Abstract
A previous study of the murine model of Schistosoma mansoni infection has implicated splenic CD19+ B lymphocytes as Fas ligand (FasL)-bearing mediators of CD4+ T-lymphocyte apoptosis. The present study shows that B-cell deficiency leads to decreased CD4+ T-cell apoptosis during infection and compares FasL expression and killer function of B-1a- and CD5− B-lymphocyte subsets. B-1a cells from uninfected mice displayed constitutive expression of FasL compared with that of CD5− B cells. FasL expression was enhanced following worm egg deposition and antigenic stimulation on both subsets of B cells. Purified B-1a cells from uninfected mice were potent effectors of CD4+ T-cell apoptosis, and the killing effect was enhanced during schistosome infection. FasL expression by splenic B cells required CD4+-T-cell help that was replaced by addition of culture supernatants from antigen-stimulated splenocytes of infected mice. The culture-supernatant-stimulated FasL expression was inhibited by anti-interleukin 10 (IL-10) and anti-IL-4 antibodies. Culture of purified B cells with recombinant IL-4 (rIL-4), rIL-10, and soluble egg antigens (SEA) led to increased expression of FasL on B-1a cells. These results suggest that FasL-expressing, splenic B-1a cells are important mediators of SEA-stimulated CD4+-T-cell apoptosis and that maximal FasL expression on B-1a cells is dependent on antigenic stimulation and the presence of IL-4 and IL-10.
Schistosoma mansoni worms produce eggs that secrete soluble egg antigens (SEA) and induce T-cell-mediated granulomatous inflammation around the disseminated eggs in mammalian hosts (6). In infected humans, chronic inflammation and subsequent fibrous healing are the major factors in morbidity and mortality (1). Similar pathology is present in the murine model of infection, which allows for analysis of the factors controlling host response to the infection. The initial immunological reaction to SEA (6 weeks of infection) consists of a Th1-type cytokine response that switches to a Th2-type profile before the peak of granuloma formation (8 to 10 weeks of infection) (27). As the infection progresses to chronicity (>14 weeks of infection), there is a spontaneous, systemic downmodulation of the inflammatory response to SEA that leads to decreased CD4+-T-cell activity and decreased granuloma formation around newly implanted eggs but enhanced SEA-specific humoral response (7, 10). Previous observations showed that splenectomized, B-cell-depleted or B-cell-deficient mice did not downmodulate granuloma size during the chronic stage of the infection (8, 13, 20, 22). The putative role of B cells in the downmodulation process is still unclear; however, B-cell-secreted IL-10 as well as immune complex formation and/or antigen sequestration have been proposed as possible regulatory pathways (3, 17, 22, 33).
Other previous studies described T-cell apoptosis in the spleens and granulomas of infected mice (12, 29). We recently reported that SEA-stimulated, CD4+-T-cell apoptosis occurs during the early (Th1) stage and continues throughout the florid and downmodulated (Th2) stages of schistosome infection (24). SEA-stimulated upregulation of Fas ligand (FasL, CD95L), an important mediator of activation-induced cell death (23, 26), was demonstrated on the surface of CD4+ and CD8+ T cells and, surprisingly, on CD19+ B cells. Furthermore, splenic B cells were prominent mediators of CD4+-T-cell apoptosis in vitro and in vivo.
The present study further establishes the importance of B cells in mediating CD4+-T-cell apoptosis in vivo during schistosome infection and examined the phenotype and activation of FasL-expressing B cells. FasL expression was constitutive on splenic B-1a (CD19+/CD5+) and was higher than that on CD5− B (CD19+/CD5−) cells, which correlated with the more potent effector function of B-1a cells in mediating CD4+-T-cell apoptosis. Maximal FasL expression on B-1a cells was dependent on antigenic stimulation with interleukin 4 (IL-4) and IL-10. FasL-mediated apoptosis by B-1a cells indicates a novel function of these cells in immune regulation during schistosome infection.
MATERIALS AND METHODS
Mice, infection, and cell preparation.
Six- to eight-week-old female CBA/J, C57BL/6, or C57BL/6 μMT (B-cell-deficient) mice (Jackson Laboratories, Bar Harbor, Maine) were injected subcutaneously with 25 to 30 cercariae of the Puerto Rican strain of S. mansoni. Infected mice received standard mouse chow and acidified water. Mice were sacrificed at the indicated times of infection, and spleens and livers were removed aseptically. Granuloma cells were dispersed from livers as described previously (28). The erythrocytes from dispersed splenocytes were removed by hypotonic shock, and remaining cells were washed. Viability of the isolated spleen and granuloma cells were determined by trypan blue exclusion to be >95% and >80%, respectively. For in vitro experiments, cells were cultured in RPMI 1640 (Sigma, St. Louis, Mo.), 10% fetal calf serum (GibcoBRL, Gaithersburg, Md.), 2 mM pyruvate, 0.05 mM 2-mercaptoethanol, 2 mM l-glutamine, 100 U of penicillin/ml, and streptomycin at 0.1 mg/ml. Depletion of splenic CD4+ T cells and purification of CD19+ B cells was accomplished with magnetic microbead separations (Miltenyi Biotech, Auburn, Calif.). Purified B-1a and CD5− B cells were prepared by T-cell depletion with anti-CD90 (Thy1.2) magnetic beads followed by positive (B-1a) and negative selection with anti-CD5 magnetic beads (Miltenyi).
Antibodies and reagents.
Conjugated, monoclonal antibodies against murine lymphocyte surface markers, anti-CD5-phycoerythrin (PE), anti-FasL-PE (Kay-10), anti-FasL-biotin (Kay-10), as well as fluorescein isothiocyanate (FITC)-conjugated annexin V reagent, FcBlock, and streptavidin-allophycocyanin (APC) were purchased from Pharmingen (San Diego, Calif.). Anti-CD4-PE and anti-CD19-FITC were purchased from Caltag (Burlingame, Calif.), and propidium iodide (PI) and IgG2b,κ-PE (MOPC-141; control antibody for anti-FasL-PE) were purchased from Sigma. Cytokine supernatants were produced by culture of splenocytes (5 × 106 cells/ml) for 24 h in the presence or absence of 10 μg of SEA/ml. Hybridoma clones producing anti-IL-2 (S4B6), anti-IL-4 (11B-11), anti-IL-10 (JES 2A5), anti-gamma interferon (IFN-γ) (R4-6A2), and anti-dinitrophenol (DNP; DNP 30) were purchased from American Type Culture Collection. Neutralizing antibodies to murine cytokines were purified from ascites by thiophilic resin chromatography (Pierce, Rockford, Ill.). SEA were prepared by homogenization of schistosome eggs as previously described (6).
Measurement of CD4+-T-cell apoptosis.
Splenocytes and granuloma cells from mice infected for 8 or 14 weeks (n = 4 per group) were isolated individually as described above. For ex vivo analysis of CD4+-T-cell apoptosis, cells were immediately stained with 0.5 μg of FcBlock for 10 min at 4°C before incubation with 0.1 μg of CD4-PE antibody for 30 min at 4°C. Labeled cells were washed once in phosphate-buffered saline (PBS) and once in annexin V labeling buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2), and then 3 μl of annexin V-FITC and 0.5 μg of propidium iodide were added to the resuspended cell pellet for 10 min at room temperature. Labeling was terminated by the addition of 0.3 ml of annexin V labeling buffer, and data were acquired immediately on a FacScan instrument (Becton Dickinson, San Jose, Calif.). Viable lymphocytes were gated by using forward scatter versus side scatter characteristics, and analysis of Th-cell apoptosis was performed by gating of the CD4+/PI− population followed by analysis of annexin V-FITC labeling. Data were plotted as the percentage of viable T cells that were positive for annexin V. For in vitro apoptosis assays, target CD4+ T splenocytes were prepared by magnetic microbead depletion of CD8+ T cells and CD19+ B cells followed by culture in media for 36 h. Effector B-1a and CD5− B cells were prepared from freshly isolated splenocytes by magnetic microbead purification of B-cell subpopulations as described above. Target cells were plated at ×105 cells/well in 96-well round-bottom plates, and effector cells were added at the indicated effector:target (E:T) ratios for an additional 24 h. Cells from two wells were pooled and washed in labeling buffer and then were stained as described above. Error bars indicate the standard deviation of four replicates from a representative of three independent experiments.
Granuloma measurements.
Livers from B-cell-deficient (μMT) and wild-type C57BL/6 mice were removed and fixed in 10% neutral buffered formalin. Paraffin-embedded livers were cut in 5-μm-thick sections separated by 300 μm (6 sections/liver) and were stained with hematoxylin and eosin. Single egg granulomas (24/mouse) with a well-circumscribed egg in the center were measured by computerized morphometry with Scion Image Beta 3 software (Frederick, Md.). The mean granuloma areas of four mice were pooled for each condition and time point. Error bars indicate standard errors of the means.
Detection of FasL surface expression on B-1a and CD5− B cells.
Freshly isolated splenocytes or purified CD19+ B cells were stained ex vivo or cultured with indicated doses of recombinant IL-4 and/or IL-10 (Peprotech, Rocky Hill, N.J.) in the presence or absence of 10 μg of SEA/ml. Cells were washed three times in PBS, resuspended in 1% paraformaldehyde, incubated for 1 h at 4°C, and washed three times with PBS and then once with labeling buffer (PBS:0.2% bovine serum albumin:0.1% sodium azide). Cells were then incubated with FcBlock for 10 min at 4°C followed by incubation with 0.5 μg of anti-FasL-biotin for 30 min at 4°C. Stained cells were washed twice with labeling buffer and then were incubated with 0.2 μg of anti-CD19-FITC, 0.5 μg of anti-CD5-PE, and 0.2 μg of streptavidin-APC. Three-color flow cytometry was performed on a FacsCaliber instrument (Becton Dickinson). Cells were gated as CD19+/CD5+ (B-1a) and CD19+/CD5− (CD5− B) populations and analyzed for FasL-APC staining. Mean fluorescence intensity (MFI) ratios were determined by dividing the sample MFI by the average MFI of the indicated control sample for each experiment. Data from a representative experiment of three performed were plotted, and error bars indicate standard deviations of triplicate samples.
Detection of FasL surface expression on CD19+ B cells.
Unseparated or CD4+-T-cell-depleted splenocytes from mice infected for 8 weeks were cultured in the presence or absence of 10 μg of SEA/ml for 36 h. Cells were paraformaldehyde fixed and washed as described above. Fixed cells were labeled with FcBlock for 10 min at 4°C followed by incubation with 0.2 μg of anti-CD19-FITC and either 0.4 μg of anti-FasL-PE or 0.4 μg of IgG2b,κ-PE (control antibody) for 30 min at 4°C. After being labeled, the cells were washed twice in PBS and counted on a FacScan instrument (Becton Dickinson). Viable cells were gated by forward scatter versus side scatter, and then CD19-FITC-positive cells were gated and analyzed for display of FasL-PE compared with data from isotype-matched control antibody staining. Alternatively, purified CD19+ B cells were cultured for 20 h in the presence of cytokine supernatants that were either untreated or pretreated with cytokine neutralizing antibodies for 30 min at 37°C prior to staining as described above. MFI ratios, compared to those of the indicated control sample, are plotted from a representative experiment of three performed. Error bars indicate standard deviations of triplicate samples.
Statistical analysis.
MFI ratios were used for data comparison in order to standardize each experiment to an intra-assay control. Data are presented as MFI ratio ± standard deviation for individual samples. Means and standard deviations of CD4+-T-cell apoptosis percentages (Fig. 1A and 4) were determined by normalization of flow cytometric data by arcsine transformation. Arcsine transformation prevents skewing of results by very high or very low percentage values, thus increasing the validity of mean and error calculations. The statistical significance of the data were determined by analysis of variance and paired Student's t test. Because of multiple pair-wise comparisons in several figures, differences were considered significant when P was less than 0.02, unless otherwise indicated.
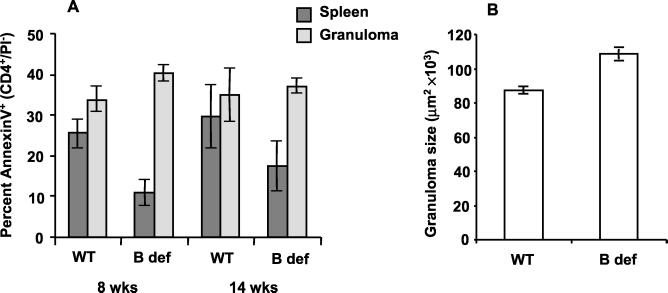
FIG. 1.
CD4+-T-cell apoptosis and granuloma downmodulation is dependent on B cells. B-cell-deficient μMT (B def) and age-matched, wild-type C57BL/6J (WT) mice were infected with S. mansoni cercariae. Mice (n = 4 for each data point) were sacrificed at the indicated times postinfection, splenocytes and granuloma cells were isolated individually from each mouse, and portions of the livers were formalin fixed and prepared for hematoxylin and eosin staining. (A) Freshly isolated splenocytes and granuloma cells were analyzed for CD4+-T-cell apoptosis by annexin V-based three-color flow cytometry. Percentage values were normalized by arcsine transformation prior to determination of the means and standard deviations of quadruplicate samples. (B) Granuloma sizes were determined from stained liver sections of mice infected for 14 weeks by computerized morphometry. Only granulomas with a single, well-circumscribed egg were included in the analysis. Error bars indicate the standard errors of the means of 24 granulomas/mouse for 4 mice/group.
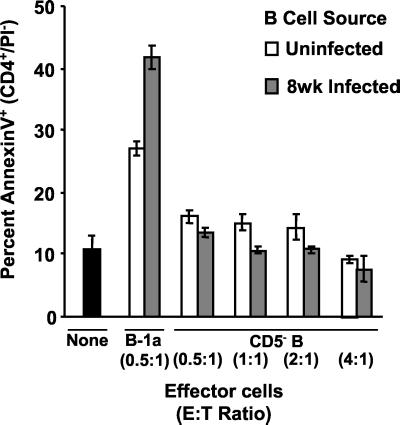
FIG. 4.
Purified B-1a cells from uninfected and schistosome-infected mice mediate apoptosis of purified CD4+ T cells. Splenocytes from mice infected for 8 weeks were cultured in media for 24 h and then were enriched for CD4+ T cells by magnetic bead depletion of CD19+ B cells and CD8+ T cells. CD4+-T-cell targets were then cultured without added cells (black bar) or mixed at the indicated ratios with B-cell subsets purified from freshly isolated splenocytes of uninfected mice and mice infected for 8 weeks. After 24 h of coculture, the cells were stained with anti-CD4-PE, annexin V-FITC, and PI and were analyzed by three-color flow cytometry. The viable CD4+ T cells (CD4+/PI−) were gated and analyzed for binding of the early apoptosis marker, annexin V. The percentages were normalized by arcsine transformation prior to determination of means and standard deviations. Reverse transformed means ± standard deviations of quadruplicate samples from a representative of three experiments are presented.
RESULTS
B-cell deficiency affects splenic CD4+-T-cell apoptosis and granuloma downmodulation.
Our previous study established a role for SEA-stimulated, FasL-bearing, splenic B cells as mediators of CD4+-T-cell apoptosis (24). As shown in Fig. 1A, infection of B-cell-deficient mice led to significant decreases in splenic CD4+-T-cell apoptosis during the acute (8 weeks) and chronic (14 weeks) stages of schistosomiasis (P < 0.01 and 0.05, respectively). As predicted by our previous observation that B-cell depletion did not affect in vitro granuloma CD4+-T-cell apoptosis, infection of B-cell-deficient mice did not lead to alterations in intragranulomatous apoptosis. However, at 14 weeks of infection granuloma downmodulation was significantly affected by the absence of B cells (Fig. 1B) (P < 0.01).
Comparison of FasL expression by freshly isolated B-1a- and CD5−-B-lymphocyte subsets.
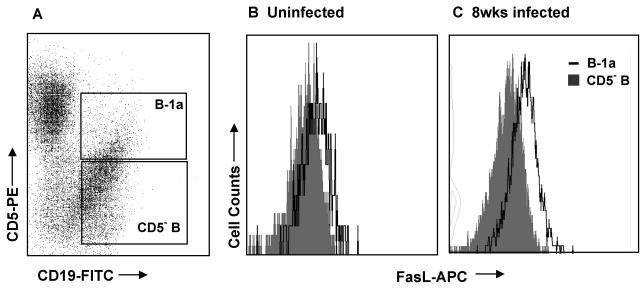
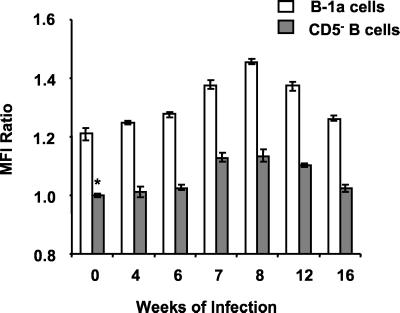
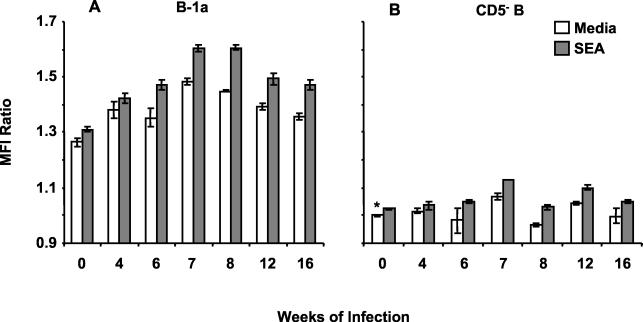
These results prompted further investigation into the phenotype of the splenic B cells expressing Fas ligand (FasL, CD95L) during murine schistosomiasis. As shown in Fig. 2A, flow cytometry was used to separately analyze the B-1a (CD19+/CD5+) and CD5− (CD19+/CD5−) B-cell subsets from freshly isolated splenocyte preparations. FasL expression was analyzed on gated B-1a and CD5− B cells by using an anti-mouse FasL-biotin and streptavidin-APC (FasL-APC) combination (Fig. 2B and C). B-1a cells from both uninfected mice and mice infected for 8 weeks expressed higher levels of FasL than CD5− B cells from the same animals. CD5− B cells from uninfected mice (Fig. 2B, shaded histogram) had the lowest intensity staining for FasL of any group tested, and therefore those MFI values were used as the standard for the determination of MFI ratios of B-1a- and CD5−-B-cell FasL expression at multiple stages of infection (Fig. 3). Splenic B-1a cells from uninfected mice (0 weeks of infection) and all stages of schistosome infection were more FasL+ than were CD5− B cells from the same animals (P < 0.001 for all points). FasL expression increased on the B-1a-cell subset during the acute, egg-induced, inflammatory stage (6 to 12 weeks) (P < 0.001 compared to that of uninfected mice) and returned to baseline during the chronic, downmodulated stage (16 weeks) of infection. An increase in FasL expression was also detected on CD5− B cells from 7 to 12 weeks of infection (P < 0.001). These results were consistent with the previous report of antigen-induced FasL expression on CD19+ B cells during schistosome infection (24).
FIG. 2.
Analysis of FasL expression on B-1a- and CD5−-B-cell subsets by three-color flow cytometry. Freshly isolated splenocytes from uninfected and schistosome-infected mice were paraformaldehyde fixed and then stained with biotinylated anti-FasL, streptavidin-APC, anti-CD19-FITC, and anti-CD5-PE. Live cells were gated from plots of forward scatter versus side scatter and then were plotted for CD19-FITC versus CD5-PE (A). B-1a (CD5+/CD19+) and CD5− B (CD5−/CD19+) cells were gated and FasL expression was compared on the subsets. Representative histograms for uninfected mice (B) and mice infected for 8 weeks (C) are shown. Because the staining appeared to be constitutive on B-1a cells, MFI was chosen for presentation of data instead of percentages of positive cells.
FIG. 3.
Surface FasL expression is increased on B-1a and CD5− B cells during schistosome infection. Freshly isolated splenocytes from mice infected for the indicated number of weeks were fixed and stained as described in the legend to Fig. 2. FasL expression was analyzed on gated B-1a and CD5− B cells. The ratios of MFI ± standard deviations of triplicate samples were determined in comparison to CD5− B cells from uninfected mice (∗) and are plotted from a representative of three experiments.
Splenic B-1a cells mediate apoptosis of CD4+ T cells.
Because a higher percentage of B-1a cells were FasL+ than were CD5− B cells, it was tested whether B-cell subsets differed in their ability to mediate CD4+-T-cell apoptosis (Fig. 4). B-1a and CD5− B cells were purified from freshly isolated splenocytes of uninfected mice and mice infected for 8 weeks by magnetic microbead separations. Target CD4+ T cells were prepared from unstimulated splenocyte cultures of 8-week-infected mice by magnetic microbead depletion of CD19+ B cells and CD8+ T cells. This method of purifying CD4+ T cells yielded target cells that did not display FasL on their surfaces and had low spontaneous apoptosis (<10%). Purified B-1a and CD5− B cells were mixed for 24 h with target CD4+ T cells at the indicated E:T ratios and then were analyzed for apoptosis by annexin V-based three-color flow cytometry (32). B-1a cells from uninfected mice were potent effectors of CD4+-T-cell apoptosis, correlating with their high level of constitutive FasL expression. B-1a cells from 8-week-infected mice showed significantly enhanced killer function (P < 0.001). In agreement with the low surface FasL expression pattern, purified CD5− B cells from uninfected mice and mice infected for 8 weeks did not induce CD4+-T-cell apoptosis even at E:T ratios eight times higher than those used for B-1a-cell-mediated apoptosis.
SEA-induced FasL expression on B-1a cells.
We previously detected increased FasL expression on SEA-stimulated, splenic CD19+ B cells. To test the induction of FasL on B-cell subsets, splenocytes from uninfected (0 weeks) and schistosome-infected (4 to 16 weeks) mice were cultured for 36 h in the presence or absence of SEA and then were analyzed by three-color flow cytometry (Fig. 5). MFI ratios were determined with the MFI for CD5− B cells without SEA stimulation as the standard. B-1a cells from uninfected and infected mice maintained a high level of FasL expression without SEA stimulus. FasL expression on in vivo SEA-primed B-1a cells (6 to 16 weeks of infection) was enhanced by SEA stimulation (P < 0.01 for 6 weeks, P < 0.002 for 12 weeks, and P < 0.001 for 7, 8, and 16 weeks of infection compared to those of unstimulated control B-1a cells from the same time point). In contrast, unstimulated CD5− B cells did not display FasL, and SEA-induced FasL expression was detected only on CD5− B cells from mice infected for 7, 8, and 12 weeks (P < 0.001).
FIG. 5.
B-1a- and CD5−-B-cell FasL expression is upregulated in vitro by treatment with SEA. Splenocytes from mice infected for the indicated number of weeks were cultured for 36 h in the absence or presence of 10 μg of SEA/ml. Cells were fixed, stained, and analyzed as described in the legend to Fig. 2. Ratios of MFI ± standard deviations of triplicate samples were determined against unstimulated CD5− B cells from uninfected mice (∗). Data from a representative of three experiments are presented.
Cytokine-stimulated FasL expression on purified B cells.
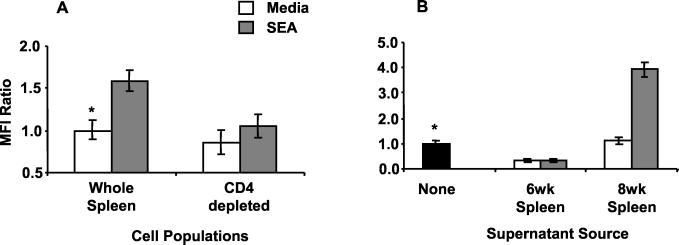
To elucidate the mechanism(s) by which SEA drives FasL expression on B cells, SEA-stimulated expression was compared between unseparated spleen cells and CD4+-T-cell-depleted populations from mice infected for 8 weeks. As shown in Fig. 6A, SEA induced significantly more FasL expression on B cells in the unseparated spleen cell cultures than with cultures devoid of CD4+ T cells (P < 0.001). Because it appeared that SEA-stimulated, B-cell FasL expression was dependent on the presence of CD4+ T cells, it was considered that CD4+-Th-cell-derived cytokines might play a role in driving FasL expression. Therefore, supernatants were collected at 24 h from unstimulated and SEA-stimulated splenocyte cultures of mice infected for 6 and 8 weeks and were mixed with magnetic bead-purified CD19+ B cells from mice infected for 8 weeks. The data shown in Fig. 6B indicated that supernatants from unstimulated and SEA-stimulated splenocytes of mice infected for 6 weeks inhibited FasL expression compared to purified B cells cultured in medium without cytokine supernatants (P < 0.001). In contrast, the supernatant from SEA-stimulated cultures of splenocytes from mice infected for 8 weeks induced FasL expression on purified B cells (P < 0.001 compared to that of control supernatant), which was comparable to the effect of SEA on unseparated spleen cells (Fig. 6A).
FIG. 6.
FasL expression on CD19+ B cells is dependent on soluble factors from CD4+ T cells. (A) Splenocytes from mice infected for 8 weeks were left unseparated or were depleted of CD4+ T cells prior to 36 h of culture in the presence or absence of 10 μg of SEA/ml. (B) Purified B cells were cultured for 20 h in the absence (black bar) or presence of 24-h culture supernatants from SEA-stimulated or unstimulated splenocytes of mice infected for 6 or 8 weeks. FasL expression was determined on paraformaldehyde-fixed cells by two-color flow cytometry using anti-CD19-FITC, anti-FasL-PE, and an isotype-matched, control IgG2b,κ-PE antibody. Ratios of MFI ± standard deviations are plotted for triplicate samples compared to data for indicated controls (∗). Data are from a representative of three experiments.
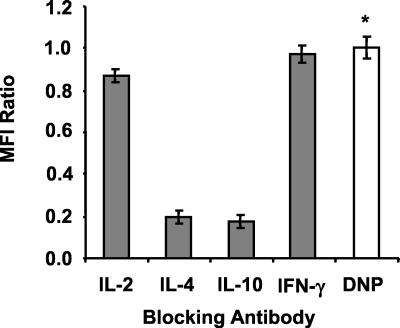
To examine the putative role of cytokines in FasL induction, neutralizing anti-cytokine antibodies were added to splenic supernatants from mice infected for 8 weeks prior to culture with purified B cells. Results shown in Fig. 7 indicated that neutralization of IL-10 or IL-4 led to decreased supernatant-induced expression of FasL on B cells compared to that of supernatants treated with isotype-control anti-DNP antibodies (P < 0.001). Treatment of supernatants with anti-IL-2 and anti-IFN-γ antibodies had no effect on B-cell FasL expression. A similar experiment with the same panel of neutralizing antibodies was carried out with cytokine supernatants from mice infected for 6 weeks. None of the antibodies influenced the suppressive effect of 6-week supernatants on FasL expression by B cells (data not shown).
FIG. 7.
Supernatant-induced, B-cell FasL expression is inhibited by anti-IL-4 and anti-IL-10 antibodies. Culture supernatants from SEA-stimulated splenocytes of mice infected for 8 weeks were pretreated for 30 min with 10-μg/ml concentrations of the indicated neutralizing antibodies prior to 20 h of culture with magnetic-bead-purified CD19+ B cells. FasL expression was evaluated by two-color flow cytometry as for Fig. 6. Ratios of MFI ± standard deviations of triplicate samples compared to those of anti-DNP control antibody (∗) are plotted. Data are from a representative of three experiments.
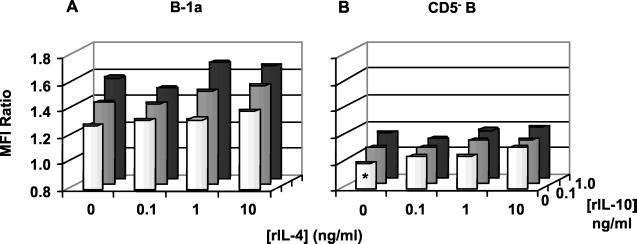
An increase in B-1a-cell FasL expression was detected following addition of recombinant IL-10 and/or IL-4 with SEA to purified B cell cultures (Fig. 8A). Both IL-10 and IL-4 had an individual, dose-dependent, stimulatory effect on SEA-induced, B-1a-cell FasL expression (P < 0.02 for 10 ng of IL-4/ml and P < 0.01 for both concentrations of IL-10 compared to that with no cytokine control), and the effect was additive when both Th2 cytokines were present together with SEA (P < 0.02 for all combinations). Th2 cytokine addition led to small but significant increases in FasL expression on the surface of the SEA-stimulated CD5−-B-cell populations (Fig. 8B) (P < 0.02 when combined and the amount of IL-4 was greater than 1 ng/ml). In contrast, FasL expression was not upregulated on either B-cell population cultured in the absence of SEA despite treatment with IL-10 and IL-4 (data not shown).
FIG. 8.
Recombinant IL-4 (rIL-4) and recombinant IL-10 induce additive FasL expression on the B-1a and CD5− B subsets of SEA-stimulated, purified CD19+ B cells. Magnetic immunobead-purified, splenic CD19+ B cells from mice infected for 8 weeks were cultured for 20 h in the presence of 10 μg of SEA/ml and the indicated doses of recombinant IL-4 and/or IL-10. Cells were paraformaldehyde fixed and stained as described in the legend to Fig. 2. FasL expression was analyzed on (A) gated B-1a (CD5+/CD19+) and (B) B (CD5−/CD19+) cells. Ratios of MFI of triplicate samples were determined in comparison to SEA-treated CD5− B cells devoid of exogenous cytokines (∗). Data from a representative of three experiments are plotted, with error bars omitted for clarity.
DISCUSSION
Previously, we demonstrated that activation-induced cell death of CD4+ T cells occurred during the acute and chronic stages of schistosome infection (24). In infected mice, increased FasL expression on CD4+ and CD8+ T cells as well as on CD19+ B cells paralleled the dynamics of increased CD4+-T-cell apoptosis. The novel finding that SEA-stimulated B cells were the major mediators of apoptosis in the spleens of infected animals prompted us to further investigate the killer role of B cells during schistosome infection. In direct agreement with the previous data, infection of B-cell-deficient mice led to significant decreases in splenic but not granuloma CD4+-T-cell apoptosis. Thus, the mechanism of intragranulomatous Th-cell apoptosis is B cell independent and is most likely dependent on FasL+/CD4+ T cells within the granuloma. Previous studies had determined a role for B cells in granuloma downmodulation (8, 13, 22), but none had suggested altered CD4+-T-cell apoptosis as a mechanism. The present study corroborated the previous reports of increased granuloma size at the chronic stage of infection in B-cell-deficient mice and introduced splenic, B-cell-mediated, CD4+-T-cell apoptosis as an additional mechanism of downregulation. The present data also suggested that granuloma size may be partially regulated by the viability and effector function of CD4+ splenic T cells. This is in agreement with previous findings made in splenectomized mice where enhanced granuloma growth was observed (20).
The splenic B-cell population can be subdivided into two groups based on the surface expression of CD5 (18). B-1a cells are CD5+ while B-1b, MZ, and B-2 cells are CD5−. B-1b and MZ cells share some similar functions with B-1a cells, but FasL expression on these specific subsets was not analyzed in this study. Analysis of FasL expression on B-1a and CD5−-B-cell subsets revealed marked differences. The dynamics of FasL expression on freshly isolated splenic B-cell subsets showed that throughout the infection, a much higher percentage of B-1a cells expressed surface FasL than did CD5− B cells. Whereas increased FasL expression was detected following egg deposition on both B-1a and CD5−-B-cell subsets, the B-1a cells were the predominant FasL-expressing cells. FasL expression on both B-cell subsets peaked during the florid Th2 phase (8 weeks) of infection, corresponding to peak granuloma formation and cell activation, and then decreased during the chronic, downmodulated stage of infection (16 weeks). These data indicated a correlation between the high level of surface FasL expression on B-1a cells and the degree of granulomatous inflammation.
A comparison of CD4+-T-cell apoptosis mediated by B-1a and CD5− B cells clearly indicated that B-1a cells were much more potent effectors than CD5− B cells. In agreement with the observed levels of FasL expression, B-1a cells from uninfected mice mediated significant CD4+-T-cell apoptosis but schistosome infection significantly increased B-1a-cell-mediated killing. The relatively high level of FasL expression and killing effect by B-1a cells from uninfected mice was surprising and indicated that B-1a cells may have a constitutive killer/effector function in nondiseased mice. These data demonstrated for the first time that FasL+ B-1a cells were capable of mediating CD4+-T-cell apoptosis and were the dominant effector B-cell subset within the spleens of schistosome-infected mice.
B-1a cells are predominantly localized in the pleural and peritoneal cavities and represent a minor subpopulation of B cells in the spleen (18, 25, 40). This subset is primarily responsive to T-cell-independent antigens and has been implicated as a major source of polyreactive autoantibodies. B-1a cells produce IL-10 in response to lipopolysaccharide stimulation and require IL-10 for normal development (21). Previously, autoantibody production, polyclonal activation of splenic B-1a cells, and homing to Peyer's patches and mesenteric ganglia were reported during schistosome infection (11, 14). It was also shown that a polylactosamine sugar component of SEA, lacto-N-fucopentaose III, induced splenic and peritoneal B-1a cells to produce IL-10 (33, 34, 35). Schistosome-infected, B-1 cell-deficient (Xid) mice showed increased mortality, elevated IFN-γ and IL-4 production, and diminished IL-10 production (16). Thus, there is previous evidence that B-1a cells are activated during schistosome infection and that they may participate in antibody production, regulation of the CD4+-T-cell response, and host survival.
SEA consistently induced elevated FasL expression on the surface of in vivo-primed splenic B-1a cells. This result correlated with our previous report of SEA-stimulated FasL induction on total splenic B cells (24). While examining FasL induction, we observed that in splenocytes depleted of CD4+ T cells fewer FasL+ B cells were induced than in unseparated cultures. The defect could be overcome by addition to purified B cells of culture supernatants from SEA-stimulated splenocytes of mice infected for 8 weeks. In contrast, addition of supernatants from mice infected for 6 weeks inhibited B-cell FasL expression. These data indicated that B-cell FasL expression was likely to be upregulated by Th2-type cytokines and inhibited by Th1-type cytokines. Further experiments proved that IL-4 and IL-10 upregulated SEA-stimulated FasL expression on B-1a cells. However, neutralization of the Th1-type cytokines, IL-2 and IFN-γ, did not have the expected enhancing effect on B-cell FasL expression. Thus, the inhibitory factors in supernatants from mice infected for 6 weeks remain undefined. These data indicate a novel regulatory function of IL-4 and IL-10 on the B-1a-cell subset.
Th1 and Th2 cytokine cross-regulation has been studied during the early granulomatous stage of schistosome infection, with antagonism of IFN-γ/IL-12 and IL-4/IL-10 production consistently noted (4, 5, 9, 31, 38). The present data indicate that a high percentage of FasL+ B-1a cells are induced by SEA at the 7th week of infection, the time when the switch from Th1 to Th2 responsiveness occurs (2, 27, 37). Previously, we demonstrated that CD4+-T-cell apoptosis was elevated at 7 weeks and peaked at 8 weeks of infection; therefore, IL-4-/IL-10-induced splenic B-1a cells are implicated as contributors to the Th1-to-Th2 switch during schistosome infection.
Moreover, the fact that B-1a cells continued to express FasL between 7 to 16 weeks of the infection and that B-cell deficiency led to decreased CD4+-T-cell apoptosis and increased granuloma size during the Th2 stage of response (8 to 16 weeks) also suggested that these B-1a cells contribute to regulation of the Th2-cell response and granulomatous downmodulation. Several studies have shown that endogenous IL-10 is important to downregulate granuloma formation during acute infection and to decrease cytokine production of both Th1- and Th2-type cytokines (4, 15, 19, 30, 36, 39). Moreover, neutralization of IL-10 led to decreased mitogen-induced apoptosis of CD4+ T cells and increased SEA-stimulated Th1 cytokine production in vitro (12). The present data indicate that autocrine and/or paracrine IL-10-mediated activation of B-1a cells can induce FasL expression. B-cell-mediated apoptosis of CD4+ T cells is a novel mechanism by which IL-10 could indirectly control the activity of both Th1- and Th2-type cells. The dependence of B-1a-cell FasL expression on CD4+ Th2 cell-derived IL-4 and IL-10 most likely serves as a negative feedback mechanism to reduce apoptosis once granuloma downmodulation and decreased inflammation has been achieved.
During schistosomal infection constant egg production provides sustained antigenic stimulation of T-effector cells and chronic granulomatous inflammation. Tight regulation of the CD4+-T-cell response is necessary for the modulation of inflammatory granuloma formation. In addition to the previously established proinflammatory roles of SEA it is now evident that SEA induces FasL expression on T and B cells, leading to activation-induced cell death of CD4+ T cells. In particular, SEA-induced IL-10 and IL-4 participate in FasL upregulation on splenic B-1a cells, which are potent mediators of CD4+-T-cell apoptosis. Additional work is needed to further establish the in vivo role for the B-1a cell-mediated regulation of granulomatous response.
Acknowledgments
This work was supported by Public Health Service grant AI-12913 from the National Institute of Allergy and Infectious Diseases.
We thank Vidya Reddy, Eric VanBuren, and Evano Piasentin for excellent technical assistance and Stephen Lerman and Nicholas Lukacs for helpful consultation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Boros, D. L. 1989. Immunopathology of Schistosoma mansoni infection. Clin. Microbiol. Rev. 2:250-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boros, D. L. 1999. T helper cell populations, cytokine dynamics, and pathology of the schistosome egg granuloma. Microbes Infect. 1:511-516. [DOI] [PubMed] [Google Scholar]
- 3.Boros, D. L., A. F. Amsden, and A. T. Hood. 1982. Modulation of granulomatous hypersensitivity. IV. Immunoglobulin and antibody production by vigorous and immunomodulated liver granulomas of Schistosoma mansoni-infected mice. J. Immunol. 128:1050-1053. [PubMed] [Google Scholar]
- 4.Boros, D. L., and J. R. Whitfield. 1998. Endogenous IL-10 regulates IFN-γ and IL-5 cytokine production and the granulomatous response in schistosomiasis mansoni-infected mice. Immunology 94:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boros, D. L., and J. R. Whitfield. 1999. Enhanced Th1 and dampened Th2 responses synergize to inhibit acute granulomatous and fibrotic responses in murine schistosomiasis mansoni. Infect. Immun. 67:1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boros, D. L., and K. S. Warren. 1970. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J. Exp. Med. 132:488-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boros, D. L., R. P. Pelley, and K. S. Warren. 1975. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J. Immunol. 114:1437-1441. [PubMed] [Google Scholar]
- 8.Cheever, A. W., J. E. Byram, S. Hieny, F. von Lichtenberg, M. N. Lunde, and A. Sher. 1985. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 7:387-398. [DOI] [PubMed] [Google Scholar]
- 9.Chensue, S. W., K. S. Warmington, J. Ruth, P. M. Lincoln, and S. L. Kunkel. 1994. Cross-regulatory role of interferon-gamma (IFN-γ), IL-4 and IL-10 in schistosome egg granuloma formation: in vivo regulation of Th activity and inflammation. Clin. Exp. Immunol. 98:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colley, D. G. 1975. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J. Immunol. 115:150-156. [PubMed] [Google Scholar]
- 11.el-Cheikh, M. C., A. C. Bonomo, M. I. D. Rossi, M. D. Pinho, and R. Borojevic. 1998. Experimental murine schistosomiasis mansoni: modulation of the B-1 lymphocyte distribution and phenotype expression. Immunobiology 199:51-62. [DOI] [PubMed] [Google Scholar]
- 12.Estaquier, J., M. Marguerite, F. Sahuc, N. Bessis, C. Auriault, and J. C. Ameisen. 1997. Interleukin 10-mediated T-cell apoptosis during the T helper type 2 cytokine response in murine Schistosoma mansoni parasite infection. Eur. Cytokine Netw. 8:153-160. [PubMed] [Google Scholar]
- 13.Ferru, I., O. Roye, M. Delacre, C. Auriault, and I. Wolowczuk. 1998. Infection of B-cell-deficient mice by the parasite Schistosoma mansoni: demonstration of the participation of B cells in granuloma modulation. Scand. J. Immunol. 48:233-240. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, E., D. Camus, F. Santoro, and A. Capron. 1981. Schistosoma mansoni: autoantibodies and polyclonal B cell activation in infected mice. Clin. Exp. Immunol. 46:89-97. [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Villanueva, P. O., X. X. Zheng, T. B. Strom, and M. J. Stadecker. 1996. Recombinant IL-10 and IL-10/Fc treatment down-regulate egg antigen-specific delayed hypersensitivity reactions and egg granuloma formation in schistosomiasis. J. Immunol. 156:3315-3320. [PubMed] [Google Scholar]
- 16.Gaubert, S., A. Viana Da Costa, C. A. Maurage, E. C. Santos-Lima, J. Fontaine, S. Lafitte, P. Minoprio, A. Capron, and J. M. Grzych. 1999. X-linked immunodeficiency affects the outcome of Schistosoma mansoni infection in the murine model. Parasite Immunol. 21:89-101. [DOI] [PubMed] [Google Scholar]
- 17.Goes, A. M., G. Gazzinelli, R. Rocha, N. Katz, and B. L. Doughty. 1991. Granulomatous hypersensitivity to Schistosoma mansoni egg antigens in human schistosomiasis. III. In vitro granuloma modulation induced by immune complexes. Am. J. Trop. Med. Hyg. 44:434-443. [DOI] [PubMed] [Google Scholar]
- 18.Hardy, R. R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595-621. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 20.Hood, A. T., and D. L. Boros. 1980. The effect of splenectomy on the pathophysiology and egg-specific immune response of Schistosoma mansoni-infected mice. Am. J. Trop. Med. Hyg. 29:586-591. [DOI] [PubMed] [Google Scholar]
- 21.Howard, M., and A. O'Garra. 1992. Biological properties of interleukin-10. Immunol. Today 13:198-200. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic, D., A. W. Cheever, M. C. Kullberg, T. A. Wynn, G. Yap, P. Caspar, F. A. Lewis, R. Clynes, J. V. Ravetch, and A. Sher. 1998. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J. Exp. Med. 187:619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 24.Lundy, S. K., S. P. Lerman, and D. L. Boros. 2001. Soluble egg antigen (SEA)-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL+ T and B cells during murine Schistosoma mansoni infection. Infect. Immun. 69:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, F., and J. F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195-201. [DOI] [PubMed] [Google Scholar]
- 26.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 27.Pearce, E. J., P. Caspar, J. M. Grzych, F. A. Lewis, and A. Sher. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth. Schistosoma mansoni. J. Exp. Med. 173:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragheb, S., R. C. Mathew, and D. L. Boros. 1987. Establishment and characterization of an antigen-specific T-cell line from liver granulomas of Schistosoma mansoni-infected mice. Infect. Immun. 55:2625-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rumbley, C. A., S. A. Zekavat, H. Sugaya, P. J. Perrin, M. A. Ramadan, and S. M. Phillips. 1998. The schistosome egg granuloma: characterization of lymphocyte migration, activation, and cytokine production. J. Immunol. 161:4129-4137. [PubMed] [Google Scholar]
- 30.Sher, A., D. Fiorentino, P. Caspar, E. Pearce, and T. Mosmann. 1991. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J. Immunol. 147:2713-2716. [PubMed] [Google Scholar]
- 31.Todt, J. C., J. R. Whitfield, S. R. Ivard, and D. L. Boros. 2000. Down-regulation of interleukin-12 and interleukin-12R expression/activity mediates the switch from Th1 to Th2 granuloma response during murine schistosomiasis mansoni. Scand. J. Immunol. 52:385-392. [DOI] [PubMed] [Google Scholar]
- 32.Van Engeland, M., L. J. W. Nieland, F. C. S. Ramaekers, B. Schutte, and C. P. Reutelingsperger. 1998. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1-9. [DOI] [PubMed] [Google Scholar]
- 33.Velupillai, P., and D. A. Harn. 1994. Oligosaccharide-specific induction of interleukin 10 production by B-220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc. Natl. Acad. Sci. USA 91:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velupillai, P., J. Sypek, and D. A. Harn. 1996. Interleukin 12 and -10 and gamma interferon regulate polyclonal and ligand-specific expansion of murine B-1 cells. Infect. Immun. 64:4557-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velupillai, P., W. E. Secor, A. M. Horauf, and D. A. Harn. 1997. B-1 cell (CD5+/B-220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J. Immunol. 158:338-344. [PubMed] [Google Scholar]
- 36.Wynn, T. A., A. W. Cheever, M. E. Williams, S. Hieny, P. Caspar, R. Kuhn, W. Muller, and A. Sher. 1998. IL-10 regulates liver pathology in acute murine schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 160:4473-4480. [PubMed] [Google Scholar]
- 37.Wynn, T. A., and A. W. Cheever. 1995. Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 7:505-511. [DOI] [PubMed] [Google Scholar]
- 38.Wynn, T. A., I. Eltoum, I. P. Oswald, A. W. Cheever, and A. Sher. 1994. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J. Exp. Med. 179:1551-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn, T. A., R. Morawetz, T. Scharton-Kersten, S. Hieny, H. C. Morse III, R. Kuhn, W. Muller, A. W. Cheever, and A. Sher. 1997. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J. Immunol. 159:5014-5023. [PubMed] [Google Scholar]
- 40.Youinou, P., J. O. Pers, C. Jamin, and P. M. Lydyard. 2000. CD5-positive B cells at the crossroads of B cell malignancy and nonorgan-specific autoimmunity. Pathol. Biol. 48:574-576. [PubMed] [Google Scholar]