Abstract
During parasitic disease such as schistosomiasis, sex hormones have an important influence on the age- and gender-dependent level of infection. Since mammal glutathione S-transferase (GST) has the ability to bind hormones and particularly sexual steroids to influence their transport, metabolism, and physiological action, we have evaluated the capacity of testosterone to bind the 28-kDa GST of the Schistosoma haematobium parasite (Sh28GST). For the first time, we have demonstrated a specific binding of testosterone to parasite GST protein with high affinity (Kd = 2.57 × 10−7 M). In addition, we have assessed the effect of this binding on Sh28GST enzymatic activity, a mechanism closely associated with the reduction of Schistosoma fecundity. We showed that testosterone has the functional ability to inhibit the Sh28GST enzymatic activity in a dose-dependent manner, suggesting that this hormone could be directly involved in an antifecundity mechanism. This effect seemed to be related to the binding of testosterone to one peptide involved in the enzymatic site (i.e., amino acids 24 to 43). During human infection, binding of sexual hormones to Schistosoma Sh28GST could play a key role in parasite metabolism, especially the decrease of fecundity, and could be involved in the sex-dependent immune response to Sh28GST that we have previously observed in infected adults.
Sex hormones seem to have an influence on the level of parasitic infection. Indeed, gender-dependent patterns of prevalence and intensity of infection after puberty have been observed for several parasite species (5). It has been suggested that this effect seems to be associated with the regulatory roles of sex steroids on antiparasite immunity (2, 24). Generally, female hormones have an influence in increasing antibody response against specific antigen, which could explain the higher resistance of women against several parasitic infections (24, 25).
During human Schistosoma infection, a chronic infection affecting 200 million individuals around the world, sex hormones and particularly the high level of testosterone after puberty could be an important immune modulator leading to the decrease of susceptibility to infection with age (10, 26). In mice infected by Schistosoma mansoni, fewer adult worms develop in males than in similarly infected females (9). In addition, female mice treated with testosterone before infection presented a reduction in worm burden, whereas no difference in antischistosome immune response was detected between treated and untreated animals (20). These authors suggest thus that effects of testosterone on specific immunity are not adequate to explain the differences in parasite loads observed between the sexes.
Schistosomes could express a hormone receptor with homology to the testosterone receptor, which could explain the direct effect of testosterone on worm development (8). Interestingly, it has been demonstrated that testosterone treatment can affect not only the development of Nippostrongylus brasiliensis helminthic worms but also the fecundity and maturity of laid eggs (27).
Glutathione S-transferases (GSTs), a family of enzymes, are able to detoxify electrophilic compounds by catalyzing the formation of glutathione conjugates (12). Mammal GSTs are also involved in the intracellular transport of a variety of endogenous metabolites, drugs, and hormones by their abilities to bind these substances (16). Particularly, GSTs are glucocorticoid-binding proteins and, thereby, may influence transport, metabolism, and action of steroids (13). It has also been demonstrated that testosterone and progesterone have the ability to bind mammal GSTs with moderate (10−6 < Kd < 10−4 M) or high (Kd < 10−6 M) affinity, respectively (16). Thus, GSTs are also involved in the transport of sexual steroids and could play a key role in the physiological action of these hormones.
The Schistosoma 28-kDa GST (28GST) is an essential enzyme for the parasite life in its host and is now a vaccine candidate against schistosomiasis (6). Immunization with recombinant 28GST Schistosoma haematobium (Sh28GST) has been shown to reduce S. haematobium fecundity in experimentally infected monkeys (4). It is well established in rodents (30) and observed during human infection (11) that this antifecundity effect is associated with the inhibition of the 28GST enzymatic activity by recognation of specific antibodies. Particularly, antibodies directed against amino acids 24 to 43 or 190 to 211 involved in the enzymatic site of the 28GST inhibited the GST activity, reducing tissue egg number and egg viability (31).
Ultrastructural localization of antigen in adult worms showed that 28GST was identified in the tegument and the parenchyma, but also in the germinal organ of both male and female parasites (17, 21). These results indicate that 28GST expression seems to be closely associated with parasite metabolism but also with the genital tract, which could explain the relationship between the inhibition of enzymatic activity and an antifecundity effect.
The aim of our study was to demonstrate the potential binding of testosterone to Sh28GST and evaluate the functional ability of this binding on the enzymatic activity of the parasite protein.
MATERIALS AND METHODS
Antigen preparation and synthetic peptides.
The recombinant Sh28GST (rSh28GST) was produced in Saccharomyces cerevisiae strain TGY73.4 containing pTG8889 (provided by Transgene SA, Strasbourg, France) exactly as previously described (28). The purity of the rSh28GST (>98%) was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining and its concentration was measured by amino acid analysis. The enzymatic activity of recombinant Sh28GST was similar to native protein as it was previously reported (28).
Three different linear peptides derived from the primary structure of the Sh28GST, amino acids 24 to 43, 115 to 131, and 190 to 211 (peptides 24–43, 115–131, and 190–211, respectively), have been constructed by Biocytex Biotechnology (Marseille, France).
Competition binding assay.
To determine the specificity of binding between testosterone and Sh28GST, competition assay was performed using unlabeled testosterone (Sigma, St. Louis, Mo.) at increasing concentrations (10−7 to 10−3 M) and 125I-testosterone (Amersham, Les Ulis, France).
Nunc-Immuno Tubes (Nunc, Roskilde, Denmark) were coated with polyclonal antibody to Sh28GST (5 μg/ml) for 2.5 h at 37°C, and after washing in phosphate-buffered saline (PBS), saturation was performed using PBS containing 0.5% (wt/vol) gelatin (Merck, Darmstadt, Germany). Previously, Sh28GST (10 μg/ml) was incubated with testosterone-3-(O-carboxymethyl)oximino-(2-[125I]iodohistamine) (1 × 10−7 M; 0.2 μCi/ml) and with unlabeled testosterone (0 or 10−7 to 10−3 M) for 4 h at 37°C, and this mixture was then incubated in the coated tubes for 2.5 h at 37°C. After washing, the radioactivity was measured in a gamma counter, and triplicate determinations of bound 125I-testosterone were performed at each concentration of unlabeled testosterone.
Affinity determination using Biacore technology.
Surface plasmon resonance analysis was used to measure the association and dissociation rate constants for the binding of Sh28GST and peptides of Sh28GST (i.e., amino acids 24 to 43, 115 to 131, and 190 to 211) to testosterone, using Biacore (Biacore AB, Uppsala, Sweden). Biotinylated testosterone was immobilized onto an SA sensor chip coated with streptavidin; 20 μl of biotinylated testosterone (20-μg/ml ethanol-water mixture, 70:30 [vol/vol]) was injected at 10 μl/min, followed by a large excess of biotin (40 μl of a solution containing 40 μg of biotin per ml in HEPES-buffered saline [HBS]; Biacore AB) to complex all remaining streptavidin on the sensor chip. The amount of immobilized testosterone was in the range of 50 to 100 pg/mm2.
Various concentration of Sh28GST protein or Sh28GST peptides in HBS (10 to 100 μg/ml) were injected on immobilized testosterone, and the sensorgrams corresponding to the binding were registered: association time of 180 s, dissociation time of 1,000 s, and flow rate of 20 μl/min. The kinetics data, Req (plateau value of the sensorgrams in resonance units [RU]), and association and dissociation rate constants were obtained from these sensorgrams, using BIAevaluation 3.01 software (Biacore AB) and the so-called global method (simultaneous analysis of the sensorgrams corresponding to all Sh28GST concentrations). Apparent equilibrium constants (dissociation constants, Kd) were calculated by the software as Kd/Ka.
Testosterone binding to synthetic peptides by colorimetric assay.
The 96-well plates (Nunc) were coated at 4°C overnight with Sh28GST protein or synthetic peptides corresponding to amino acids 24 to 43, 115 to 131, or 190 to 211 of the Sh28GST (5 μg/ml in carbonate buffer, pH 9). After saturation in PBS containing 0.5% (wt/vol) gelatin (Merck) and three washes in PBS-Tween (Merck), peroxidase-labeled testosterone (200 μg/ml in PBS; Sigma) was then incubated 1.5 h at 37°C. Colorimetric development was carried out by using ABTS (2,2′-azino-bis [3-ethylbenzthiazoline 6-sulfonic acid] diammonium; Sigma) in 50 mM citrate buffer (pH 4) containing 0.003% H2O2, and optical density (OD) was measured at 405 nm. Results were expressed as ΔOD, representing the difference between OD values in the presence and in the absence of coated peptides. The OD value in the absence of peptides thus represented the background of binding of peroxidase-labeled testosterone on gelatin. Triplicate determinations were performed, and the results were expressed as the mean ± standard deviation (SD) of ΔOD reading values.
GST activity assay.
The GST-catalyzed reaction was performed using 1-chloro-2,4-dinitrobenzene (CNDB; Sigma) as a substrate according to Habig et al. (12). Sh28GST (6 μg/ml in 50 mM potassium phosphate, pH 6.5) was incubated with 20 μl of water-soluble testosterone (Sigma) at increasing concentrations (0 and 10−4 to 5 × 10−1 M) for 1 h at 37°C in Immulon 3 flat-bottomed plates (Dynatech Lab). After incubation, the enzymatic reaction was carried out in 200 μl of reaction buffer containing 5 mM glutathione (Sigma) and 0.36 mM CNDB in 50 mM potassium phosphate, pH 6.5. The absorbance was measured at 340 nm in a spectrophotometer (Labsystems Multiskan) every 15 s for up to 2 min. Triplicate determinations were performed at each concentration of testosterone, and the results are expressed as the mean ± SD of ΔOD/minute reading values. Student’s t test was used to compare the mean between each testosterone concentration and the value without hormones. Differences were considered significant at P < 0.05.
RESULTS
Specific binding between testosterone and Sh28GST protein.
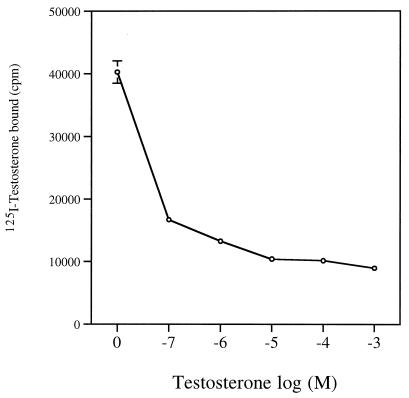
The binding of 125I-testosterone with Sh28GST was analyzed in the presence of increasing concentrations of unlabeled testosterone, and radioactivity values were measured (Fig. 1). High cpm value was observed in the absence of nonlabeled testosterone (0 M). The binding decreased in a dose-dependent manner in the presence of 10−7 to 10−3 M unlabeled testosterone, with a plateau from 10−5 M, resulting in a competition mechanism.
FIG. 1.
Specific binding of testosterone to Sh28GST. Radioactivity counts were determined after incubation of 125I-testosterone and Sh28GST protein (unlabeled testosterone at 0 M; see Materials and Methods). To determine the specificity of the binding, competition assay were performed using unlabeled testosterone incubated with increasing concentrations (10−7 to 10−3 M) and 125I-testosterone at one fixed concentration. Triplicate determinations were performed at each concentration of unlabeled testosterone, and results are expressed as the mean cpm ± SD.
Increasing concentrations of the unlabeled hormone were also able to inhibit the binding of [125I]testosterone, indicating that testosterone bound specifically to Sh28GST. This binding was confirmed using Biacore technology, indicating a high resonance unit (RU = 50), and its affinity was calculated in terms of Kd (Table 1). A strong Kd value (5.7 × 10−7 M) was recorded, indicating an important affinity of the testosterone-Sh28GST binding. In addition, a similar Kd value was observed with different concentrations of Sh28GST (data not shown)
TABLE 1.
Biacore study of the binding of Sh28GST protein and peptides of Sh28GST to immobilized testosteronea
| Protein | Plateau value (RU) | Ka (1/Ms) | Kd (1/s) | Kd (M) |
|---|---|---|---|---|
| Sh28GST | 50 | 3.28 × 103 | 1.87 × 10−3 | 5.7 × 10−7 |
| Peptide 24–43 | 81 | 44 | 3.25 × 10−4 | 7.4 × 10−6 |
| Peptide 115–131 | 5 | ND | ND | ND |
| Peptide 190–211 | 14 | ND | ND | ND |
Values correspond to peptide concentrations of 100 μg/ml and to an Sh28GST concentration of 10 μg/ml. ND, not determined.
Inhibition of Sh28GST enzymatic activity by testosterone.
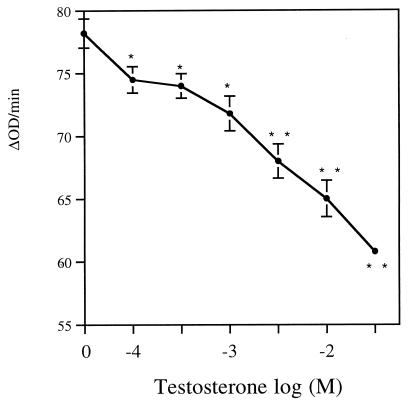
To evaluate the functional role of specific binding of testosterone to Sh28GST, we have assessed the effect of testosterone on the enzymatic activity of Sh28GST (Fig. 2). The activity of GST enzyme was significantly lower in the presence of 10−4 M testosterone compared to activity measured without hormone (P < 0.05). The GST activity decreased strongly when testosterone concentrations were scaled up (10−4 to 5 × 10−1 M), indicating that the inhibition of Sh28GST activity was clearly dose dependent (P < 0.01 for 5 × 10−2 to 5 × 10−1 M).
FIG. 2.
Inhibition of Sh28GST enzymatic activity by testosterone. Sh28GST protein was incubated in the presence of testosterone at increasing concentrations (0 to 5 × 10−1 M). Enzymatic activity of Sh28GST was evaluated at each concentration of testosterone as described in Materials and Methods. Triplicate determinations were performed, and the results are expressed as the mean ± SD of ΔOD/minute reading values. Student’s t test was used to compare the mean between each testosterone concentration and the value without hormones. *, P < 0.05; **, P < 0.01.
Specific binding of testosterone to peptide 24–43 involved in the enzymatic site of Sh28GST.
It has been demonstrated that three different peptides represent the major epitopes of Schistosoma Sh28GST, i.e., amino acids 24 to 43, 115 to 131, and 190 to 211. Indeed, immune response to peptide 115–131 was particularly involved in rodent protection against schistosoma infection in terms of reduction of worm burden (29). In contrast, peptide 24–43 and peptide 190–211 sequences participate in the enzymatic site of Sh28GST, and specific antibodies directed to these two peptides have the capacity to neutralize Sh28GST enzymatic activity (31).
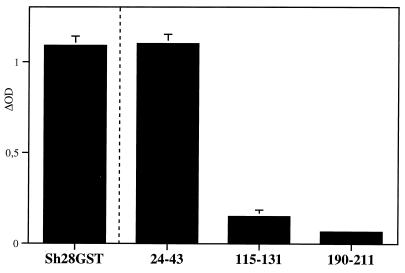
The binding of testosterone with three different peptides derived from the primary structure of the Sh28GST was evaluated by colorimetric assay using testosterone labeled with peroxidase (Fig. 3). In contrast to peptide 115–131 and peptide 190–211, strong binding was only observed with the peptide 24–43 sequence. This specific binding was very similar to those indicated with total Sh28GST protein used as a positive control.
FIG. 3.
Specific binding of testosterone to Sh28GST peptides. Synthetic peptides 24–43, 115–131, and 190–211 were incubated with testosterone (peroxidase labeled). Sh28GST protein was incubated as a positive control. Colorimetric development was carried out, and absorbance (OD) was measured at 405 nm. Results were expressed as ΔOD value after subtraction of background (as described in Materials and Methods). Triplicate determinations were performed, and the results are expressed as the mean ± SD of ΔOD reading values.
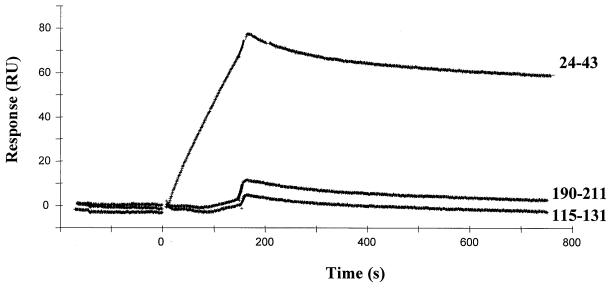
The affinity of binding was quantified by Biacore, and sensorgrams are presented in Fig. 4. An important resonance unit value was observed only for peptide 24–43 (RU = 81), indicating a specific binding with high Kd (7.4 × 10−6 M) (Table 1). In contrast to the other two peptides, very low baseline of RU was recorded, and the Kd value could thus not be calculated.
FIG. 4.
Sensorgrams of the binding of testosterone to Sh28GST peptides using Biacore technology. Biotinylated testosterone was immobilized onto an SA sensor chip coated with streptavidin. Synthetic peptides of Sh28GST (peptides 24–43, 115–131, and 190–211) were injected (100 μg/ml) on immobilized testosterone, and the sensorgrams corresponding to association time (180 s) and dissociation time (1,000 s) were evaluated for each peptide. Results are expressed as resonance units (RU) corresponding to the plateau value for the complex as described in Materials and Methods.
DISCUSSION
Mammal GSTs have the ability to bind hormones and particularly sexual steroids, to influence their transport, metabolism, and action (13, 16). Direct binding of testosterone for mammal GST with moderate affinity (10−6 < Kd < 10−4 M) has been demonstrated (16). In our study, we have demonstrated for the first time a specific binding between testosterone and a parasite GST enzyme, the S. haematobium 28-kda GST. We have shown a higher affinity of this binding (Kd = 5.7 × 10−7 M) compared to those observed with mammal GST.
Interestingly, it has been demonstrated previously in mice infected by Schistosoma that males developed lower worm burdens and survived longer after infection than female mice (9). This phenomenon could involve the effect of testosterone on the failure of schistosomule development, independently of the influence of this hormone on the specific immune response (20). Thus, 28GST could be a parasite receptor of testosterone, and the binding with 28GST could act as a carrier of hormone in the parasite. This mechanism could play a role in the negative effect of this steroid on schistosome maturation.
In addition, we have demonstrated the functional ability of this binding on GST enzymatic activity, where testosterone has the capacity to inhibit the Sh28GST activity in a dose-dependent manner. In animal models, specific antibodies to 28GST induced after immunization and particularly antibodies directed to the two epitopes involved in the enzymatic site (i.e., residues 24 to 43 and 190 to 211) have the capacity to inhibit 28GST enzymatic activity which was associated with an antifecundity effect (30, 31). We have demonstrated in our study that testosterone specifically bound the peptide 24–43 (Kd = 7.4 × 10−6) in contrast to two other major peptides of Sh28GST (i.e., 115 to 131 and 190 to 211). The specific binding to this peptide could explain the inhibition of Sh28GST activity observed.
During human infection, testosterone could thus play a role in parasite fecundity not only by acting on the antischistosome immunity (24) but also by directly inhibiting the 28GST enzymatic activity. This direct effect of sexual steroid has been demonstrated previously, where estradiol had the capacity to inhibit the enzymatic activity of a rat GST (3). In human schistosomiasis, an age-dependent reduction of parasite fecundity was particularly observed after puberty during S. haematobium infection (1). In addition, a negative correlation between intensity of infection, in terms of egg counts, and testosterone levels was recorded, but in this study, this relationship could not be dissociated from the effect of age on either parameter (26). Nevertheless, all these results observed during human infection could be in accordance with our hypothesis suggesting the age-dependent effect of testosterone on parasite fecundity in inhibiting 28GST enzymatic activity.
Several mechanisms could explain a direct antifecundity effect of testosterone. In particular, the important association observed between GST protein, testosterone, and muscular mechanisms during parasite fecundity could be involved. Morrison et al. have demonstrated that progesterone significantly decreased in vitro schistosome egg production in reducing the muscular tension of the parasite (19). This steroid-dependent mechanism appeared thus strikingly involved in egg laying, suggesting that steroid hormones could inhibit the fecundity of the parasite in directly decreasing the muscular tension. In addition, a relationship between mammal GSTs and muscle cells has been reported, indicating that GST activity increased during muscular toxicity or atrophy (15, 18). In flight muscles of Drosophila melanogaster, the interaction of GST with troponin was closely involved in myosin cross-bridge acting in muscular contractions (7). An influence of estradiol and testosterone has also been demonstrated on the expresssion of hamster GST in smooth muscle cells (14). All these results suggest a close relationship between muscle cells, GST protein, and an effect of steroid hormones on muscular contractions.
In schistosome adult worms, ultrastructural localization of 28GST indicated that this protein was highly expressed not only in the tegumental surface and in genital organs but also in the parenchyma of the parasite, in which two types of muscle cells were stained (17). Schistosoma 28GST could thus be strikingly associated with muscular organs of the parasite and the inhibition of its enzymatic activity could be involved in the antifecundity effect in decreasing muscle tension. Binding of testosterone to 28GST in inhibiting GST activity could participate directly in this antifecundity mechanism.
In addition, we have previously demonstrated during human schistosomiasis that immune response to 28GST was gender dependent, with a specificity of epitope recognition (22, 23). The binding of testosterone to 28GST could influence the orientation of the specific immune response according to the sex of infected individuals. The 28GST protein could thus act as a hormone carrier in “increasing” or “facilitating” the access of sexual steroids such as testosterone to 28GST-specific immune cells. This hypothesis is now under investigation.
In the present study, we have demonstrated a binding of testosterone to parasite GST, specifically to one Sh28GST peptide involved in the enzymatic site. This specific binding seems to have the ability to inhibit Sh28GST enzymatic activity. This functional binding could play a direct role in the decrease of parasite fecundity in acting not only on worm metabolism but also directly on parasite reproductive organs. In human schistosomiasis, this GST-dependent effect of sexual steroids could have an influence on the age- and sex-dependent level of infection.
Acknowledgments
This work was supported by the Region Nord-Pas-de-Calais, the Institut Pasteur de Lille, CNRS, and INSERM. F. Remoué holds a fellowship from the Region Nord-Pas-de-Calais.
Editor: W. A. Petri, Jr.
Footnotes
In memory of our colleague Jean-Claude Mani.
REFERENCES
- 1.Agnew, A., A. J. Fulford, M. T. Mwanje, K. Gaghuchi, V. Gutsmann, F. W. Krijger, R. F. Sturrock, B. J. Vennervald, J. H. Ouma, A. E. Butterworth, and A. M. Deelder. 1996. Age-dependent reduction of schistosome fecundity in Schistosoma haematobium but not Schistosoma mansoni infections in humans. Am. J. Trop. Med. Hyg. 55:338–343. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J., and W. H. Stimson. 1988. Sex hormones and the course of parasitic infection. Parasitol. Today 4:189–193. [Google Scholar]
- 3.Aravinda, S., B. Gopalakrishnan, C. S. Dey, S. M. Totey, C. H. Pawshe, D. Salunke, K. Kaur, and C. Shaha. 1995. A testicular protein important for fertility has glutathione S-transferase activity and is localized extracellularly in the seminiferous tubules. J. Biol. Chem. 270:15675–15685. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger, D., A. Warter, B. Sellin, V. Lindner, R. J. Pierce, J. P. Chippaux, and A. Capron. 1999. Vaccine potential of a recombinant glutathione S-transferase cloned from Schistosoma haematobium in primates experimentally infected with an homologous challenge. Vaccine 17:319–326. [DOI] [PubMed] [Google Scholar]
- 5.Bundy, D. A. P. 1988. Gender-dependent patterns of infection and disease. Parasitol. Today 4:186–189. [DOI] [PubMed] [Google Scholar]
- 6.Capron, A. 1998. Schistosomiasis: forty years’ war on the worm. Parasitol. Today 14:379–384. [DOI] [PubMed] [Google Scholar]
- 7.Clayton, J. D., R. M. Cripps, J. C. Sparrow, and B. Bullard. 1998. Interaction of troponin-H and glutathione S-transferase-2 in the indirect flight muscles of Drosophila melanogaster. J. Muscle Res. Cell. Motil. 19:117–127. [DOI] [PubMed] [Google Scholar]
- 8.de Mendonça, R., H. Escriva, D. Bouton, V. Laudet, and R. J. Pierce. 2000. Hormones and nuclear receptors in schistosome development. Parasitol. Today 16:233–240. [DOI] [PubMed] [Google Scholar]
- 9.Eloi-Santos, S., N. J. Olsen, R. Correa-Oliveira, and D. G. Colley. 1992. Schistosoma mansoni: mortality, pathophysiology, and susceptibility differences in male and female mice. Exp. Parasitol. 75:168–175. [DOI] [PubMed] [Google Scholar]
- 10.Fulford, A. J. C., M. Webster, J. H. Ouma, G. Kimani, and D. W. Dunne. 1998. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol. Today 14:23–26. [DOI] [PubMed] [Google Scholar]
- 11.Grzych, J. M., D. Grezel, C. B. Xu, J. L. Neyrinck, M. Capron, J. H. Ouma, A. E. Butterworth, and A. Capron. 1993. IgA antibodies to a protective antigen in human schistosomiasis mansoni. J. Immunol. 150:527–635. [PubMed] [Google Scholar]
- 12.Habig, W. H., M. J. Pabst, and W. B. Jacoby. 1974. Glutathione-S-transferase: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249:73. [PubMed] [Google Scholar]
- 13.Homma, H., and I. Listowsky. 1985. Identification of Yb-glutathione-S-transferase as a major rat liver protein labeled with dexamethasone 21-methanesulfonate. Proc. Natl. Acad. Sci. USA 82:7165–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson, C. E., J. E. DeHaven, B. A. Schulte, and J. S. Norris. 1999. Exogenous 17b-estradiol blocks alpha and mu but not pi class glutathione S-transferase immunoreactivity in epithelium of Syrian hamster vas deferens. J. Histochem. Cytochem. 47:91–98. [DOI] [PubMed] [Google Scholar]
- 15.Kondo, H., M. Miura, and Y. Itokawa. 1993. Antioxidant enzyme systems in skeletal muscle atrophied by immobilization. Pflugers Arch. 422:404–406. [DOI] [PubMed] [Google Scholar]
- 16.Listowsky, I., M. Abramovitz, H. Homma, and Y. Niitsu. 1988. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab. Rev. 19:305–318. [DOI] [PubMed] [Google Scholar]
- 17.Liu, J. L., J. Fontaine, A. Capron, and J.-M. Grzych. 1996. Ultrastructural localization of Sm28 GST protective antigen in Schistosoma mansoni adult worms. Parasitology 113:377–391. [DOI] [PubMed] [Google Scholar]
- 18.Misra, P., S. K. Srivastava, S. S. Singhal, S. Awasthi, Y. C. Awasthi, and P. J. Boor. 1995. Glutathione S-transferase 8–8 is localized in smooth muscle cells of rat aorta and is induced in an experimental model of atherosclerosis. Toxicol. Appl. Pharmacol. 133:27–33. [DOI] [PubMed] [Google Scholar]
- 19.Morrison, D. D., E. A. Vande Waa, and J. L. Bennett. 1986. Effects of steroids and steroid synthesis inhibitors on fecundity of Schistosoma mansoni in vitro. J. Chem. Ecol. 12:1901–1908. [DOI] [PubMed] [Google Scholar]
- 20.Nakazawa, M., M. R. Fantappie, G. L. Freeman, J. S. Eloi-Santos, N. J. Olsen, W. J. Kovacs, W. E. Secor, and D. G. Colley. 1997. Schistosoma mansoni: suceptibility differences between male and female mice can be mediated by testosterone during early infection. Exp. Parasitol. 85:233–240. [DOI] [PubMed] [Google Scholar]
- 21.Porchet, E., A. McNair, A. Caron, J. P. Kusnierz, K. Zemzoumi, and A. Capron. 1994. Tissue expression of the Schistosoma mansoni 28 kDa glutathione S-transferase. Parasitology 109:565–572. [DOI] [PubMed] [Google Scholar]
- 22.Remoué, F., F. Rogerie, M. C. Gallissot, H. L. Guyatt, J. L. Neyrinck, M. M. Diakkhate, M. Niang, A. E. Butterworth, C. Auriault, A. Capron, and G. Riveau. 2000. Sex-dependent neutralizing humoral response to Schistosoma mansoni Sh28GST antigen in infected human population. J. Infect. Dis. 181:1855–1859. [DOI] [PubMed] [Google Scholar]
- 23.Remoué, F., D. To Van, A. M. Schacht, M. Picquet, O. Garraud, J. Vercruysse, A. Ly, A. Capron, and G. Riveau. 2001. Gender-dependent specific immune response during chronic human schistosomiasis haematobia. Clin. Exp. Immunol. 124:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts, C. W., A. Satoskar, and J. Alexander. 1996. Sex steroids, pregnancy-associated hormones and immunity to parasitic infection. Parasite Today 12:382–388. [DOI] [PubMed] [Google Scholar]
- 25.Schuurs, A. H. W. M., and H. A. M. Verheul. 1990. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 35:157–172. [DOI] [PubMed] [Google Scholar]
- 26.Skelly, P. J., W. Evan Secor, M. G. Reis, E. A. Ramos, T. M. Carmo, E. E. Peixoto, and D. A. Harn. 1994. Failure of schistosomiasis to significantly decrease testosterone levels in Brazilian men. Am. J. Trop. Med. Hyg. 51:40–44. [DOI] [PubMed] [Google Scholar]
- 27.Tiuria, R., T. Kaneyuki, Y. Horii, S. Makimura, K. Tsuchiya, and Y. Nawa. 1997. Long term survival of damaged Nippostrongylus brasiliensis adult worms in the testosterone Indian soft-furred rat, Millardia meltada. Parasite Immunol. 19:455–459. [DOI] [PubMed] [Google Scholar]
- 28.Trottein, F., C. Godin, R. J. Pierce, B. Sellin, M. G. Taylor, I. Gorrillot, S. M. Sampaio, J. P. Lecocq, and A. Capron. 1992. Interspecies variation of schistosome 28 kDa glutatione S-transferase. Mol. Biochem. Parasitol. 54:63–72. [DOI] [PubMed] [Google Scholar]
- 29.Wolowczuk, I., C. Auriault, M. Bossus, D. Boulanger, H. Gras-Masse, C. Mazingue, R. Pierce, D. Grezel, G. D. Reid, A. Tartar, and A. Capron. 1991. Antigenicity and imunogenicity of a multiple peptidic construction of the Schistosoma mansoni Sm-28 GST antigen in rat, mouse and monkey. J. Immunol. 146:1987–1995. [PubMed] [Google Scholar]
- 30.Xu, C.-B., C. Verwaerde, J.-M. Grzych, J. Fontaine, and A. Capron. 1991. A monoclonal antibody blocking the Schistosoma mansoni 28-kDa glutathione S-transferase activity reduces female worm fecundity and egg viability. Eur. J. Immunol. 21:1801–1807. [DOI] [PubMed] [Google Scholar]
- 31.Xu, C. B., C. Verwaerde, H. Gras-Masse, J. Fontaine, M. Bossus, F. Trottein, I. Wolowczuk, A. Tartar, and A. Capron. 1993. Schistosoma mansoni 28-kDa glutathione S-transferase and immunity against parasite fecundity and egg viability. Role of the amino- and carboxyl-terminal domains. J. Immunol. 150:940–949. [PubMed] [Google Scholar]