Abstract
Nitric oxide (NO) is a toxic molecule of the immune system which contributes to the control of microbial pathogens. Additional functions of NO in innate and adaptive immunity have recently been described; these functions include the modulation of the cytokine response of lymphocytes and the regulation of immune cell apoptosis. In addition to direct microbicidal actions, NO has immunoregulatory effects relevant to the control of infections. In turn, infected macrophages and macrophage-regulating lymphocytes may undergo apoptosis during infection by Salmonella spp. In this work we investigated the ability of attenuated strains of Salmonella enterica serovar Enteritidis with different protective capacities to induce intestinal inducible nitric oxide synthase (iNOS) and apoptosis in Peyer's patches (PP) in mice. Results showed that the intestinal iNOS activity correlated with increased apoptosis in PP. Furthermore, the ability to induce intestinal NO production and apoptosis within the first few hours after immunization seemed to correlate with the protective capacity of mutant E/1/3 of S. enterica serovar Enteritidis. It was found that nonprotective mutant C/2/2, which was unable to induce intestinal NO production, also failed to induce apoptosis in PP. Moreover, aminoguanidine treatment at the time of immunization resulted in inhibition of the NO production and apoptosis induced by protective mutant E/1/3 and completely abolished protection against challenge. These results suggest that the induction of iNOS in the intestinal mucosa by attenuated mutant E/1/3 of S. enterica serovar Enteritidis at the time of immunization is necessary to generate a protective immune response.
The intestinal mucosa is an important route of entry for microbial pathogens. Following bacterial entry, intestinal epithelial cells rapidly initiate the innate immune response, upregulating the expression of proinflammatory molecules (22). Thus, within the first few hours after bacterial invasion, the intestinal mucosa produces mediators that have the potential to orchestrate the onset of an early inflammatory response (14, 16). Some of these mediators also have the potential to induce cellular apoptosis (9).
At the interface between the innate and acquired immune systems lies the high-output isoform of inducible nitric oxide synthase (iNOS). Indeed, much of the attention on the killing of intracellular pathogens has recently focused on this remarkable molecular machine. Enteroinvasive bacteria directly activate expression of iNOS and NO production in intestinal epithelial cells (25, 29). The role of the epithelial cell-derived NO in the pathogenesis of enteric infections with invasive bacteria is still a matter of discussion. However, the evidence suggests an antimicrobial role of NO in the intestine. NO and/or its redox products can be cytostatic for enteric pathogens such as Salmonella spp. and may decrease microbial entry into epithelial cells by increasing the protective barrier through the release of epithelial mucus (3) and the induction of intestinal fluid secretion (20, 28).
In addition to direct microbicidal actions, NO has immunoregulatory effects relevant to the control of infections (21, 24). Infected macrophages and macrophage-regulating lymphocytes may undergo apoptosis during infection by Salmonella spp. Whether such apoptosis is best understood as the host depriving the pathogen of the protected niche or the pathogen disarming the host (27) is not clear. The effect of NO on cell apoptosis is controversial; low levels of NO produced by activated macrophages delay apoptosis (17), whereas high levels of NO can cause either apoptotic or necrotic death of host cells by reacting with a wide range of molecular targets (5). Thus, the state of macrophage activation and NO production may affect not only the ability of macrophages to kill intracellular pathogens, but also the susceptibility of macrophages to apoptosis. Furthermore, apoptosis induction by bacterial infection of macrophages may contribute to the initiation of the immune response (30). In this work, we studied the effect of inhibition of NO on the early stages of the immune response induced by attenuated mutants of Salmonella enterica serovar Enteritidis.
Temperature-sensitive (ts) mutants isolated in our laboratory are able to replicate at 26 to 28°C but show impaired growth at 37°C (nonpermissive temperature). Some ts mutants cannot replicate at the nonpermissive temperature, whereas others are able to sustain limited replication at 37°C before ceasing growth. The latter are known as coasters, and upon inoculation into a host, their activity resembles that seen in the early stages of the natural infection (19). E/1/3 and C/2/2 are two isogenic mutants of the coaster phenotype. It was demonstrated that the ts mutant E/1/3 of S. enterica serovar Enteritidis induced excellent protection against lethal challenge in vaccinated mice and chickens (7, 8, 12). Attenuated mutant C/2/2 is highly safe but does not induce protective immunity in mice (13).
Innate immunity plays an important role not only in the control of Salmonella spp. infections but also in the modulation of the subsequent acquired immune responses. In this study, the early responses of the murine intestine to attenuated mutants of S. enterica serovar Enteritidis in vivo were compared. Protective mutant E/1/3 and nonprotective strain C/2/2 were used to study early induction of iNOS and cellular apoptosis in the murine intestine in vivo.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old BALB/c mice were purchased from the Instituto Nacional de Tecnolog|$$|Aa|fia Agropecuaria Castelar, Buenos Aires, and kept in our animal house throughout the experiments.
Bacteria.
The wild-type strain of S. enterica serovar Enteritidis and attenuated derivatives ts E/1/3 and C/2/2 were used in the experiments. We have reported earlier that ts mutant E/1/3 protects mice and chickens from challenge with the virulent S. enterica serovar Enteritidis strain, whereas the C/2/2 mutant is nonprotective. Groups of 20 mice were inoculated intragastrically with 109 CFU of E/1/3 or C/2/2 mutant or the wild-type strain of S. enterica serovar Enteritidis. Animals received two bacterial inoculations a week apart (day 1 and day 7). To test the effect of aminoguanidine (AG) on the safety of the ts mutants, 10 mice of each group were injected intraperitoneally with 1 mg of AG daily for 30 days, starting at day 0. Results are depicted in Table 1.
TABLE 1.
Safety of protective and nonprotective mutants of S. enterica serovar Enteritidisa
| Bacteria | No. of survivors/10 per group after oral infection of mice treated with AG at:
|
|
|---|---|---|
| 0 mg | 1 mg | |
| None | 10 | 10 |
| Wild type | 0b | 0b |
| E/1/3 (protective) | 10 | 10 |
| C/2/2 (nonprotective) | 10 | 10 |
Animals were immunized intragastrically with bacteria on days 1 and 7 and received 0 or 1 mg of AG daily for 30 days, starting at day 0.
All mice died within 5 days after bacterial inoculation.
Inoculations and samples.
Mice were inoculated intragastrically with 109 CFU of the bacterial suspension per animal. Animals were sacrificed 6 h after inoculation, and the intestines were removed immediately. For NOS activity determination, approximately 100 mg of small intestine containing at least one Peyer's patch (PP) was removed and placed immediately in Kreb's solution. Tissues for hematoxylin-eosin staining were fixed in formalin. Samples for enzyme-linked immunosorbent assay (ELISA) determinations were frozen immediately after removal.
Protection experiments.
Groups of 10 mice were immunized with two oral doses (a week apart) of 109 CFU of the attenuated strains of S. enterica serovar Enteritidis E/1/3 and C/2/2 per animal. Nonimmunized animals were included in the control group. Twenty-one days later, mice were challenged orally with 106 CFU of the virulent strain of S. enterica serovar Enteritidis per animal. To test the involvement of nitric oxide in the early stages of the immune response, a group of animals were injected with AG at the time of immunization with the protective mutant E/1/3. Mice received two intraperitoneal injections with 1 mg of AG 24 h and 30 min before each immunization.
NOS activity.
NOS activity was measured by the production of [14C]citrulline from [14C]arginine according to the procedures described by Bredt and Synder (4). Briefly, after 20 min of incubation in Kreb's Ringer bicarbonate solution (KRB), tissues were transferred to 500 μl of warmed KRB equilibrated with 5% CO2 in oxygen in the presence of [14C]arginine (0.5 μCi). Tissues were incubated for 20 min under carbogen (95% O2 and 5% CO2) at 37°C. Tissues were then homogenized in 1 ml of medium containing 20 nmol of HEPES (pH 7.4), 0.5 mmol of EDTA, 1 mmol of dithiothreitol, 1 mmol of leupeptin, and 0.2 mmol of phenylmethylsulfonyl fluoride per liter at 4°C. After centrifugation for 10 min at 2,000 × g, supernatants were transferred to 2-ml columns of Dowex AG-50 WX-8. [14C]citrulline was eluted with 3 ml of distilled water and quantified by liquid scintillation counting.
Apoptosis.
PP were fixed in 10% buffered neutral formalin embedded in paraffin and cut. Some sections were stained with hematoxylin and eosin. Apoptosis was detected by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL). Sections for TUNEL reactions were processed as described for the in situ cell death detection kit (Boehringer Mannheim Biochemicals). Briefly, tissue sections were heated at 60°C for 30 min, followed by washing in xylene and rehydration through a graded series of ethanol and redistilled water. Sections were treated with proteinase K (20 μg/ml in 10 mM Tris-HCl, pH 7.4) for 30 min at room temperature, washed, and overlaid with premixed TUNEL reaction mixture containing the components for the end labeling. The sections were labeled for 1 h at 37°C, washed three times with phosphate-buffered saline (PBS), mounted, and examined under light microscopy.
Percent apoptotic tissue.
Apoptotic cells were counted in hematoxylin-eosin-stained sections using the morphometric procedure described by Zhou et al. (31). Briefly, sections were examined under light microscopy. Cells were scored as apoptotic if they exhibited cellular shrinkage with concurrent cytoplasmic eosinophilia, nuclear pyknosis, and fragmentation with associated apoptotic bodies. Digitized images of randomly selected fields were obtained and characterized morphometrically in terms of percent apoptotic lymphoid tissue. Images were captured, saved as PICT files, and analyzed using a double-density 25/125-point grid. The tissue beneath each grid point was categorized as normal lymphoid tissue, apoptotic lymphoid tissue, or other (extracellular space, blood vessels, etc.). The number of points in each category was totaled individually for every animal. The percentage of apoptotic tissue for each animal was calculated from the area of lymphoid tissue: % area(apoptotic) = (area(apoptotic)/[area(normal) + area(apoptotic)]) × 100.
Cell death detection by ELISA.
DNA fragmentation was assessed by using a sandwich ELISA (cell death detection ELISA kit; Boehringer Mannheim, Indianapolis, Ind.). Briefly, single-cell suspensions were prepared by smashing PP with a glass plunger against a fine stainless steel wire net submerged in ice-cold PBS. Approximately 5 × 104 cells were lysed, and the cytosolic oligonucleosomes were quantitated using a biotin-coupled mouse monoclonal antihistone antibody as the capturing antibody, peroxidase-conjugated mouse monoclonal anti-DNA antibody as the detecting antibody, and ABTS (2,2′-azino-di[3-ethylbenzthiazoline]-sulfonate) as the developing reagent. The relative increase in nucleosomes in the cytoplasm was expressed as an enrichment factor, calculated as the ratio of specific absorbance in lysates from treated mice compared with that in control animals, as described by the manufacturer.
Statistics.
Student's t test was used to compare mean values. Medians were compared using the Wilcoxon rank sum test.
RESULTS
AG does not affect the safety of attenuated mutants E/1/3 and C/2/2 of S. enterica serovar Enteritidis.
The main features of the attenuated strains of S. enterica serovar Enteritidis are depicted in Table 1. Both mutants are highly safe; mice inoculated with attenuated strain E/1/3 or C/2/2 showed no signs of disease. In fact, all animals survived the oral administration of doses as high as 109 CFU. The 50% lethal dose (LD50) of both mutants is >1010 CFU. On the other hand, animals receiving 109 CFU of the wild-type strain of S. enterica serovar Enteritidis died within 5 days after inoculation. The LD50 of the wild-type strain of S. enterica serovar Enteritidis is 104 CFU. Daily treatment with AG did not modify the safety of the mutants.
Wild-type strain and protective mutant of S. enterica serovar Enteritidis induce NOS activity in murine intestine.
The production of [14C]citrulline from [14C]arginine was investigated in the murine intestine 6 h after oral administration of S. enterica serovar Enteritidis. Differences in NOS activity was found between animals inoculated with protective mutant E/1/3 and nonprotective mutant C/2/2 (Table 2). No differences in the enzymatic activity were found between animals immunized with nonprotective mutant C/2/2 and untreated mice. On the other hand, NOS activity induced by both wild-type and the attenuated mutant E/1/3 of S. enterica serovar Enteritidis was significantly higher (P < 0.05) than that found in control mice. It is likely that this increase was due to the expression of inducible NOS (iNOS), since enzymatic activities in immunized mice treated with AG and inoculated with the wild-type strain and the protective mutant E/1/3 were similar to those found in control mice (Table 2).
TABLE 2.
Intestinal NOS induced by protective and nonprotective mutants
| Bacteria | Mean intestinal NOS activity (pmol/g) ± SE (n = 6) in mice treated with AG at:
|
|
|---|---|---|
| 0 mg | 1 mg | |
| None | 259 ± 56 | 264 ± 60 |
| Wild type | 515 ± 70a | 313 ± 62 |
| E/1/3 (protective) | 599 ± 44a | 300 ± 45 |
| C/2/2 (nonprotective) | 322 ± 65 | 247 ± 55 |
Significantly different (P < 0.05) from value for nontreated control group, as calculated by the Student t test.
Increased apoptosis in PP of mice immunized with the protective mutant E/1/3.
The ability of attenuated strains of S. enterica serovar Enteritidis to induce apoptosis in murine PP was evaluated using different assays.
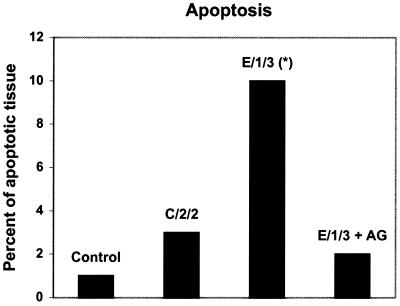
Apoptotic cells were counted in hematoxylin-eosin-stained sections using a morphometric procedure described in Materials and Methods (Fig. 1). Results showed that the percentage of apoptotic lymphoid tissue in PP of animals immunized with protective mutant E/1/3 (median, 10%; range, 7 to 12%) was significantly higher (P < 0.01) than that found in control mice (median, 1%; range, 0.5 to 2%). Animals immunized with mutant E/1/3 and treated with AG showed a percentage of apoptotic tissue similar to that of the control group.
FIG. 1.
Morphometry of apoptotic cells in PP of mice immunized with protective and nonprotective mutants of S. enterica serovar Enteritidis. Mice were inoculated intragastrically with 109 CFU/animal of the bacterial suspension. Animals were sacrificed 6 h after inoculation, and PP were removed aseptically. Apoptotic cells were counted in hematoxylin-eosin-stained sections using the morphometric procedures described in Materials and Methods. Mice treated with AG received two intraperitoneal inoculations (1 mg), 24 h and 30 min before immunization. Data are medians (n = 5). Asterisk indicates values significantly different (P < 0.01) from those for control mice. Results are representative of three separate experiments.
Histopathologic changes induced by S. enterica serovar Enteritidis in PP were evident 6 h after oral administration. Scattered small focal aggregates of leukocytes undergoing apoptotic death were found in the PP of mice inoculated with nonprotective mutant C/2/2 (data not shown). Affected foci contained shrunken, apoptotic cells with nuclear chromatin condensation, margination, or fragmentation that were dispersed among apoptotic bodies. Similar but more extensive cellular apoptosis was evident in the PP of mice inoculated with protective mutant E/1/3 and the wild-type strain of S. enterica serovar Enteritidis (data not shown).
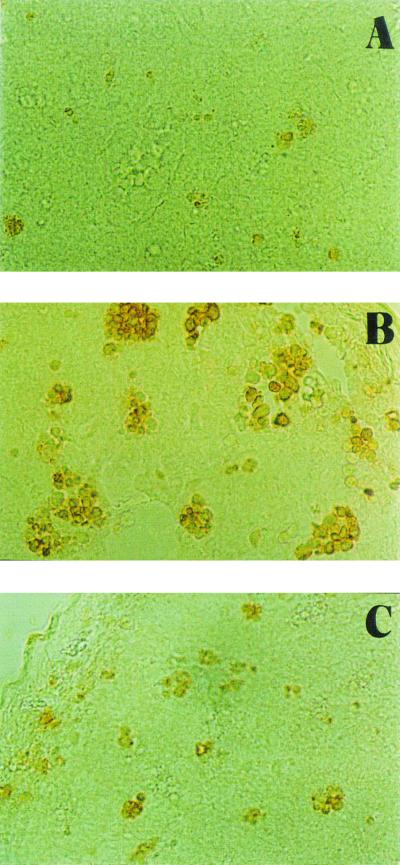
TUNEL reactions were performed in PP sections (Fig. 2). There was a dramatic increase in the number and size of TUNEL-positive foci in the PP of mice immunized with protective mutant E/1/3 compared with those observed in mice receiving the nonprotective mutant C/2/2. The number and size of TUNEL-positive foci in PP from animals immunized with E/1/3 and treated with AG were not different from those seen in control mice (data not shown).
FIG. 2.
TUNEL-positive reactions were observed in PP from mice immunized with attenuated strains of S. enterica serovar Enteritidis as well as in control mice, although there were differences in localization. In control animals (A), apoptotic cells appeared evenly distributed, whereas mice immunized with S. enterica serovar Enteritidis mutants showed apoptosis in germinal centers. Affected foci in PP of mice inoculated with protective mutant E/1/3 (B) were increased in number and size compared with those seen in animals receiving the nonprotective mutant C/2/2 (C).
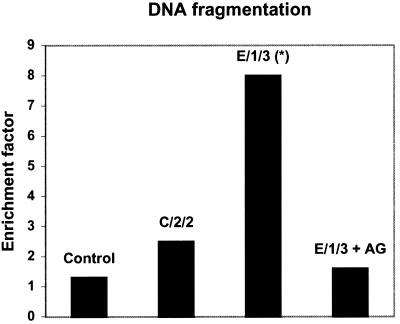
Similar results were obtained when DNA fragmentation was assessed in PP of animals immunized with the attenuated strains of S. enterica serovar Enteritidis (Fig. 3). Again, statistically significant differences (P < 0.05) were found in mice immunized with the protective mutant E/1/3 compared with the control group. DNA fragmentation in the PP of mice inoculated with nonprotective mutant C/2/2 was no different from that seen in the control animals. AG inhibited the increase in DNA fragmentation induced by protective mutant E/1/3.
FIG. 3.
Apoptosis in PP of mice immunized with protective and nonprotective mutants of S. enterica serovar Enteritidis. Apoptosis was assessed using a cell death detection ELISA. The y axis represents DNA fragmentation, as measured by the enrichment factor (see Materials and Methods). Mice treated with AG received two intraperitoneal inoculations (1 mg), 24 h and 30 min before immunization. Data are median enrichment factors for six animals. Asterisk indicates significantly different values (P < 0.05) versus control mice. Results are representative of three separate experiments.
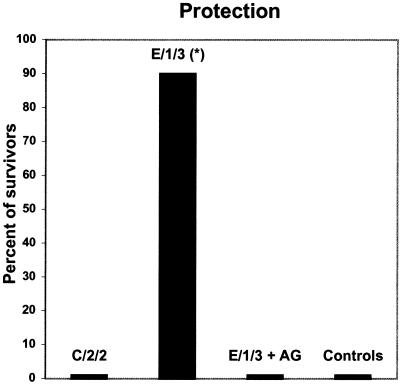
AG treatment at the time of immunization inhibits the protection induced by attenuated mutant E/1/3.
The protective capacity of mutant E/1/3 was investigated in mice treated with AG. The inhibition of NO production at the time of immunization completely abolished protection induced by mutant E/1/3; the survival rate of AG-treated mice (0 of 10) was similar to that of control mice (0 of 10) (Fig. 4). Animals from these groups died within 5 days after challenge. On the other hand, 30 days after challenge, the survival rate of animals immunized with E/1/3 was 9 of 10 (90% protection). Differences between the survival rates of mice immunized with E/1/3 and AG-treated or control mice were significant (P < 0.01).
FIG. 4.
Effect of AG on the protection induced by attenuated mutant E/1/3 of S. enterica serovar Enteritidis. Mice were immunized with two oral doses of 109 CFU of the attenuated mutants E/1/3 and C/2/2 of S. enterica serovar Enteritidis. Twenty-one days later, mice were challenged orally with 106 CFU/animal of the virulent strain of S. enterica serovar Enteritidis. Mice treated with AG received two intraperitoneal inoculations (1 mg), 24 h and 30 min before each immunization with the protective mutant E/1/3.
DISCUSSION
During the course of infection with pathogens such as Salmonella spp., intestinal epithelial cells upregulate the expression of certain host genes (11, 16). This epithelial cell inflammatory program induces the expression of proinflammatory molecules such as NO. Although the NO pathway probably evolved to protect the host from infection, its suppressive effects on lymphocyte proliferation and damage to other normal host cells confer on NO the same destructive/protective duality. Therefore, NO toxicity is not a constant value, and NO may confer cell protection as well. In part, this is understood by transcription and translation of protective proteins, such as cyclooxygenase-2. Alternatively, protection may result as a consequence of diffusion-controlled NO-O2− (superoxide) interaction that redirects the apoptosis-initiating activity of NO towards protection.
In this regard, NO is endowed with the unique ability to initiate and to block apoptosis, depending on multiple variables (6). Proapoptotic effects (e.g., in macrophages or T lymphocytes) were observed with high concentrations of exogenous NO. NO might promote apoptotic events by upregulating the cell surface receptor Fas or its ligand FasL, although it can also induce apoptosis without measurable modulation of Fas or FasL (1). On the other hand, an antiapoptotic function of endogenous NO generated by iNOS has been observed in B-cell lines, macrophages, and T lymphocytes (2). In mouse macrophages, nontoxic concentrations of NO lead to the activation of the transcription factors NF-κB and AP-1 and the subsequent expression of cyclooxygenase-2, which protect macrophages against apoptosis (26).
Wild-type S. enterica serovar Enteritidis and its derivative, the protective mutant E/1/3, induced intestinal iNOS-derived NO within the first few hours after inoculation. Results showed that the intestinal iNOS activity induced by S. enterica serovar Enteritidis correlated with increased apoptosis in PP. Furthermore, the ability to induce intestinal NO production and apoptosis in PP soon after immunization seemed to correlate with the protective capacity of mutant E/1/3. It was found that nonprotective mutant C/2/2, which was unable to induce intestinal NO production, also failed to induce apoptosis in PP. Moreover, AG treatment at the time of immunization resulted in the inhibition of NO production and apoptosis induced by protective mutant E/1/3, and completely abolished protection against challenge.
To date, there is no doubt that reactive nitrogen intermediates are integral parts of signaling pathways of immunological importance, regulating cytokine responses and cell survival and contributing to tissue damage, as seen in autoimmune diseases. Recent studies provided support for the importance of iNOS in immunoregulation and suggested that chronic stimulation of iNOS in vivo may have profound effects on subsequent immune responses (15). The mechanisms through which NO could regulate the initiation of a protective immune response were not investigated, although the induction of apoptosis in PP cannot be ruled out. In recent experiments, Yrlid and Wick showed that apoptosis induced during bacterial infection is not a quiescent death that allows the bacteria to escape recognition by the immune system, but rather may contribute to an antimicrobial immune response (30). The authors demonstrated that bacterially induced apoptosis of infected cells results in presentation of a bacterium-encoded antigen on both major histocompatibility complexes I and II by bystander dendritic cells (30).
Attenuated strains of Salmonella spp. can be used as vehicles for delivery and expression of vaccine antigens. Murine models of infection have been used extensively to evaluate both the pathogenicity of and immune responses against Salmonella spp. Early studies performed by Eisenstein and coworkers tested a panel of attenuated strains of S. enterica serovar Enteritidis and S. enterica serovar Dublin and found that in many cases NO induction correlated with immunosuppression and protection. In one case, the PhoP mutant, protection occurred without NO induction (10, 18, 23).
It was also reported that treatment with AG inhibits immunosuppression and potentiates Salmonella infection (18). Moreover, inhibition of NO resulted in 90% mortality in mice inoculated with highly attenuated mutant SL3235 (18). With a different mouse model of infection, we showed that the ability to induce intestinal NO correlated with the protective capacity of the attenuated mutants of S. enterica serovar Enteritidis. Further, treatment with AG (1 mg daily) did not modified the safety of the E/1/3 and C/2/2 mutants. Note that PhoP mutants were not studied in the present work, and, in addition, important differences exist between the murine model used by the Eisenstein group and ours (route of immunization, AG treatment, method for measuring NO, etc.). Nevertheless, it is clear from both animal models that NO actively participates in the initiation of the immune response induced by Salmonella spp. Our results suggest that NO is involved in the very early phase of the immune response, since inhibition of iNOS at the time of immunization inhibited protection.
The role of innate immune responses in promoting specific immune responses is an interesting area for research that may define important principles of bacterial interactions with immune cells. Here we present evidence that induction of NOS in the intestinal mucosa by attenuated mutant E/1/3 of S. enterica serovar Enteritidis is necessary to generate a protective immune response.
Acknowledgments
The excellent technical assistance of Mar|$$|Aa|fia Isabel Bernal is gratefully acknowledged.
This work was supported in part by Ministerio de Salud, Subsecretaría de Investigación y Tecnología, “Beca Carrillo-Oñativia,” and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Editor: E. I. Tuomanen
REFERENCES
- 1.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan, C. 2000. The function of nitric oxide in the immune system, p. 443-493. In M. B. Heidelberg (ed.), Handbook of experimental pharmacology: nitric oxide. Springer, Berlin, Germany.
- 3.Branka, J. E., G. Vallette, A. Jarry, and C. L. Laboisse. 1997. Stimulation of mucin exocytosis from human epithelial cells by nitric oxide: evidence for a cGMP-dependent and a cGMP-independent pathway. Biochem. J. 323:521-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredt, D. S., and S. H. Snyder. 1989. Nitric oxide mediates glutamate-linked enhancement of cyclic GMP levels in the cerebellum. Proc. Natl. Acad. Sci. USA 86:9030-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brune, B., A. Von Knethen, and K. B. Sandau. 1998. Nitric oxide and its role in apoptosis. Eur. J. Pharmacol. 26:261-272. [DOI] [PubMed] [Google Scholar]
- 6.Brune, B., A. Von Knethen, and K. B. Sandau. 1999. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 6:969-975. [DOI] [PubMed] [Google Scholar]
- 7.Cerquetti, M. C., and M. M. Gherardi. 2000. Vaccination of chickens with a temperature-sensitive mutant of Salmonella enteritidis. Vaccine 6:1140-1145. [DOI] [PubMed] [Google Scholar]
- 8.Cerquetti, M. C., and M. M. Gherardi. 2000. Orally administered attenuated Salmonella enteritidis reduces cecal carriage of virulent challenge organisms. Vet. Microbiol. 76:185-192. [DOI] [PubMed] [Google Scholar]
- 9.Chung, H. T., H. O. Pae, B. M. Choi, T. R. Billiar, and Y. M. Kim. 2001. Nitric oxide as a bioregulator of apoptosis. Biochem. Biophys. Res. Commun. 282:1075-1079. [DOI] [PubMed] [Google Scholar]
- 10.Eisenstein, T. K., J. J. Jr Meissler, S. I. Miller, and B. A. Stocker. 1998. Immunosuppression and nitric oxide production induced by parental live Salmonella vaccines do no correlate with protective capacity: a phoP::Tn10 mutant does not suppress but does protect. Vaccine 16:24-32. [DOI] [PubMed] [Google Scholar]
- 11.Elewaut, D., J. A. DiDonato, J. K. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 163:1457-1466. [PubMed] [Google Scholar]
- 12.Gherardi, M. M., V. E. García, D. O. Sordelli, and M. C. Cerquetti. 1993. Protective capacity of a temperature-sensitive mutant of Salmonella enteritidis after oral and intragastric inoculation in a murine model. Vaccine 11:19-24. [DOI] [PubMed] [Google Scholar]
- 13.Gherardi, M. M., D. O. Sordelli, and M. C. Cerquetti. 1995. Relevance of local and systemic humoral immunity to efficacy of immunization with live-attenuated Salmonella enteritidis. Vaccine Res. 4:19-27. [Google Scholar]
- 14.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn, D. A., D. C. Archer, D. P. Gold, and C. J. Kelly. 2001. Adjuvant immunotherapy is dependent on inducible nitric oxide synthase. J. Exp. Med. 193:1261-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, Y. M., R. V. Talanian, J. Li, and T. R. Billiar. 1998. Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme). J. Immunol. 161:4122-4128. [PubMed] [Google Scholar]
- 18.MacFarlane, A. S., M. G. Schwacha, and T. K. Eisenstein. 1999. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophages and polymorphonuclear leukocytes influx into the spleen. Infect. Immun. 67:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris Hooke, A. 1994. Temperature-sensitive mutants of bacterial pathogens: isolation and use to determine host clearance and in vivo replication rates. Methods Enzymol. 235:448-457. [DOI] [PubMed] [Google Scholar]
- 20.Mourad, F. H., L. J. O'Donnell, E. A. Andre, C. P. Bearcroft, R. A. Owen, M. L. Clark, and M. J. Farthing. 1996. l-Arginine, nitric oxide, and intestinal secretion: studies in rat jejunum in vivo. Gut 39:539-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer, C. A., and S. I. Miller. 2001. Molecular pathogenesis of Salmonellae, p. 265-333. In E. A. Groisman (ed.), Principles of bacterial pathogenesis, Academic Press, New York, N.Y.
- 23.Schwacha, M. G., J. J. Meissler, Jr., and T. K. Eisenstein. 1998. Salmonella typhimurium infection in mice induces nitric oxide-mediated immunosuppression through a natural killer cell-dependent pathway. Infect. Immun. 66:5862-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiloh, M. U., and C. F. Nathan. 2000. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 3:35-42. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez-Torres, A., and F. C. Fang. 2001. Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol. 9:29-33. [DOI] [PubMed] [Google Scholar]
- 26.von Knethen, A., D. Callsen, and B. Brune. 1999. NFκB and AP-1 activation by nitric oxide attenuated apoptosis cell death in RAW 264.7 macrophages. Mol. Biol. Cell 10:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, K. T., A. B. Vaandrager, J. De Vente, M. W. Musch, H. R. De Jonge, and E. B. Chang. 1996. Production and localization of cGMP and PGE2 in nitroprusside-stimulated rat colonic ion transport. Am. J. Physiol. 270:C832-C840. [DOI] [PubMed]
- 29.Witthoft, T., L. Eckmann, J. M. Kim, and M. F. Kagnoff. 1998. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. 275:G564-G571. [DOI] [PubMed]
- 30.Yrlid, U., and M. J. Wick. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191:613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, H. R., J. R. Harkema, J. A. Hotchkiss, D. Yang, R. A. Roth, and J. J. Pestka. 1999. Lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol) synergistically induce apoptosis in murine lymphoid organs. Toxicol. Sci. 53:253-263. [DOI] [PubMed] [Google Scholar]