points from the steepest portion of this graph and multiplying the OD value at each of these two points by the corresponding dilution value. The two products were averaged, and the resulting antibody level was expressed in arbitrary units (17).
Percent parasitemias.
Daily tail snips for blood smears were performed to determine the percent parasitemias, starting 3 days after challenge and continuing until the day 38, with one exception. One mouse in trial 2 had a high parasitemia on day 38 and was monitored until there was zero parasitemia for 3 days in a row, i.e., to day 58. The blood smears were stained with modified Giemsa stain (Sigma Diagnostics, St. Louis, Mo.), and the percent parasitemias were determined in the following manner. When a scan of the blood smear showed more than ∼3% parasitemia, 200 RBC were counted, and the number of parasitized RBC was determined. If it appeared there was <3% parasitemia, then 10 fields of 200 cells per field (i.e., 2,000 cells) were scanned, and the number of parasitized cells was determined.
Virulence study.
As the trials progressed, it was noted that some of the mice self-cured in spite of parasitemia levels that were as high as 75%. To determine whether the parasites in these mice had changed to a less-virulent strain, parasites from these mice were collected by tail snip, diluted with normal saline, and injected i.p. into naive mice. Total red cell counts and percent parasitemias were determined as described above. Two immunologically naive mice received a dose of 7 × 106 parasites from a donor immunized with alum-ODN-yMSP119, i.p. (mouse 006). Two other naive mice received a dose of 8 × 106 parasites from a donor immunized with Freund-yMSP119 given s.c. (mouse 016). Daily parasitemia levels were monitored as described above, beginning 2 days after challenge.
RESULTS
Vaccine formulation: adsorption of protein to alum in the presence or absence of ODN.
To determine the optimal conditions for adsorption of protein alone and protein-ODN to alum, binding assays were performed with various pH and buffer combinations (Table 1). Adsorption of ODN alone to alum was highest when sodium acetate buffer (pH 7.0) was used. Maximum adsorption of yMSP119 occurred at pH 6.0 with sodium acetate buffer, while EGF3 had maximum adsorption in PBS at pH 6.0. When ODN was added to either protein, adsorption of the protein was highest when sodium acetate buffer at pH 6.0 was used. Although the adsorption of the ODN to alum in the presence of protein was lower than that of ODN alone, it remained highest when sodium acetate (pH 6.0) was used.
To determine whether protein deadsorbs from alum when buffer conditions change, as would be expected in vivo, the percentage of protein released in PBS (pH 7.4) was determined as described above. The amount of yMSP119 released was 72%, and the amount of EGF3 released was 41%. The presence or absence of ODN had little effect on the amount of protein released.
The optimal concentration of alum was determined as follows. Mixtures of alum and yMSP119 were made in sodium acetate buffer (pH 6.0) with increasing concentrations of alum (Fig. 1). For immunizations the concentration of the material in the sodium acetate buffer included ODN (300 μg/ml), yMSP119 (500 μg/ml), EGF3 (250 μg/ml), and alum (4 mg/ml), and each immunization was 100 μl.
Cellular immune responses.
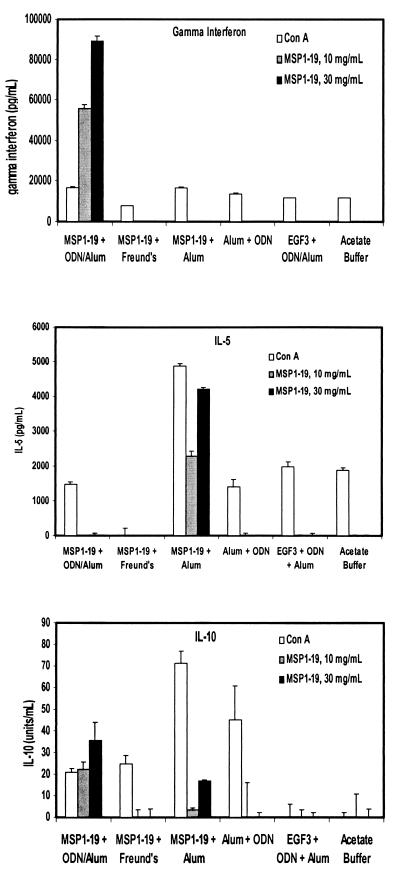
To determine the contribution of cytokines associated with Th1 or Th2 immune responses, the levels of IFN-γ, IL-5, and IL-10 were measured in the supernatant fluid collected from cultured splenocytes specifically stimulated with yMSP119 (Fig. 2). Only in mice vaccinated with alum-ODN-yMSP119 were IFN-γ levels detected. Both the alum-ODN-yMSP119 group and the alum-yMSP119 group had detectable levels of IL-10, while only the alum-yMSP119 group had detectable levels of IL-5.
FIG. 2.
Cytokine responses. Splenocytes were harvested from mice immunized with material as indicated and then stimulated with ConA as a control or yMSP119. Supernatants were analyzed for IFN-γ (pg/ml), IL-5 (pg/ml), and IL-10 (units/ml) by ELISA.
Humoral immune responses: yMSP119-specific total and isotype-specific IgG levels.
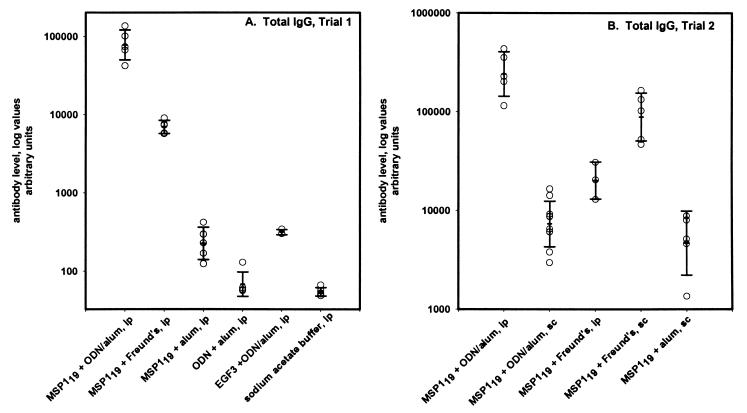
To determine the level of antibody response and the isotype associated with Th1 or Th2 immune responses, both total IgG and isotype IgG1, IgG2a, IgG2b, and IgG3 yMSP119-specific antibody responses were measured. In trial 1, the total IgG level was highest in the alum-ODN-yMSP119 i.p. group: it was 12-fold higher than that in the Freund-yMSP119 i.p. group and more than 300 times the level in the alum-yMSP119 i.p. group (Fig. 3A). The yMSP119-specific IgG response in animals that received EGF3 was similar to that of the alum-yMSP119 group and likely represents production of antibody to one of the cysteine-rich EGF-like domains in MSP1 (5, 31). In trial 2, the alum-ODN-yMSP119 i.p. response was again 12 times higher than that of the Freund-yMSP119 i.p. group but only three times higher than that of the Freund-yMSP119 s.c. group. Of note, the alum-ODN-yMSP119 i.p. group had antibody levels 30 times greater than those of the s.c. group. The response of the Freund-yMSP119 i.p. group was almost five times lower than that of the comparable s.c. group (Fig. 3B).
FIG. 3.
Total yMSP119-specific IgG. (A) Results from trial 1. All immunizations were done i.p. (B) Results from trial 2. Routes of immunization as indicated. The bars represent geometric means of arbitrary units ± 1 standard deviation (SD).
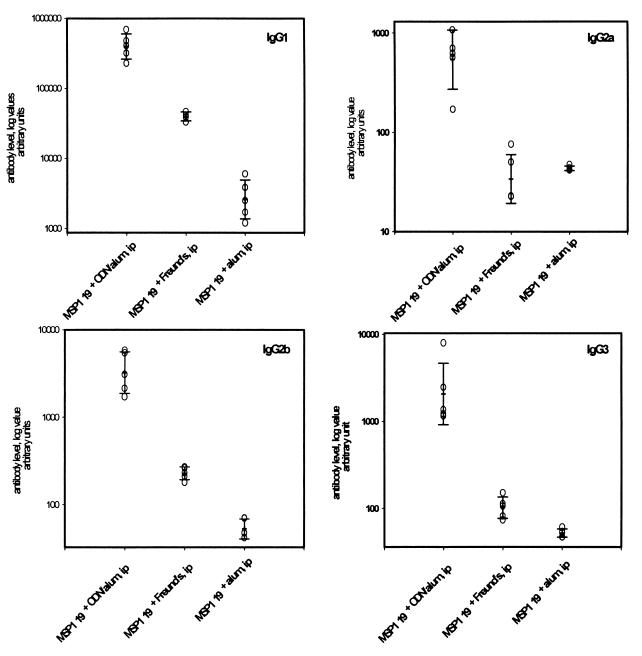
Antibody levels for all IgG isotypes (IgG1, IgG2a, IgG2b, and IgG3) were greater in the alum-ODN-yMSP119 i.p. group compared with either the Freund-yMSP119 i.p. group or the alum-yMSP119 i.p. group (Fig. 4). Specifically, in comparison with the Freund-yMSP119 s.c. group or the alum-yMSP119 i.p. group, the alum-ODN-yMSP119 i.p. group elicited a higher IgG2a (Th1 type) response; however, the ratio of the alum-ODN-yMSP119 i.p. group geometric mean for IgG1, IgG2a, IgG2b, and IgG3 antibody levels to those of the alum-yMSP119 i.p. group were 152.0, 12.4, 61.6, and 40.0, respectively, suggesting that a Th2-type response (IgG1 isotype) is the dominant antibody isotype response in the alum-ODN-yMSP119 i.p. group (Fig. 4).
FIG. 4.
Isotypes of yMSP119-specific antibodies. The bars represent geometric means of arbitrary units ± 1 SD.
Protective immune responses: parasite challenge and antibody responses.
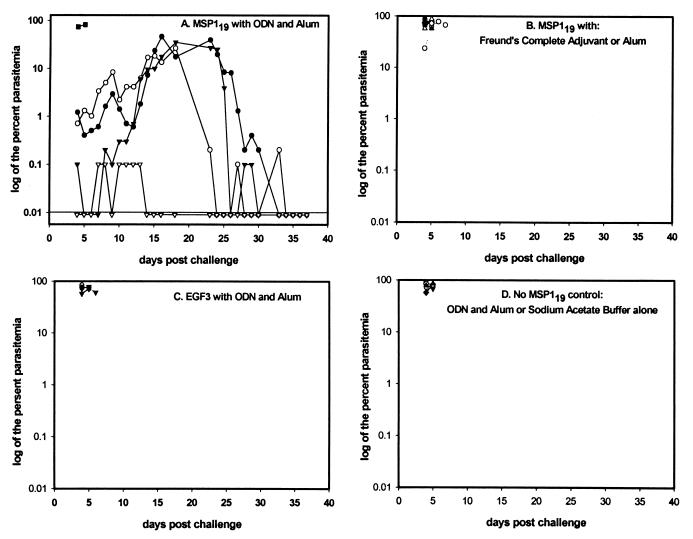
The ultimate test of a potential vaccine is its ability to control pathogens in a safe and efficacious manner. In trial 1, the only mice that survived the challenge of 107 P. yoelii 17XL were mice immunized with alum-ODN-yMSP119 i.p. (Fig. 5). Of the five mice in that group (Fig. 5A), one mouse died within 5 days of challenge, with a parasitemia of 82%, one mouse had a transient parasitemia of 0.1%, and three mice developed patent but delayed parasitemias; all four of these latter mice self-cured. The parasitemias in mice in the other groups were similar: all developed high parasitemias and died within 5 to 7 days (Fig. 5B, C, and D). A challenge of 107 P. yoelii parasites is an extraordinarily large inoculum; in our experience, no previous murine studies with this virulent parasite have ever shown mice surviving such a large challenge.
FIG. 5.
Trial 1, percent parasitemias. (A) Five mice immunized with yMSP119-ODN-alum. The line at 0.01 represents the level of detection for parasitemia. (B to D) All of the mice in the other groups died 5.8 ± 0.7 days after challenge.
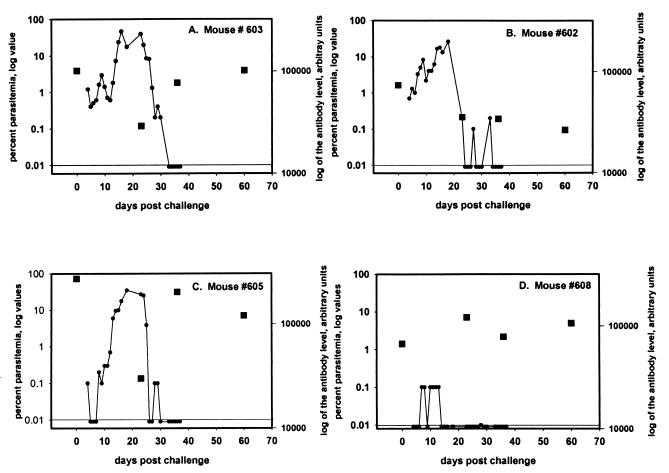
Antibody responses were determined three times (days 23, 36, and 60) during the course of infection in the four surviving mice (Fig. 6). In the one mouse with low-level parasitemia, the antibody level did not change substantially from the day of challenge (day 0) level (Fig. 6D). In the other three mice, all of which developed patent parasitemias of >0.1%, the antibody levels declined as the parasitemias increased.
FIG. 6.
Trial 1. Individual mouse parasitemias and yMSP119-specific total IgG antibody levels were monitored throughout the course of infection in the four mice that survived a challenge with 107 P. yoelii 17XL. The line at 0.01 represents the level of detection for parasitemia. The squares represent antibody levels.
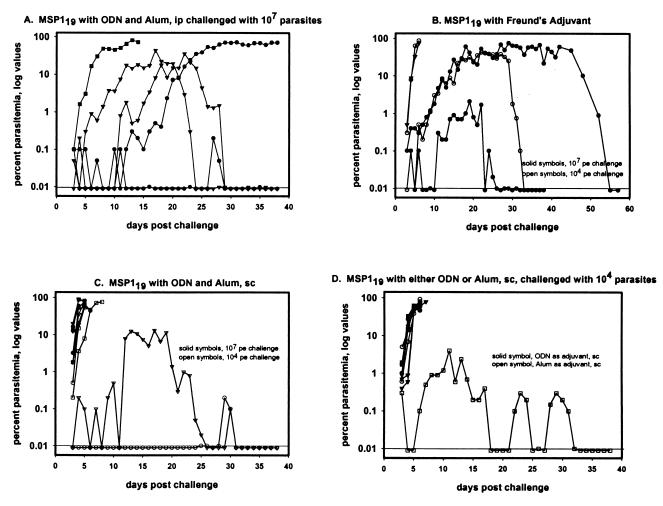
When the study was repeated in trial 2, three of five mice immunized with alum-ODN-yMSP119 i.p. survived the challenge of 107 P. yoelii 17XL (Fig. 7A and Table 2). One mouse had a peak parasitemia of 0.1% and then self-cured. The two other surviving mice in this group developed a delayed parasitemia on day 10 and day 15 that peaked at 43 and 36%, respectively, and subsequently self-cured. Of the two mice that died, one had a parasitemia of 81% and died on day 14 and the other died on day 37 after a delay in developing a high parasitemia that peaked at 72% on day 31.
FIG. 7.
Trial 2, percent parasitemia. The line at 0.01 represents the level of detection for parasitemia. pe, parasitized erythrocytes.
In trial 2, three groups of mice were immunized with yMSP119 in Freund adjuvant; one group was immunized i.p. and, as in trial 1, challenged with 107 P. yoelii 17XL parasites; another group was immunized s.c. and challenged with 107 P yoelii 17XL parasites; and a third group was also immunized s.c. but challenged with only 104 P. yoelii 17XL parasites (see Table 2 for details concerning immunization and challenge regimens and Fig. 7 for parasitemia data). Mice that received i.p. immunizations with yMSP119 in Freund’s adjuvant did not survive a challenge of 107 P. yoelii 17XL; all died with high parasitemias within 5 days of the challenge (Table 2). Of the mice that received s.c. immunizations, three of five mice survived the challenge with either 104 or 107 parasites (Fig. 7B and Table 2).
It was noted that high antibody levels were associated both with overall survival and with a delay of parasitemia. Spearman’s rank correlation comparing the level of total IgG antibody specific to yMSP119 to the days of survival was highly significant (P < 0.001; statistics program Stato 6.0).
Change in virulence of breakthrough parasites.
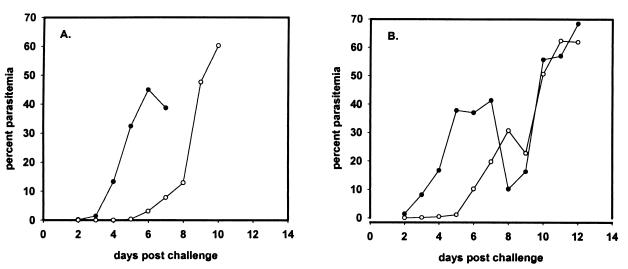
Several mice in the alum-ODN-yMSP119 i.p. group and in the Freund-yMSP119 s.c. group developed high parasitemias and self-cured (Fig. 7). To determine whether there was a change in virulence in these parasites, a few drops of blood from the tail vein of two mice that had developed high parasitemias were injected into each of two naive mice. The four recipient mice all died with high parasitemias; however, the time to death was delayed by at least 2 to 7 days (11.2 ± 2.4 days after infection) when historically compared with the parasite challenge of naive mice in trial 2 (7.2 ± 0.4 days after infection) (Fig. 8).
FIG. 8.
Virulence of parasites from each of two mice, with high levels of parasitemia, injected into two naive mice. (A) Percent parasitemias of two mice challenged with 7 × 106 parasites collected from mouse 006. (B) Percent parasitemias of two mice challenged with 8 × 106 parasites collected from mouse 016.
Administration of ODN 2 days before challenge.
In trial 2, six mice received i.p. injections of 30 μg of ODN 2 days before the parasite challenge. Three mice received a challenge of 104 P. yoelii 17XL parasites, and three mice received a challenge of 107 parasites. The average number of days to death for the mice that received a challenge of 104 was 12.0 ± 6.1 days; for the mice that received a challenge of 107, the average number of days to death was 9.0 ± 2.0 days. The average number of days to death for the mice in trial 2 that did not receive any immunizations and were challenged with 104 parasites was 7.2 ± 0.4 days (data provided for review).
DISCUSSION
Adsorption of protein, ODN, or protein with ODN to alum.
Proteins and alum have pH-dependent surface charges that play an important role in the adsorption of proteins by alum (4). Pretreatment of alum (pI 11) with phosphate anions reduces the positive surface charge of alum and may increase the adsorption of basic proteins such as lysozyme (pI 11) (39). In vitro, the adsorption of yMSP119 (pI 4.8) was significantly reduced when PBS buffer of any pH was used; in contrast, sodium acetate buffer enhanced adsorption. EGF3, on the other hand, with a pI of 8.4, had higher level of adsorption to alum in the presence of PBS and a lower level of adsorption when buffered with sodium acetate. The adsorption of ODN, a DNA molecule with many negatively charged phosphate ions, was also highest when buffered with sodium acetate and negligible when buffered with PBS. The phosphate anions in ODN presumably neutralized the charge on the alum and increased the adsorption of EGF3, whereas the ODN phosphate anions had minimal to no effect on the adsorption of yMSP119 in sodium acetate buffer (Table 1).
In 1977 the World Health Organization recommended adsorption of 80% or more of tetanus toxoid and diphtheria toxoid on aluminum adjuvants. The U.S. Minimum Requirement (1956) for adult tetanus and diphtheria toxoid is at least 75% adsorption of diphtheria toxoid component on the aluminum adjuvants (38). With these values in mind, we sought to obtain a binding of 75 to 80% of yMSP119. By increasing the amount of alum to 4 mg/ml, we were able to increase the percent adsorption of yMSP119 to 78% (Fig. 1). The amount of ODN adsorbed when 4 mg of alum per ml was used in sodium acetate buffer (pH 6.0) was 98%, and the amount of EGF3 bound under these conditions was 99%. There were no apparent adverse effects in mice that were immunized with 400 μg of alum. These data suggest that pre-vaccine formulation testing for adsorption to alum is important to maximize the actual amount of antigen bound to alum and delivered at the time of immunization. When the buffering conditions of the alum-bound protein, with or without ODN, were changed by using PBS (pH 7.4), there was a release of the protein into the solution. The degree to which protein release occurs and the implications of that protein release in vivo remain unclear, in part because the adsorption of proteins to alum is dependent on complex interactions of multiple substances with different alum adsorption coefficients (13). Attempts to predict the release of proteins in vitro by bathing alum-bound proteins in interstitial fluid have been made (42), but there are many variables that confound this model in vivo. The adsorption of antigens and coadjuvants (i.e., ODN) to alum is best maximized by adjusting buffering conditions prior to immunization, thus enhancing the depot effect of this adjuvant.
Immune responses and protection.
It is unclear what mechanisms are required for the protection of C57BL/6 mice from challenge with P. yoelii 17XL; both innate and humoral immunity appear to be essential for the control of blood-stage infection by P. yoelii 17XL in the murine infection (46). IFN-γ plays a critical role in early parasite control (9, 10, 37, 46). In trial 1, mice that survived the parasite challenge—i.e., mice immunized with alum-ODN-yMSP119—secreted very high levels of IFN-γ from their yMSP119-stimulated splenocytes, while the level of IFN-γ produced by splenocytes of mice from the other groups was undetectable. In addition, injection of ODN alone is known to increase IFN-γ levels transiently in murine intracellular bacterial infection models, and these mice were protected from various intracellular pathogens (22, 23, 27). In a recent study by Gramzinski et al. (10), ODN alone stimulated a strong Th1 response that was protective against a P. yoelii sporozoite challenge. However, in our blood-stage model, ODN alone was not protective. Two days prior to blood-stage P. yoelii challenge in trial 2, six mice were injected with ODN alone. These mice died with high parasitemias, but the prepatent period was longer compared with that for mice who received no immunizations prior to challenge (data submitted for review), suggesting at least a transient protective property associated with increased IFN-γ levels.
Increased MSP119 antibody levels have correlated with protection to challenge from P. yoelii 17XL (6, 15), and transfer of MSP119-specific antibody has similarly been associated with protection (6, 29, 43). In trial 1, the only mice that survived the parasite challenge were mice immunized with alum-ODN-yMSP119 and with antibody levels in the range of 67,000 to 136,000 arbitrary units. Mice immunized i.p. with Freund adjuvant and yMSP119 had antibody levels in the range of 5,700 to 9,000 arbitrary units; none of these mice survived lethal challenge. In trial 2, a comparison of the length of survival in days versus the antibody level was statistically significant (P < 0.001), and high antibody levels were associated with protection.
Thus, in summary, mice with high antibody levels contained the initial infection by delaying an increase in the parasitemia compared with all other groups (Fig. 5 and 7) and, in the antibody response and parasitemia throughout the infection (Fig. 6), there appears to be an initial consumption of antibody with increasing parasitemia. In two of three mice (Fig. 6A and C), there was a recovery of the antibody level associated with undetectable levels of parasitemia. The third mouse (Fig. 6B), with no recovery of antibody levels, contained the infection but took longer for the percent parasitemia to drop to undetectable levels. The fourth surviving mouse (Fig. 6D) maintained a high antibody level and had a low to undetectable level of parasitemia throughout the observation period.
IFN-γ levels were extremely high in the alum-ODN-yMSP119 group, a finding clearly indicative of the Th1 response typically found with ODN stimulation of the immune response (12, 24, 40), although the predominant antibody isotype was IgG1, and the level of IL-10 was elevated in this group— both indicators of the Th2-type response seen when alum is used as the adjuvant (2, 3). The highest rate of survival after parasite challenge was seen in mice with the highest combined Th1 and Th2 response, i.e., mice that received the alum-ODN-yMSP119 immunizations.
Immune responses and the site of immunization.
Differences were noted in the level of antibody production based on the adjuvant and the site of immunization. When alum-ODN-yMSP119 was injected i.p. both the Th1 and the Th2 immune responses were striking, and yet when the same preparation was injected s.c. there was minimal antibody response. Conversely, when yMSP119 was combined with Freund adjuvant and injected i.p. the response was minimal and yet, when it was injected s.c., the antibody level was high. It has been suggested that the mechanism of action of repository adjuvants such as Freund adjuvant or aluminum salts is primarily the slow release of antigen into draining lymph nodes (likely to the follicular dendritic cells of the draining lymph node) to stimulate continued antibody production by follicular B cells (2). One difference between s.c. and i.p. injections is the site of the draining lymph nodes: cervical and submandibular sites for s.c. injections (near the nape of the neck) and a mesenteric site for i.p. injections. Another possible difference is the immunologic cellular milieu of the two sites; the peritoneum of a mouse is rich with CD5+ B-1 cells, whereas the s.c. site is not. CD5+ B-1 cells are known to produce IL-10 (36), are associated with production of autoimmune antibodies (11, 35), and are functionally a part of the first-line defense by producing “low-affinity, broad-specificity germ line antibodies that react with ubiquitous organisms” (20). Peritoneal B-1 cells may have influenced a localized cytokine response when alum-ODN-yMSP119 was injected i.p.
In summary, we have shown that by combining the adjuvants ODN and alum with the recombinant protein yMSP119 under buffer conditions that maximize adsorption, we could control a large inoculum of a lethal murine Plasmodium species, P. yoelii 17XL, in a mouse-parasite challenge model. The apparent mechanism of protection is a combination of Th1 and Th2 responses, as evidenced by a high IFN-γ level and undetectable levels of IL-5 (Th1), as well as high IgG1 antibody levels and elevated IL-10 (Th2) in mice immunized i.p. with this combination. It is particularly interesting that we were able to generate such a strong combined Th1/Th2 response with an adjuvant combination that may be acceptable for use in humans.
Acknowledgments
We thank Carole A. Long and Louis H. Miller (LPD, NIAID, NIH) and Stanley L. Hem (Purdue University) for helpful discussions, Pat Caspar for assistance with the cytokine assays, and Sandy Cooper for invaluable help with the animal care and procedures. We also thank Nancy Shulman for excellent editorial assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Brazlolot-Millan, C. L., R. Weeratna, A. M. Krieg, C. A. Siegrist, and H. L. Davis. 1998. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl. Acad. Sci. USA 95:1555–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer, J. M., and J. Alexander. 1997. Cytokines and the mechanisms of action of vaccine adjuvants. Cytokines Cell. Mol. Ther. 3:233–246. [PubMed] [Google Scholar]
- 3.Brewer, J. M., M. Conacher, C. A. Hunter, M. Mohrs, F. Brombacher, and J. Alexander. 1999. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 163:6448–6454. [PubMed] [Google Scholar]
- 4.Chang, M. F., J. L. White, S. L. Nail, and S. L. Hem. 1997. Role of the electrostatic attractive force in the adsorption of proteins by aluminum hydroxide adjuvant. J. Pharm. Sci. Technol. 51:25–29. [PubMed] [Google Scholar]
- 5.Chitarra, V., I. Holm, G. A. Bentley, S. Petres, and S. Longacre. 1999. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 AÅ resolution, a highly protective malaria vaccine candidate. Mol. Cell 3:457–464. [DOI] [PubMed] [Google Scholar]
- 6.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236–243. [PubMed] [Google Scholar]
- 7.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870–876. [PubMed] [Google Scholar]
- 8.Deml, L., R. Schirmbeck, J. Reimann, H. Wolf, and R. Wagner. 1999. Immunostimulatory CpG motifs trigger a T helper-1 immune response to human immunodeficiency virus type-1 (HIV-1) gp 160 envelope proteins. Clin. Chem. Lab. Med. 37:199–204. [DOI] [PubMed] [Google Scholar]
- 9.De Souza, J. B., K. H. Williamson, T. Otani, and J. H. Playfair. 1997. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect. Immun. 65:1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gramzinski, R. A., D. L. Doolan, M. Sedegah, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa, K., M. Asano, S. A. Shinton, M. Gui, D. Allman, C. L. Stewart, J. Silver, and R. R. Hardy. 1999. Positive selection of natural autoreactive B cells. Science 285:113–116. [DOI] [PubMed] [Google Scholar]
- 12.Heeg, K., and S. Zimmermann. 2000. CpG DNA as a Th1 trigger. Int. Arch. Allergy Immunol. 121:87–97. [DOI] [PubMed] [Google Scholar]
- 13.Heimlich, J. M., F. E. Regnier, J. L. White, and S. L. Hem. 1999. The in vitro displacement of adsorbed model antigens from aluminium-containing adjuvants by interstitial proteins. Vaccine 17:2873–2881. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:659–660. [DOI] [PubMed] [Google Scholar]
- 15.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400–3411. [PubMed] [Google Scholar]
- 16.Hoffman, S. L., and L. H. Miller. 1996. Malaria vaccine development, a multi-immune response approach, p.1–13. In S. Hoffman (ed.), Perspectives on malaria vaccine development. American Society for Microbiology, Washington, D.C.
- 17.Hoffmann, K. F., S. L. James, A. W. Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927–938. [PubMed] [Google Scholar]
- 18.Jankovic, D., P. Caspar, M. Zweig, M. Garcia-Moll, S. D. Showalter, F. R. Vogel, and A. Sher. 1997. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp120. J. Immunol. 159:2409–2417. [PubMed] [Google Scholar]
- 19.Jones, T. R., N. Obaldia III, R. A. Gramzinski, Y. Charoenvit, N. Kolodny, S. Kitov, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 1999. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine 17:3065–3071. [DOI] [PubMed] [Google Scholar]
- 20.Kantor, A. B., A. M. Stall, S. Adams, L. A. Herzenberg, L. A. Herzenberg. 1992. Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. USA 89:3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keitel, W. A., K. E. Kester, R. L. Atmar, A. C. White, N. H. Bond, C. A. Holland, U. Krzych, D. R. Palmer, A. Egan, C. Diggs, W. R. Ballou, B. F. Hall, and D. Kaslow. 1999. Phase I trial of two recombinant vaccines containing the 19kd carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (msp-1[19]) and T helper epitopes of tetanus toxoid. Vaccine 18:531–539. [DOI] [PubMed] [Google Scholar]
- 22.Klinman, D. M., A.-K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 93:2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieg, A. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12:35–43. [DOI] [PubMed] [Google Scholar]
- 25.Krieg A. M. 2001. Now I know my CpGs. Trends Microbiol. 9:249–252. [DOI] [PubMed] [Google Scholar]
- 26.Krieg, A. M., A.-K. Yi, and G. Hartman. 1999. Mechanisms and therapeutic applications of immune stimulatory CpG DNA. Pharmacol. Ther. 84:113–120. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M., L. Love-Homan, A.-K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428–2434. [PubMed] [Google Scholar]
- 28.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling, I. T., S. A. Ogun, P. Momin, R. L. Richards, N. Garcon, J. Cohen, W. R. Ballou, and A. A. Holder. 1997. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine 15:1562–1567. [DOI] [PubMed] [Google Scholar]
- 30.Lipford, G. B., M. Bauer, C. Blank, R. Reiter, H. Wagner, and K. Heeg. 1997. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur. J Immunol. 27:2340–2344. [DOI] [PubMed] [Google Scholar]
- 31.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1–14. [DOI] [PubMed] [Google Scholar]
- 32.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216–1224. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann, T. R., J. H. Schumacher, D. F. Fiorentino, J. Leverah, K. W. Moore, and M. W. Bond. 1990. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor by using a solid phase radioimmunosorbent assay. J. Immunol. 145:2938–2945. [PubMed] [Google Scholar]
- 34.Moss, R. B., J. Diveley, F. Jensen, and D. J. Carlo. 2000. In vitro immune function after vaccination with an inactivated, gp120-depleted HIV-1 antigen with immunostimulatory oligodeoxynucleotides. Vaccine 18:1081–1087. [DOI] [PubMed] [Google Scholar]
- 35.Murakami, M., H. Yoshioka, T. Shirai, T. Tsubata, and T. Honjo. 1995. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int. Immunol. 7:877–882. [DOI] [PubMed] [Google Scholar]
- 36.O’Garra, A., and M. Howard. 1992. IL-10 production by CD5 B cells. Ann. N. Y. Acad. Sci. 651:182–199. [DOI] [PubMed] [Google Scholar]
- 37.Patterson, P. S., S. C. Bosshardt, V. Udhayukumar, L. Xiao, M. Kidd, R. L. Hunter, and A. A. Lal. 1999. Prolonged expression of IFN-gamma induced by protective blood-stage immunization against Plasmodium yoelii malaria. Vaccine 18:173–180. [DOI] [PubMed] [Google Scholar]
- 38.Powell, M., and M. J. Newman. 1995. Vaccine design: the subunit and adjuvant approach,1–42. In R. T. Borchardt (ed.), Pharmaceutical biotechnology, vol. 6. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 39.Rinella, J. V., Jr., J. L. White, and S. L. Hem. 1996. Treatment of aluminum hydroxide adjuvant to optimize the adsorption of basic proteins. Vaccine 14:298–300. [DOI] [PubMed] [Google Scholar]
- 40.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849–854. [DOI] [PubMed] [Google Scholar]
- 41.Scott, P., P. Natovitz, R. L. Coffman, E. Pearce, and A. Sher. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeber, S. J., J. L. White, and S. L. Hem. 1991. Solubilization of aluminum-containing adjuvants by constituents of interstitial fluid. J. Parenter. Sci. Technol. 45:156–159. [PubMed] [Google Scholar]
- 43.Spencer-Valero, L. M., S. A. Ogun, S. L. Fleck, I. T. Ling, T. J. Scott-Finnigan, M. J. Blackman, and A. A. Holder. 1998. Passive immunization with antibodies against three distinct epitopes on Plasmodium yoelii merozoite surface protein-1 suppresses parasitemia. Infect. Immun. 66:3925–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stowers, A. W., D. B. Keister, O. Muratova, and D. C. Kaslow. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 68:5530–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor-Robinson, A. W., and E. C. Smith. 1999. A role for cytokines in potentiation of malaria vaccines through immunological modulation of blood stage infection. Immunol. Rev. 171:105–123. [DOI] [PubMed] [Google Scholar]
- 47.Tian, J.-H., L. H. Miller, D. C. Kaslow, J. Ahlers, M. F. Good, D. W. Alling, J. A. Berzofsky, and S. Kumar. 1996. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J. Immunol. 157:1176–1183. [PubMed] [Google Scholar]
- 48.Weeratna, R. D., M. J. McCluskie., Y. Xu, and H. L. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755–1762. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 1998. Malaria. WHO fact sheet no. 94. World Health Organization, Geneva, Switzerland.