Abstract
Bordetella dermonecrotic toxin (DNT) is known to activate the small GTPase Rho through deamidation or polyamination. In this study, we examined whether Rac and Cdc42, the two other members of the Rho family, serve as intracellular targets for the toxin. Immunoprecipitation and immunoblot assays revealed that DNT deamidated or polyaminated intracellular Rac and Cdc42. After the modifications, both Rac and Cdc42 lost their GTP-hydrolyzing, but not GTP-binding, activities. The interactions of the modified Rac and Cdc42 with their respective effectors were strictly dependent on GTP. MC3T3-E1 cells treated with DNT at high concentrations demonstrated extensive formations of lamellipodia and filopodia, which indicate the intracellular activation of Rac and Cdc42, respectively.
Bordetella dermonecrotic toxin (DNT), one of the virulence factors produced by bacteria of the Bordetella species, is known to cause morphological changes accompanied by anomalous formations of actin cytoskeletons in several cultured cells (3, 6). It has been demonstrated that DNT is essentially a transglutaminase catalyzing deamidation or polyamination of the Rho family GTPases, which are known to function as molecular switches for various cellular processes, including reorganization of actin cytoskeletal systems, by shuttling between inactive GDP-bound and active GTP-bound forms (2, 10, 15). The GTPases in the GDP-bound form exchange GDP for GTP upon various stimulations, transduce signals downstream by interacting with effector proteins, and thereafter revert to the GDP-bound inactive form by hydrolyzing the bound GTP. The Rho family GTPases include Rho, Rac, and Cdc42, which are known to conduct the reorganization of actin cytoskeletal systems such as actin stress fibers and focal adhesions, lamellipodia, and filopodia, respectively (13, 16, 18-20, 22). The GTP-hydrolyzing activity of Rho was reduced after treatment with DNT, which made Rho constitutively active (2). Moreover, the polyaminated Rho, even in the GDP-bound form, interacted with a downstream effector ROCK (15). We consider that these functional alterations of Rho lead to the marked formation of actin stress fibers and focal adhesion in DNT-treated cells (2, 6).
In contrast to the good understanding of the effect of DNT on Rho mentioned above, it has not been discussed to date whether the toxin modifies intracellular Rac and Cdc42 and alters their functions, although it has already been reported that they can serve as substrates for the toxin in vitro (2, 15). In this study, we examined whether DNT modifies these GTPases in vivo as well as in vitro and alters their function after the modifications and whether one can observe the formation of lamellipodia and filopodia in DNT-treated cells, which indicate activation of Rac and Cdc42, respectively.
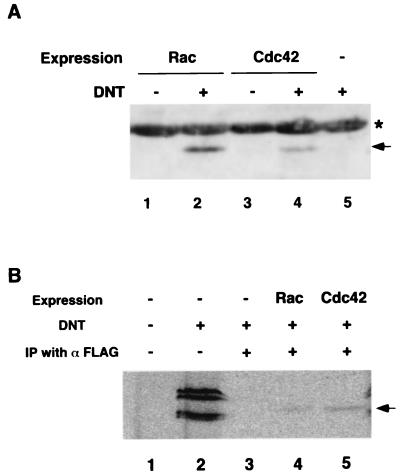
First, we examined whether DNT modifies FLAG-tagged Rac and Cdc42 exogenously expressed in C3H10T1/2 cells. The expression vectors encoding FLAG-tagged Rac1 and FLAG-tagged Cdc42, pMEPyoriF-Rac and pMEPyoriF-42, respectively, were constructed by replacement of the Rho gene in pMEPyoriF-RhoA (2) by the Rac1 or Cdc42 gene. C3H10T1/2 cells transfected with pMEPyoriF-Rac or pMEPyoriF-42 were incubated for 2 days and then treated with DNT purified by a method described previously (4). The cells were washed and lysed with lysis buffer (10 mM Tris-HCl, pH 7.8, containing 1% NP-40, 0.15 M NaCl, and 1 mM EDTA) at 4°C for 1 h. The lysates were incubated at 4°C for 2 h with anti-FLAG M2-agarose gel (Sigma) prewashed with 5% skim milk and suspended in the lysis buffer. The agarose gel was washed with the lysis buffer and boiled in a twofold concentrated sodium dodecyl sulfate (SDS) sample buffer, and the supernatant after centrifugation was subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Proteins in the gel were electrotransferred onto a polyvinylidene difluoride membrane. The deamidated GTPases on the membrane were blotted by a CDP-Star system (Tropix, Bedford, Mass.) with rabbit anti-63E antibody, which specifically recognizes the deamidated GTPases of the Rho family (2), and alkaline phosphatase-labeled anti-rabbit immunoglobulin G. As shown in Fig. 1A, FLAG-tagged Rac and Cdc42 were found to be deamidated in response to the DNT treatment of the cells. To elucidate whether the GTPases intracellularly undergo polyamination as well as deamidation, we loaded C3H10T1/2 cells with [14C]putrescine to metabolically label intracellular polyamines and attempted to detect the polyamination by autoradiography after SDS-PAGE as described before (15). From lysates of cells preloaded with [14C]putrescine and treated with DNT, several 14C-polyaminated proteins were detected (Fig. 1B, lanes 1 and 2). These results indicate that DNT polyaminates some cellular proteins besides Rho. Next, we introduced pMEPyoriF-Rac or pMEPyoriF-42 into the cells 2 days before the preloading with [14C]putrescine and carried out the immunoprecipitation of FLAG-Rac and FLAG-Cdc42 after treatment of the cells with DNT for 24 h. The FLAG-Rac1 and the FLAG-Cdc42 immunoprecipitated from the DNT-treated cells were found to be polyaminated, although the polyaminated FLAG-GTPases were not efficiently recovered, as reported before (Fig. 1B) (15). These results indicate that Rac1 and Cdc42 as well as Rho can serve as substrates for DNT in vivo.
FIG. 1.
In vivo deamidation (A) and polyamination (B) of Rac and Cdc42 by DNT. (A) C3H10T1/2 cells expressing FLAG-tagged Rac1 or Cdc42 were treated with DNT at 5 ng/ml for 20 h and subjected to immunoprecipitation with anti-FLAG antibody. The precipitates were subjected to SDS-PAGE followed by immunoblot analysis with anti-63E antibody. The arrows and asterisk indicate the deamidated GTPases and nonspecifically reactive bands, respectively. (B) C3H10T1/2 cells expressing the FLAG-GTPases were preloaded with [14C]putrescine and treated with DNT at 10 ng/ml for 24 h. The cell lysates or the immunoprecipitated (IP) FLAG-GTPases were subjected to SDS-PAGE followed by autoradiography. Lane 2 shows that several kinds of endogenous proteins were 14C-polyaminated in the DNT-treated cells. The arrow indicates 14C-polyaminated FLAG-Rac1 and FLAG-Cdc42.
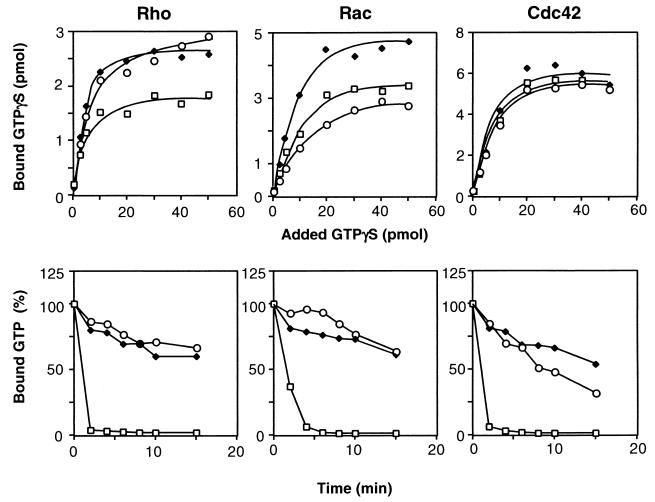
It was previously reported that the modification of Rho by DNT resulted in a reduction of the GTP-hydrolyzing activity but not GTP-binding activity, which makes Rho constitutively active (2, 15). Therefore, we examined whether this is also the case for the modified Rac1 and Cdc42. The γ-35S-labeled GTP-binding and [γ-32P]GTP-hydrolyzing activities were determined by methods described previously (2, 21). Rac1 and Cdc42 were purified from lysates of Escherichia coli harboring pET3aRac and pET3aCdc42, respectively (2). The GTP-hydrolyzing activity was assayed in the presence of the catalytic fragment of p50 rho GTPase-activating protein (GAP) at a molar ratio of 1 to 25 of the GTPase. An expression vector for the catalytic domain of p50 rho GAP (amino acids 198 to 439) (12) was a gift from A. Hall, University College London, London, United Kingdom. The GTP-hydrolyzing activities of Rac1 and Cdc42 were obviously lowered by treatment with DNT, whereas their GTP-binding activities remained almost intact or were only slightly potentiated (Fig. 2). The GTP-hydrolyzing activity of Cdc42 was not markedly reduced by DNT treatment when compared with that of Rac or Rho. This is probably due to the lower sensitivity of Cdc42 to DNT, as demonstrated previously (2, 15), because Cdc42Glu61, the mutant equivalent to Cdc42 deamidated by DNT, showed no GTP-hydrolyzing activity (data not shown).
FIG. 2.
The GTP-binding (upper panels) and GTPase (lower panels) activities of the Rho GTPases treated with DNT. The recombinant GTPases (5 μM) were treated with DNT (50 nM) in the presence or absence of 250 μM spermidine and examined for γ-35S-labeled GTP binding and GTPase activity. Ordinates express the amounts of γ-35S-labeled GTP bound to the GTPases for 2 h (upper panels) and the percentage of [γ-32P]GTP remaining bound to the GTPases after the indicated periods of incubation (lower panels). Open squares, control; closed diamonds, polyaminated GTPase; open circles, deamidated GTPase. Three independent experiments were carried out, and representative data are shown.
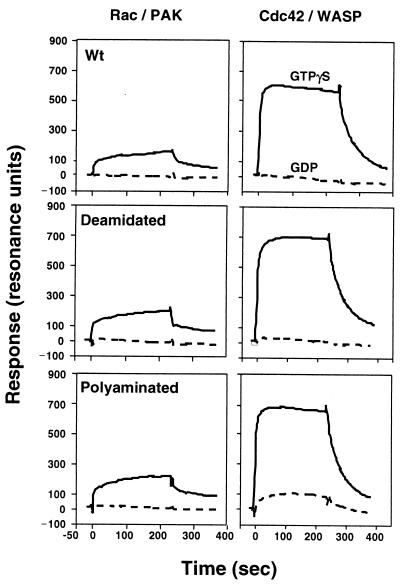
It was previously reported that the polyaminated Rho gained the ability to interact with the downstream effector ROCK independently of the bound guanine nucleotides and thereby could stimulate the formation of stress fibers in the DNT-treated cells (15). Therefore, we examined the interactions of Rac1 and Cdc42 modified by DNT with their downstream effectors, PAK and N-WASP. Glutathione S-transferase-tagged Rac/Cdc42 binding domains (GBD) of PAK and N-WASP were purified with a glutathione-Sepharose 4B (Amersham Pharmacia Biotech) column from lysates of E. coli harboring pGEXPAK-GBD and pGEXGBD(N-WASP), respectively, which were provided by C. Sasakawa, Institute of Medical Science, University of Tokyo, Tokyo, Japan. The interactions of Rac1 and Cdc42 with PAK-GBD and N-WASP-GBD were monitored with a BIAcore system (BIAcore, Uppsala, Sweden) as previously described (15). The deamidated or polyaminated Rac and Cdc42, like the intact forms, bound to PAK-GBD and N-WASP-GBD, respectively, in a GTP-dependent manner (Fig. 3). Although the polyaminated Cdc42 in the GDP form barely bound to N-WASP-GBD, the binding amount was only one-seventh that of the GTP form. Since PAK is a common effector for both Rac and Cdc42 (14, 22), we also examined the interaction between Cdc42 and PAK-GBD but obtained results similar to those with Rac and PAK-GBD (data not shown). Many potential effectors for Rho, Rac, and Cdc42 have been identified, although their functions are not fully understood (22). Each GTPase has multiple effectors that are common to several GTPases or specific to a particular one. Further work may be required to elucidate which effectors interact with the polyaminated GTPases independently of the bound guanine nucleotide, or whether such GTP-independent interaction may be specific to the polyaminated Rho and ROCK.
FIG. 3.
Binding of Rac to PAK (left panels) and Cdc42 to N-WASP (right panels) monitored by a BIAcore system.
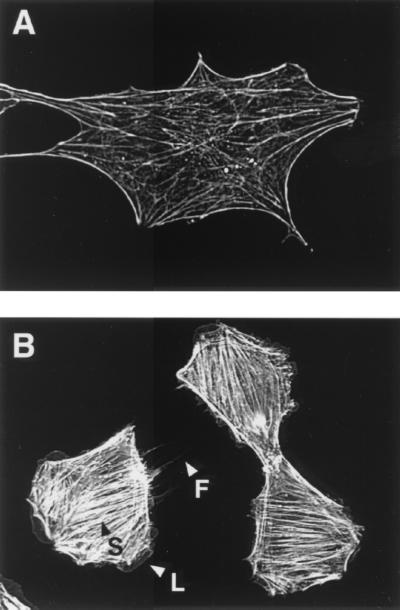
The results presented above imply that DNT renders intracellular Rac and Cdc42 constitutively active by impairing their GTP-hydrolyzing activity. Cdc42 is known to regulate the formation of filopodia in cells through the downstream N-WASP (13, 16, 20). Rac is also considered to positively regulate the formation of lamellipodia, although there has been debate as to the downstream effectors involved in this process (1, 9, 11, 17, 23, 24). However, the formation of lamellipodia and filopodia in DNT-treated cells had not been reported. In this study, we found that DNT markedly induced the formation of filopodia and lamellipodia as well as stress fibers when applied at even higher concentrations than those reported so far (Fig. 4). Of 31 cells treated with DNT, 29 and 22 cells showed apparent lamellipodia and filopodia, respectively, compared to only 1 and 2 cells of 30 control cells, which indicates that intracellular Rac and Cdc42, in addition to Rho, are actually activated by the treatment of cells with DNT. DNT at a concentration as high as 5 μg/ml was required for these actin cytoskeletons to appear, whereas 5 ng/ml was enough for stress fibers (2, 6). It remains unknown why much more DNT was necessary to produce distinct lamellipodia and filopodia than to produce stress fibers. It was reported that Rho and Rac were more preferentially modified by DNT in vitro than was Cdc42 (2, 15). However, this does not necessarily agree with the order of appearance of each actin cytoskeletal structure. The polyaminated Rho that interacts with ROCK in a GTP-independent manner might dominantly cause the stress fiber formation as previously reported (15). Alternatively, because stress fibers are a prominent component of the actin cytoskeleton, whereas filopodia and lamellipodia are temporal structures observed in moving cells, the former may be more easily observed than the latter in the DNT-treated cells. In either case, we need to address this issue by monitoring different indices for the activation states of the intracellular GTPases.
FIG. 4.
Formation of actin cytoskeletons in MC3T3-E1 cells treated with DNT. MC3T3-E1 cells in a subconfluent state were incubated in the presence (B) or absence (A) of DNT at 5 μg/ml for 24 h, and the actin fibers with rhodamine-phalloidin were observed under a fluorescence microscope. Arrowheads: S, stress fibers; L, lamellipodia; F, filopodia.
Taken together with previous findings (2, 15), the results presented here imply that DNT affects the cells by causing the overall activation of the Rho family GTPases, Rho, Rac, and Cdc42. In addition to inducing the cytoskeletal reorganization, DNT has been shown to possess a variety of biological activities, causing the promotion of DNA and protein syntheses and inhibition of osteoblastic differentiation (3, 5, 7, 8). To better understand the pathophysiology in cells influenced by DNT, one ought to elaborately correlate the toxic effects with each signaling pathway regulated by the Rho family GTPases. Such efforts may also reveal new roles of Rho GTPase signaling in various aspects of the cellular processes.
Acknowledgments
We thank C. Sasakawa for pGEXPAK-GBD and pGEXGBD(N-WASP) and A. Hall for the expression vector for p50 rho GAP.
This work was supported in part by a grant-in-aid for scientific research (B) (no. 13470059) from The Japan Society for the Promotion of Science.
Editor: J. T. Barbieri
REFERENCES
- 1.Honda, A., M. Nogami, T. Yokozeki, M. Yamazaki, H. Nakamura, H. Watanabe, K. Kawamoto, K. Nakayama, A. J. Morris, M. A. Frohman, and Y. Kanaho. 1999. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein Arf6 in membrane ruffle formation. Cell 99:521-532. [DOI] [PubMed] [Google Scholar]
- 2.Horiguchi, Y., N. Inoue, M. Masuda, T. Kashimoto, J. Katahira, N. Sugimoto, and M. Matsuda. 1997. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl. Acad. Sci. USA 94:11623-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horiguchi, Y., T. Nakai, and K. Kume. 1991. Effects of Bordetella bronchiseptica dermonecrotic toxin on the structure and function of osteoblastic clone MC3T3-E1 cells. Infect. Immun. 59:1112-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horiguchi, Y., T. Nakai, and K. Kume. 1990. Simplified procedure for purification of Bordetella bronchiseptica dermonecrotic toxin. FEMS Microbiol. Lett. 66:39-43. [DOI] [PubMed] [Google Scholar]
- 5.Horiguchi, Y., T. Okada, N. Sugimoto, Y. Morikawa, J. Katahira, and M. Matsuda. 1995. Effects of Bordetella bronchiseptica dermonecrotizing toxin on bone formation in calvaria of neonatal rats. FEMS Immunol. Med. Microbiol. 12:29-32. [DOI] [PubMed] [Google Scholar]
- 6.Horiguchi, Y., T. Senda, N. Sugimoto, J. Katahira, and M. Matsuda. 1995. Bordetella bronchiseptica dermonecrotizing toxin stimulates assembly of actin stress fibers and focal adhesions by modifying the small GTP-binding protein rho. J. Cell Sci. 108:3243-3251. [DOI] [PubMed] [Google Scholar]
- 7.Horiguchi, Y., N. Sugimoto, and M. Matsuda. 1994. Bordetella bronchiseptica dermonecrotizing toxin stimulates protein synthesis in an osteoblastic clone, MC3T3-E1 cells. FEMS Microbiol. Lett. 120:19-22. [DOI] [PubMed] [Google Scholar]
- 8.Horiguchi, Y., N. Sugimoto, and M. Matsuda. 1993. Stimulation of DNA synthesis in osteoblast-like MC3T3-E1 cells by Bordetella bronchiseptica dermonecrotic toxin. Infect. Immun. 61:3611-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joneson, T., M. McDonough, D. Bar-Sagi, and L. Van Aelst. 1996. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274:1374-1376. [DOI] [PubMed] [Google Scholar]
- 10.Kashimoto, T., J. Katahira, W. R. Cornejo, M. Masuda, A. Fukuoh, T. Matsuzawa, T. Ohnishi, and Y. Horiguchi. 1999. Identification of functional domains of Bordetella dermonecrotizing toxin. Infect. Immun. 67:3727-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 12.Lancaster, C. A., P. M. Taylor-Harris, A. J. Self, S. Brill, H. E. Erp, and A. Hall. 1994. Characterization of rhoGAP. A GTPase-activating protein for rho-related small GTPases. J. Biol. Chem. 269:1137-1142. [PubMed] [Google Scholar]
- 13.Machesky, L. M., R. D. Mullins, H. N. Higgs, D. A. Kaiser, L. Blanchoin, R. C. May, M. E. Hall, and T. D. Pollard. 1999. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA 96:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 15.Masuda, M., L. Betancourt, T. Matsuzawa, T. Kashimoto, T. Takao, Y. Shimonishi, and Y. Horiguchi. 2000. Activation of Rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 19:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miki, H., T. Sasaki, Y. Takai, and T. Takenawa. 1998. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391:93-96. [DOI] [PubMed] [Google Scholar]
- 17.Miki, H., H. Yamaguchi, S. Suetsugu, and T. Takenawa. 2000. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408:732-735. [DOI] [PubMed] [Google Scholar]
- 18.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389-399. [DOI] [PubMed] [Google Scholar]
- 19.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401-410. [DOI] [PubMed] [Google Scholar]
- 20.Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, and M. W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97:221-231. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 22.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 23.Takenawa, T., and H. Miki. 2001. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114:1801-1809. [DOI] [PubMed] [Google Scholar]
- 24.Van Aelst, L., T. Joneson, and D. Bar-Sagi. 1996. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J. 15:3778-3786. [PMC free article] [PubMed] [Google Scholar]