Abstract
Proinflammatory cytokines play a critical role in innate host defense against extracellular bacteria. However, little is known regarding the effects of these cytokines on the adaptive humoral response. Mice injected with a neutralizing anti-tumor necrosis factor alpha (TNF-α) monoclonal antibody (MAb) at the time of primary immunization with intact Streptococcus pneumoniae (strain R36A) showed a substantial reduction in both the primary immunoglobulin G (IgG) response specific for the cell wall protein, pneumococcal surface protein A (PspA), as well as in the development of PspA-specific memory. In contrast, anti-TNF-α MAb injected only at the time of secondary immunization with R36A failed to alter the boosted anti-PspA response. TNF-α was required only within the first 48 to 72 h after primary immunization with R36A and was induced both by non-B and non-T cells and by lymphoid cells, within 2 to 6 h after immunization, with levels returning to normal by 24 h. Thus, the early innate release of TNF-α was critical for optimal stimulation of the subsequent adaptive humoral response to R36A. Additional proinflammatory (interleukin 1 [IL-1], IL-6, IL-12, and gamma interferon [IFN-γ]) as well as anti-inflammatory (IL-4 and IL-10) cytokines were also transiently induced. Mice genetically deficient in IL-6, IFN-γ, or IL-12 also showed a reduced IgG anti-PspA response of all IgG isotypes. In contrast, IL-4−/− and IL-10−/− mice immunized with R36A showed a significant elevation in the IgG anti-PspA response, except that there was decreased IgG1 in IL-4−/− mice. In this regard, a marked enhancement in the induction of proinflammatory cytokines was observed in the absence of IL-10, relative to controls. Ig isotype titers specific for the phosphorycholine determinant of C-polysaccharide were similarly regulated, but to a much more modest degree. These data suggest that proinflammatory and anti-inflammatory cytokines differentially regulate an in vivo protein- and polysaccharide-specific Ig response to an extracellular bacteria.
Infections with extracellular bacteria are a global source of morbidity and mortality (3). Resolution of these infections in the naive host is mediated primarily through phagocytosis and intracellular killing by neutrophils and macrophages which are recruited to the site of infection (33). Phagocytosis in turn is facilitated by opsonins, such as natural antibody and acute-phase proteins, such as complement and C-reactive protein, which are induced early after infection (37). These components of the innate antibacterial response are induced and/or activated in part by proinflammatory cytokines, such as interleukin 1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), IL-12, and gamma interferon (IFN-γ) (20, 30). Subsequent to initial bacterial exposure, immunity develops primarily through induction of immunoglobulin G (IgG), specific for both protein and polysaccharide antigens expressed by the bacterial pathogen (3).
Although the protective role of proinflammatory cytokines in early innate defense against extracellular bacteria is well established, little is known concerning the cytokines that potentially mediate the adaptive humoral response to these agents. Further, the potential link between early innate release of cytokines in response to these pathogens and subsequent adaptive humoral immunity is unknown. That such a link may exist is suggested, in part, by the early recruitment, maturation, and migration of antigen-presenting cells (APCs), in response to proinflammatory mediators, including cytokines and chemokines (7, 67). Anti-inflammatory cytokines such as IL-4 and IL-10 may also be induced in response to extracellular bacteria (68). Although these type 2 cytokines are widely regarded as inducers of humoral responses during allergic and antihelminthic Ig responses, they could potentially down-regulate humoral responses during infections with extracellular bacteria, in part through direct and indirect inhibitory effects on APC recruitment and function (15, 23, 28, 41). Finally, the parameters that regulate protein- versus polysaccharide-specific Ig responses to an intact extracellular bacteria are largely unknown. In this regard, in vitro studies suggest that the physiological cytokine requirements for these two types of response may differ (73). This is based, in part, on the strong membrane Ig-mediated signals delivered to B cells by multivalent polysaccharide antigens, and in contrast, the recruitment of cognate T-cell help, including CD40/CD40-ligand interactions, during Ig responses to proteins (53).
In light of these unresolved issues, we set out to determine the endogenous cytokine requirements for both protein- and polysaccharide-specific Ig isotype responses to an intact extracellular bacteria, the unencapsulated variant (strain R36A) of the virulent Streptococcus pneumoniae capsular type 2 strain D39 (5). Immunity to S. pneumoniae can be mediated by a number of bacterial proteins, the phosphorycholine (PC) determinant of the cell wall teichoic acid (C-polysaccharide), as well as capsular polysaccharide (9-12, 26, 57). In this report we investigated the role of various endogenous pro- and anti-inflammatory cytokines in the regulation of Ig isotype responses specific for the pneumococcal cell wall protein, pneumococcal surface protein (PspA) and for PC. We demonstrate that proinflammatory cytokines such as IL-6, TNF-α, IL-12, and IFN-γ individually have stimulatory effects on the humoral response to R36A that are largely independent of Ig isotype. In contrast, IL-10 downregulates Ig responses in an isotype-nonspecific manner, whereas IL-4 appears to regulate Ig class switching. The anti-PspA response to R36A is significantly more sensitive to this cytokine regulation than is the Ig isotype response to PC.
MATERIALS AND METHODS
Mice.
C57BL/6, 129B6, IL-4−/−, IL-6−/−, IL-10−/−, IL-12−/−, and IFN-γ−/− female mice were purchased from Jackson Labs (Bar Harbor, Maine). Mice were used at 6 to 10 weeks of age and were maintained in a pathogen-free environment.
Reagents.
Neutralizing rat IgG1 anti-mouse TNF-α MAb (XT22) (1), neutralizing rat IgG1 anti-mouse IFN-γ MAb (XMG-6) (16), and control rat IgG1 anti-Escherichia coli β-galactosidase MAb (GL113) were purified from ascites by ammonium sulfate precipitation, followed by protein G purification. Neutralizing rat IgG1 anti-mouse IL-10 (JES5-2A5) and control MAb (R3-34, nonreactive rat IgG1) were purchased from BD PharMingen (San Diego, Calif.). Recombinant PspA, a kind gift of Luba Grinberg (Biosynexus, Inc., Rockville, Md.) was expressed in Saccharomyces cerevisiae BJ3505 as a His6-tagged fusion protein and purified by Ni-nitrilotriacetic acid (NTA) affinity chromatography (81). The expressed protein includes amino acids 4 to 299 of the mature protein. Recombinant pneumococcal surface adhesin A (PsaA), a kind gift of James C. Paton (Adelaide University, Adelaide, S. A., Australia), was expressed in E. coli as a His6-tagged fusion protein and purified by Ni-NTA affinity chromatography (60). PC-bovine serum albumin (PC-BSA) and PC-keyhole limpet hemocyanin (PC-KLH), kind gifts of Andrew Lees (Biosynexus, Inc.), were synthesized as described previously (82).
Preparation of, and immunization with, R36A.
A nonencapsulated variant (strain R36A) of virulent S. pneumoniae capsular type 2 (strain D39) (5) was grown in Todd-Hewitt broth to mid-log phase and stored at −70°C. For immunization, frozen bacteria were thawed and subcultured on blood agar plates. One to two characteristic colonies were selected and suspended in 200 ml of Todd-Hewitt broth, placed in a shaker water bath at 37°C for 4 to 6 h until an optical density (absorbance at 650 nm) of 0.6 was achieved as measured by a spectrophotometer (Spectronic 100; Bausch & Lomb, Rochester, N.Y.). The 200-ml prep of R36A was then heat killed by incubation in a 60°C water bath for 10 h (1 h/20 ml). Sterility was confirmed by culture. This bacterial stock containing 109 CFU/ml was aliquoted and frozen at −70°C until used for immunization. Mice were immunized intraperitoneally (i.p.) with various doses (CFU equivalents) of heat-killed bacteria in 250 μl of phosphate-buffered saline (PBS). Serum samples for measurement of antigen-specific Ig isotype titers were prepared from blood obtained through the tail vein.
Measurement of serum antigen-specific Ig isotype titers.
Immulon 2 plates were coated with PC-KLH or PC-BSA (5 μg/ml) and Immulon 4 plates were coated with PspA (5 μg/ml) or PsaA (1 μg/ml) in 1× PBS for 1 h at 37°C or overnight at 4°C. Plates were then blocked with blocking buffer (1× PBS plus 0.5% BSA) at 37°C for 30 min or 4°C overnight. Threefold dilutions of serum samples in blocking buffer were then added starting at a 1/50 serum dilution. After 1 h of incubation at 37°C, plates were washed three times with PBT (1× PBS plus 0.1% Tween 20). Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgM, IgG3, IgG1, IgG2b, and IgG2a antibodies (final concentration in blocking buffer, 200 ng/ml) were then added, and plates were incubated at 37°C for 1 h. Plates were washed five times with PBT. Substrate (4-methylumbelliferyl phosphate) was then added (50 μg/ml; 50 μl/well) and fluorescence was read on a MicroFLUOR enzyme-linked immunosorbent assay (ELISA) reader (Dynatech Laboratories, Inc., Chantilly, Va.).
Magnetic cell sorting.
Spleens were first enzymatically digested with a lipopolysaccharide (LPS)-free mixture of Liberase CI and DNase I (Roche Diagnostics Corp, Indianapolis, Ind.). Spleens were then macerated and passed through a wire mesh filter. The resulting cell suspension was pelleted, and red blood cells were lysed using ACK lysing buffer (Invitrogen, Rockville, Md.). Cells were then washed once with LPS-free Dulbecco’s phosphate-buffered saline (BioWhittaker, Frederick, Md.) plus LPS-free EDTA (Sigma, St. Louis, Mo.). B cells and T cells were separated from whole spleen cells by magnetic cell sorting with MACS (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Briefly, to 107 spleen cells was added 10 μl (each) of MACS MicroBeads conjugated to rat IgG2b anti-mouse Thy-1.2 MAb (30H12) and rat IgG2a anti-mouse B220 MAb (RA3-6B2) and cells were subjected to a magnetic field for cell separation.
Real-time reverse transcription-PCR (RT-PCR) for measurement of cytokine-specific mRNA.
Total RNA was extracted from isolated spleen cell populations using RNAzol B (TEL-TEST, Inc., Friendswood, Tex.). Total RNA was then reverse transcribed using the Superscript II Preamplification System for first-strand cDNA synthesis (Invitrogen) according to the manufacturer's instructions. One hundred eighty nanograms of RNA was subsequently used as a template for each real-time PCR. All PCRs for cytokine-specific mRNA were performed on an ABI PRISM 7700 Sequence Detector System (PE Applied Biosystems, Foster City, Calif.) using proprietary cytokine-specific primers and probes from ABI Applied Biosystems (Rockville, Md.). Relative cytokine mRNA levels were determined by normalization of the signal with that for rRNA. In initial studies, twofold dilutions of cDNA generated a linear signal curve over at least a 30-fold range of cDNA concentrations.
Cytokine-specific ELISA.
The concentrations of specific cytokines released into the media of spleen cell cultures were measured by optimized standard sandwich ELISA. Recombinant cytokines used as standards, as well as the capture MAbs, biotinylated MAbs used for detection, and streptavidin-alkaline phosphatase were purchased from BD PharMingen. Streptavidin-alkaline phosphatase was used in combination with 4-methylumbelliferyl phosphate (Sigma) as a substrate to detect the specific antibody binding. Standards were included in every plate, and the samples were tested in duplicate. The limits of detection of the respective ELISAs were as follows: IL-4, 5 pg/ml; IL-6, 4 pg/ml; IL-10, 150 pg/ml; IL-12 (p40/70), 6 pg/ml; TNF-α, 12 pg/ml; and IFN-γ, 80 pg/ml.
Statistics.
Data are expressed as the arithmetic means of Ig titers of individual serum samples plus or minus the standard errors of the means (SEM). Differences between treatment groups were considered significant at P values of <0.05 using the Student t test.
RESULTS
Endogenous TNF-α is required for an optimal anti-PspA, anti-PsaA, and anti-PC response to R36A.
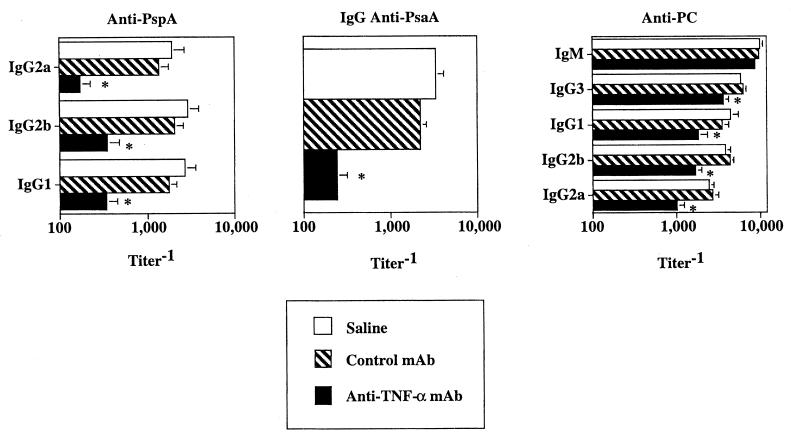
Although the proinflammatory cytokine TNF-α plays a key role in stimulating the innate immune response to extracellular bacteria, its potential to regulate an adaptive humoral immune response to these pathogens in vivo is not known. In particular, little is known regarding the potential requirements for endogenous cytokines in mediating protein versus polysaccharide-specific Ig responses to an intact bacterium. To address this issue, mice were injected i.p. with a neutralizing anti-TNF-α MAb, an isotype-matched control MAb, or saline followed 1 day later by i.p. immunization with an intact, heat-killed nonencapsulated variant of S. pneumoniae, capsular type 2 (strain R36A). Mice were bled 7 and 14 days after primary R36A immunization for determination of anti-PC (day 7) and anti-PspA and anti-PsaA Ig (day 14) isotype titers. Previous studies in our laboratory showed that the primary anti-PC response was maximal by day 7, whereas the primary anti-PspA response was maximal by day 14 (82, 83). As shown in Fig. 1, mice treated with anti TNF-α MAb had 6- to 11-fold reductions in all IgG anti-PspA and IgG anti-PsaA isotypes, whereas a more moderate but significant 2- to 3-fold reduction in the IgG anti-PC isotype response relative to control MAb or saline was observed. No significant reduction in the IgM anti-PC response was seen in mice treated with anti-TNF-α MAb relative to control MAb or saline. In additional studies, we observed that the anti-TNF-α-mediated reductions in anti-PspA and anti-PC responses were maintained over at least a 6-week period (data not shown), indicating that anti-TNF-α did not merely delay but suppressed the humoral response to R36A. These data are the first to demonstrate that endogenous TNF-α is required for an optimal protein- and polysaccharide-specific Ig isotype response to an intact extracellular bacterium in vivo.
FIG. 1.
Endogenous TNF-α is a key positive regulator of the in vivo anti-PspA, anti-PsaA and, to a lesser extent, anti-PC response to R36A. Mice were injected i.p. with 0.5 mg of anti-TNF-α MAb (XT22) or the same amount of control rat IgG1 MAb (GL113) or saline. Twenty-four hours later mice were injected i.p. with R36A (2 × 108 CFU/mouse). Sera were collected 1 week after primary immunization with R36A for determination of anti-PC titers and 2 weeks after immunization for determination of anti-PspA and anti-PsaA titers by ELISA. Values represent arithmetic means ± SEM of eight mice per group. *, P < 0.05. Data are representative of three similar experiments.
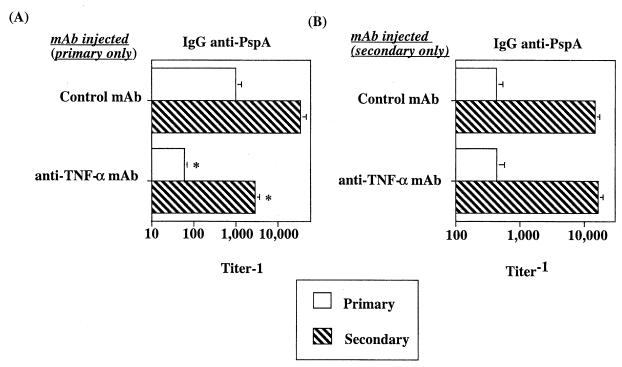
TNF-α is required for the development of PspA-specific memory but not for elicitation of the anti-PspA memory response upon secondary immunization.
We next wished to determine whether endogenous TNF-α was also required for the development of PspA-specific memory. Thus, we injected anti-TNF-α or control MAb at the time of primary immunization with R36A and collected sera 2 weeks later (primary). Mice were then rechallenged 6 weeks later with R36A alone. After rechallenge, secondary IgG anti-PspA titers were determined 7 days later (82, 83). As a control, we showed that mice immunized with R36A 6 weeks after injection of anti-TNF-α MAb alone generated a primary anti-PspA response equivalent to that of mice receiving a control MAb, indicating effective clearance of anti-TNF-α MAb after a 6-week period (data not shown). As illustrated in Fig. 2A, anti-TNF-α MAb significantly inhibited (10- to 12-fold) the primary IgG anti-PspA response relative to that found for mice treated with control MAb, as demonstrated also in Fig. 1. After boosting with R36A 6 weeks later, mice receiving control MAb during the primary showed secondary IgG anti-PspA titers >10-fold higher than those observed at the height of the primary. In contrast, mice that received anti-TNF-α MAb during the primary developed a secondary anti-PspA response that was only at the level of a normal primary. This indicated that anti-TNF-α inhibited the generation of PspA-specific memory but did not induce tolerance.
FIG. 2.
TNF-α is required for an optimal primary anti-PspA response and the development of PspA-specific memory, but not for elicitation of a secondary response to R36A. (A) Mice were injected i.p. with 0.5 mg of anti-TNF-α MAb or control MAb. Twenty-four hours later mice were injected i.p. with R36A (2 × 108 CFU/mouse). Six weeks after R36A immunization, mice were boosted with R36A alone (2 × 108 CFU/mouse). Sera were collected 2 weeks after primary immunization with R36A (primary) and 1 week after boosting with R36A (secondary) for determination of anti-PspA titers. Values represent arithmetic means ± SEM of eight mice per group. *, P < 0.05. (B) Mice were injected i.p. with R36A alone (2 × 108 CFU/mouse). Two weeks later primed mice were injected i.p. with 0.5 mg of anti-TNF-α MAb and/or 0.5 mg of control MAb, followed 24 h later by boosting with R36A (108 CFU/mouse). Values represent the arithmetic means ± SEM of eight mice per group. *, P < 0.05. One experiment was performed.
We further wished to determine the potential requirement for TNF-α during the elicitation of a memory IgG anti-PspA response. Thus, anti-TNF-α or control MAb was injected at the time of boosting of mice that had previously been immunized with R36A alone and thus were allowed to develop memory. As shown in Fig. 2B, anti-TNF-α had no significant effect on the elicitation of the memory IgG anti-PspA response. Thus, endogenous TNF-α is required for the primary anti-PspA response and the development of PspA-specific memory, but not for the elicitation of a memory response.
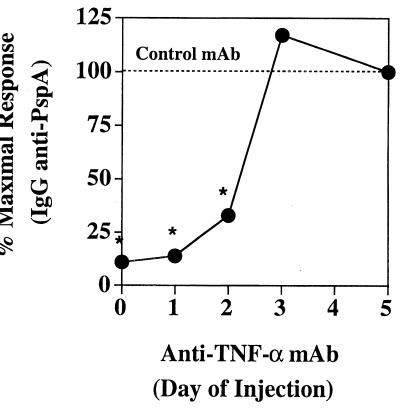
TNF-α is required relatively early after primary R36A immunization.
We next wished to know the time period after immunization during which TNF-α was required for induction of an optimal primary PspA-specific Ig response to R36A. To accomplish this, groups of five mice were given a single injection of anti-TNF-α MAb, each at a different time (0 to 5 days) after primary immunization with R36A, and sera were obtained 14 days after the initial R36A immunization for determination of serum IgG anti-PspA titers. A separate group of mice received control MAb at the time of R36A immunization for comparison. As illustrated in Fig. 3, injection of anti-TNF-α MAb on days 0, 1, and 2 following R36A immunization significantly inhibited the primary IgG anti-PspA response, whereas it had no significant effect when injected on day 3 or thereafter. These data indicate that TNF-α is required only within the first 48 to 72 h after primary immunization with R36A.
FIG. 3.
TNF-α is required within the first 48 to 72 h after R36A immunization for induction of an anti-PspA response. Mice were immunized i.p. with R36A (108 CFU/mouse), and then distinct sets of mice were injected i.p. with 0.5 mg of either anti-TNF-α MAb or control MAb at different times (days 0 to 5). Sera were collected 2 weeks after primary immunization with R36A for the determination of anti-PspA titers. Values represent the arithmetic means ± SEM of five mice per group. *, P < 0.05. Data are representative of two similar experiments.
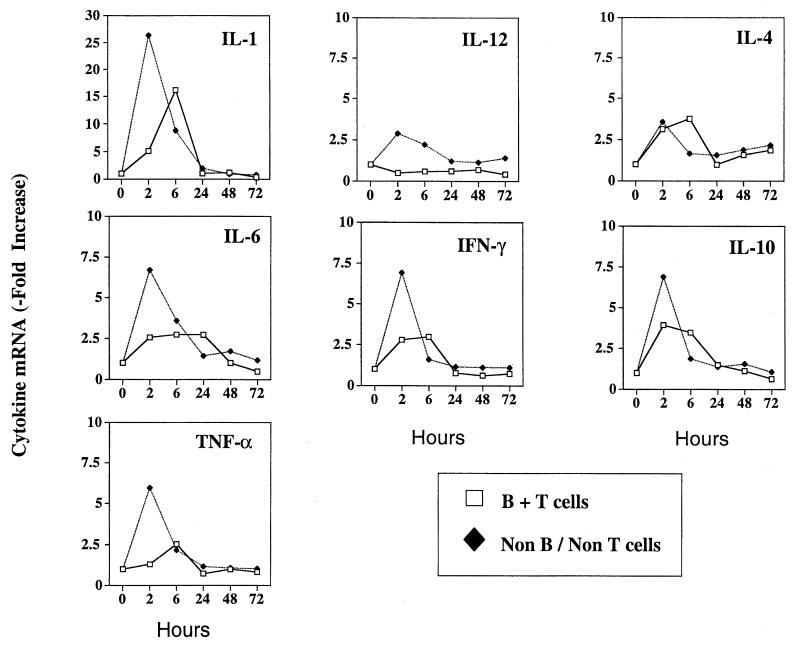
R36A rapidly coinduces both pro- and anti-inflammatory cytokines in vivo.
In light of the Ig-inductive action of TNF-α within the first 48 to 72 h after R36A immunization, we were interested to know the time during which this and other pro- and anti-inflammatory cytokines were induced and their cellular source(s). To accomplish this we combined magnetic cell sorting with real-time RT-PCR for semiquantitation of cytokine-specific mRNA in isolated lymphoid (B + T) and nonlymphoid (non-B and non-T) cell populations. For this analysis, spleen cells were obtained at different times (0 to 72 h) after R36A immunization. As illustrated in Fig. 4, R36A induced a rapid (2 to 6 h) and transient expression of both pro- (IL-1, IL-6, TNF-α, IFN-γ, and IL-12) and anti-inflammatory (IL-4 and IL-10) cytokines within the spleen. A bimodal pattern of cytokine induction was observed, with one peak seen by 2 h after immunization within the non-B- and non-T-cell compartment, and the second peak seen by 6 h within the B- + T-cell compartment. Relative expression levels of cytokines were typically higher in the non-B- and non-T-cell compartment, and B + T cells failed to make IL-12. Cytokine mRNA expression generally returned to preimmunization levels by 24 h and remained at those levels up to 72 h after immunization. As demonstrated in Fig. 9, enhancement of specific cytokine mRNA levels was correlated with induction of the corresponding cytokine protein. These data thus demonstrate a rapid and transient induction of multiple pro- and anti-inflammatory cytokines from both splenic lymphoid and nonlymphoid cells after R36A immunization. Further, the data strongly suggest that the TNF-α was responsible for stimulating the adaptive, humoral response to R36A that originated as part of the early innate response to this bacterium.
FIG. 4.
R36A induces a mixed cytokine response in vivo within 2 to 6 h. Real-time RT-PCR analysis of cytokine mRNA expression was performed on freshly harvested splenic non-B, -non-T cells and B + T cells by magnetic sorting at different time points after immunization with R36A (2 × 108 CFU/mouse). Relative levels of cytokine-specific mRNA were standardized based on rRNA levels. Cytokine-specific mRNA levels in sorted spleen cells from unimmunized mice were arbitrarily assigned a value of 1. Data are representative of two similar experiments.
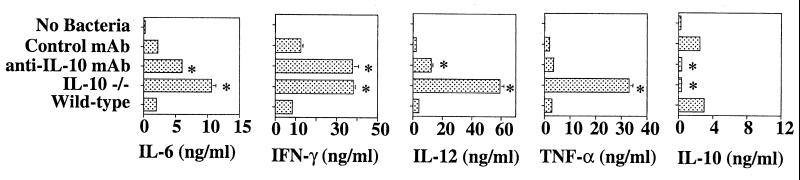
FIG. 9.
Endogenous IL-10 suppresses proinflammatory cytokine induction in response to R36A. Spleen cells from wild-type and IL-10−/− mice were cultured at 107 cells/ml in the presence of 2 × 108 CFU/ml of R36A for 24 h. In addition, wild-type spleen cells were cultured with anti-IL-10 MAb or control MAb (10 μg/ml). Concentrations of IL-6, IL-10, IL-12, IFN-γ, and TNF-α in the culture supernatants were measured by ELISA. The results are representative of two independent experiments and are shown as means ± SEM of triplicate wells. *, P < 0.05 in Student's t test.
Endogenous proinflammatory cytokines, in addition to TNF-α, stimulate the humoral response to R36A.
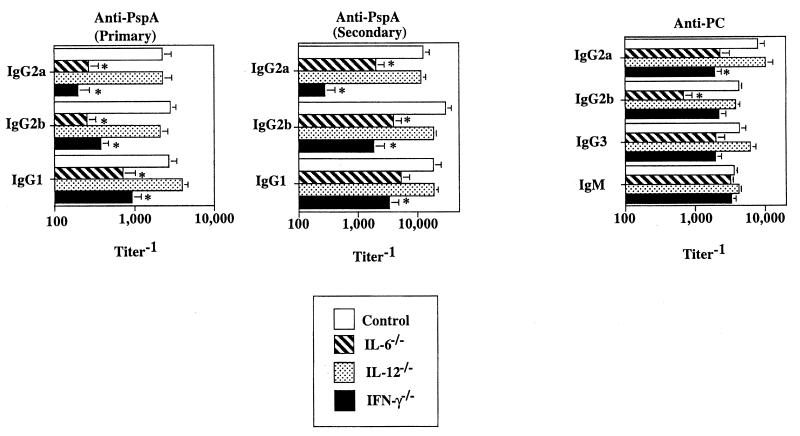
In the next set of experiments, we wished to determine whether other proinflammatory cytokines, observed early after R36A immunization, had a regulatory effect on the anti-PspA and anti-PC responses to R36A. To accomplish this, we immunized mice genetically deficient in IL-6 (IL-6−/−), IL-12 (IL-12−/−), or IFN-γ (IFN-γ−/−) and compared their humoral responses to R36A with those of wild-type controls. Mice were immunized with R36A on day 0 and boosted on day 14, and serum was obtained after each immunization for analysis of the primary and secondary Ig responses. As shown in Fig. 5, mice genetically deficient in IL-6 or IFN-γ showed significantly reduced primary and secondary anti-PspA responses (8- to 12-fold) of all IgG isotypes relative to control mice. Similar results were obtained when a neutralizing anti-IFN-γ MAb was used (data not shown). In contrast, we found more modest reductions or no changes in the anti-PC response in these mutant mice. Specifically, a significant reduction in IgG2b anti-PC titers (≈sixfold) was observed for IL-6−/− mice, and IgG2a anti-PC was reduced (three- to fourfold) in mice lacking IFN-γ (Fig. 5). No significant reduction in the anti-PspA or anti-PC responses was observed for IL-12−/− mice relative to controls.
FIG. 5.
Proinflammatory cytokines stimulate the humoral response to R36A. IL-6−/−, IL-12−/−, IFN-γ−/−, and control mice were immunized i.p with R36A (2 × 108 CFU/mouse). Sera were collected on day 7 (primary anti-PC), day 14 (primary anti-PspA), and day 21 (secondary anti-PspA) after immunization. Values are expressed as the arithmetic means ± SEM of eight mice per group. *, P < 0.05. Data are representative of two similar experiments.
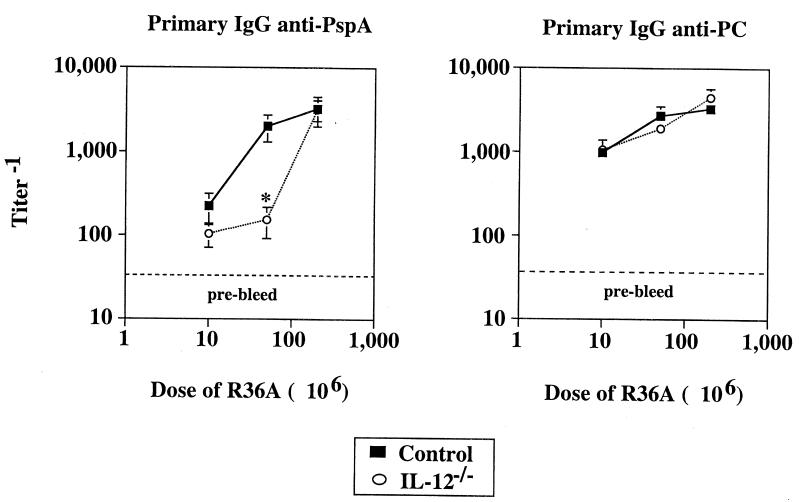
The requirement for IL-12 for induction of humoral immunity is dependent on the immunizing dose of R36A.
As illustrated in Fig. 5, we showed that the IgG anti-PspA response was IL-12 independent. In this and other experiments reported herein, we used a single dose of R36A (2 × 108 CFU per mouse), which we determined induces an optimal primary Ig response in wild-type mice (data not shown). We next wished to determine whether decreasing the strength of immunization with R36A might reveal a requirement for IL-12. IL-12−/− and control mice were immunized with three different doses of R36A (1 × 107, 5 × 107, and 2 × 108 CFU per mouse), and IgG anti-PspA and anti-PC responses were determined. As shown in Fig. 6, a reduction in the dose of R36A from 2 × 108 to 5 × 107 CFU per mouse resulted in a 2- to 3-fold reduction in IgG anti-PspA titers in wild-type mice, whereas 1 × 107 CFU of R36A per mouse led to an additional 10-fold reduction in PspA-specific IgG to a level comparable to that observed for unimmunized mice. Consistent with data shown in Fig. 5, IL-12−/− and wild-type mice had equivalent serum titers of IgG anti-PspA in response to 2 × 108 CFU of R36A. However, at the lower R36A dose of 5 × 107 CFU, IgG anti-PspA titers were 10-fold lower in IL-12−/− mice than in controls. IgG anti-PC titers were similar in IL-12−/− and control mice at all three doses of R36A. These data suggest that only optimal immunization of IL-12−/− mice with R36A induces a level of helper activity for Ig induction sufficient to compensate for the lack of IL-12. These data, in combination with data illustrated in Fig. 5, also suggest that at 2 × 108 CFU of R36A, IFN-γ is able to augment the IgG anti-PspA response in an IL-12-independent manner. In contrast, the relative reduction in the humoral response observed for IL-6−/− mice, compared to controls, was unaffected by the dose of R36A used for immunization (data not shown).
FIG. 6.
Role of R36A dose in induction of anti-PspA and anti-PC responses in IL-12−/− mice. IL-12−/− and control mice (five per group) were immunized i.p. with three different doses of R36A (10 × 106, 50 × 106, and 200 × 106 CFU/mouse). Sera were collected on day 7 (anti-PC) and day 14 (anti-PspA) after immunization. Values represent the arithmetic means ± SEM. *, P < 0.05. One experiment was performed.
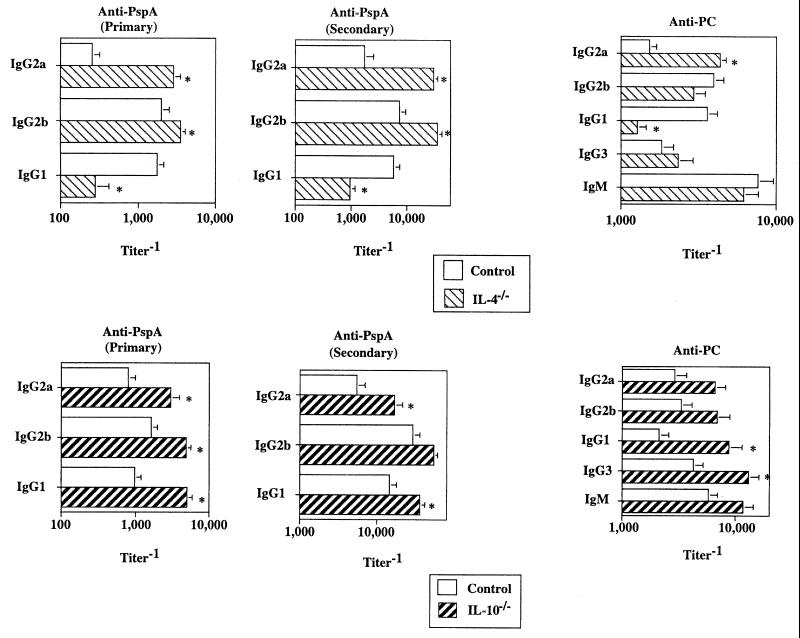
The anti-inflammatory cytokines IL-10 and IL-4 inhibit humoral immunity and regulate Ig class switching, respectively, in response to R36A.
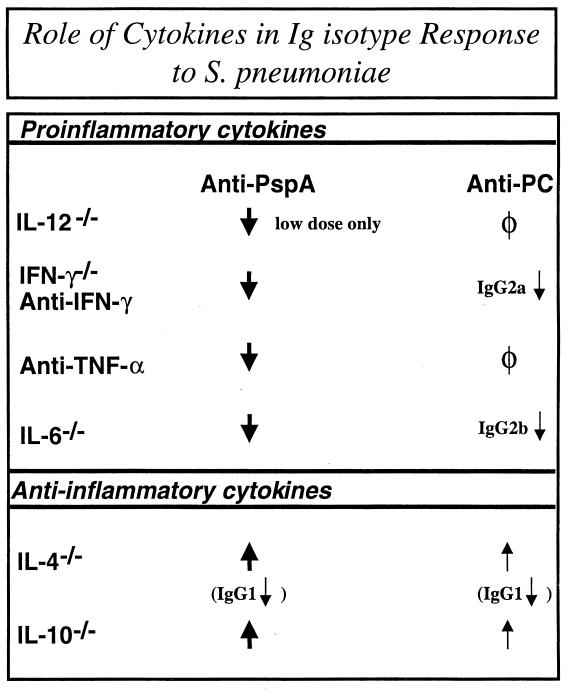
We next wished to determine the potential role of the anti-inflammatory cytokines IL-10 and IL-4 in the anti-PspA and anti-PC responses to R36A. As mentioned earlier, IL-10 and IL-4 were coinduced with the proinflammatory cytokines after R36A immunization. When IL-10−/− or IL-4−/− mice were immunized with R36A, significant elevations (5- to 11-fold) of both primary and secondary anti-PspA IgG responses were observed (Fig. 7). In contrast, IgG anti-PC titers were modestly elevated (two- to fourfold) in both sets of these mutant mice (Fig. 7). An exception was noted for the reduced titers (approximately sixfold) of PspA-specific IgG1 and PC-specific IgG1 in the absence of IL-4. Mice doubly mutant in IL-4 and IL-10 showed no additional alterations in Ig isotype titers relative to that observed for either IL-4−/− or IL-10−/− mice (data not shown). These data strongly suggest that endogenous IL-10 exerts an Ig isotype-nonspecific suppression on the humoral response to R36A, whereas IL-4 regulates Ig class switching (36). As with the proinflammatory cytokines, the effects of IL-4 and IL-10 generally were more pronounced and consistent for the anti-PspA than for the anti-PC response, suggesting potential quantitative differences in the endogenous cytokine regulation of protein- versus polysaccharide-specific Ig responses to intact bacteria. These data are summarized in Fig. 8.
FIG. 7.
Role of anti-inflammatory cytokines in the humoral response to R36A . IL-4−/−, IL-10−/−, and control mice were immunized i.p. with R36A (2 × 108 CFU/mouse). Sera were collected on day 7 (primary anti-PC), day 14 (primary anti-PspA), and day 21 after boosting with R36A (secondary anti-PspA). Values represent the arithmetic means ± SEM of seven mice per group. *, P < 0.05. Data are representative of two similar experiments.
Endogenous IL-10 inhibits induction of proinflammatory cytokines.
In a final set of experiments we wished to determine whether the enhancement in the humoral response to R36A observed for IL-10−/− mice might, at least in part, be secondary to the ability of endogenous IL-10 to downregulate proinflammatory cytokine induction (41). Spleen cells from wild-type versus IL-10−/− mice were cultured in the presence or absence of R36A, and cytokine concentrations in culture supernatant were measured 24 h later. In addition, spleen cells from wild-type mice were cultured with R36A in the presence of a neutralizing anti-IL-10 versus control MAb. Spleen cells from wild-type and IL-10−/− mice cultured in medium alone made no detectable cytokines (Fig. 9). Addition of R36A to wild-type spleen cell cultures resulted in the induction of IL-6, IFN-γ, IL-12, TNF-α, and IL-10; IL-4 was not detected (≤5 pg/ml). In contrast, spleen cells from IL-10−/− mice showed a striking upregulation of R36A-induced IL-6, IFN-γ, IL-12, and TNF-α relative to wild-type controls. IL-6, IFN-γ, and IL-12 were also upregulated in wild-type spleen cells upon addition of anti-IL-10 MAb relative to control MAb. These data suggest that endogenous IL-10 inhibits Ig responses to R36A, at least in part, by downregulating the expression of Ig-inducing, proinflammatory cytokines.
DISCUSSION
Extracellular bacteria rapidly induce the release of proinflammatory cytokines, including IL-1, IL-6, TNF-α, IL-12, and IFN-γ from multiple cell types which participate in the protective, innate response to these pathogens (20, 30). Dendritic cells (DCs), macrophages, NK cells, NK-T and TCR-γ/δ-T cells, mast cells, neutrophils, endothelial cells, and fibroblasts can collectively release these mediators and subsequently become activated in response to them. Anti-inflammatory cytokines, such as IL-4 and IL-10, can also be released in response to bacterial challenge (68). Following the innate response to extracellular bacteria, long-lived, adaptive immunity develops, and this is primarily mediated by antibody. The events which mediate the innate response to pathogens can have a profound influence on the quality and intensity of the subsequent adaptive immune response (51). In this regard, little is known concerning the potential impact of innate cytokine release on the subsequent development of the adaptive Ig response to an extracellular bacterium. In this report we demonstrate that a number of proinflammatory cytokines independently regulate, in a positive manner, induction of both protein- and polysaccharide-specific Ig isotypes in response to S. pneumoniae in vivo. In contrast, the effects of the anti-inflammatory cytokines IL-10 and IL-4 on the humoral response to S. pneumoniae appear to be isotype-nonspecific suppression and regulation of Ig isotype switching, respectively. Our detailed studies of one proinflammatory cytokine, TNF-α, typically induced in response to numerous bacterial pathogens, strongly suggest a link between this cytokine released during the early, innate response and the subsequent development of adaptive humoral immunity.
We demonstrate that endogenous TNF-α strongly stimulates the primary anti-PspA response and the development of PspA-specific memory in response to R36A. TNF-α also enhances the anti-PC response, but to a lesser extent. In contrast, the elicitation of the secondary anti-PspA response, which occurs with significantly more rapid kinetics than the primary (83), is not affected by neutralization of endogenous TNF-α at the time of secondary challenge. This suggests that primary and secondary humoral responses have different cytokine requirements, perhaps due to the increased efficiency and strength of the latter. Kinetic studies indicate that the Ig-inducing effects of TNF-α are completed within the first 48 to 72 h after primary R36A immunization. The major source of this TNF-α appears to be splenic non-B and non-T cells, which upregulate TNF-α mRNA by 2 h following R36A immunization, with levels returning to near normal by 6 h and remaining so up to 72 h. Although we did not evaluate other tissue sources of TNF-α, the spleen appears to be a major source of early primary Ig production in response to systemic administration of S. pneumoniae (17). The splenic non-B and non-T cells producing TNF-α may include mast cells, neutrophils, DCs, and macrophages, all of which are activated early in response to extracellular bacteria (47, 63, 69). Lipoteichoic acid (LTA), peptidoglycan, and bacterial DNA, all expressed by S. pneumoniae, are all known inducers of TNF-α (31, 63, 75) at least in part through engagement of distinct Toll-like receptors (2). Thus, early TNF-α synthesis by one or more of these innate immune cells appears necessary for induction of downstream events leading ultimately to optimal primary Ig responses and the development of immunologic memory.
TNF-α exhibits a multitude of proinflammatory effects that could influence the development of an adaptive response. TNF-α promotes DC maturation and antigen presentation, and through induction of chemokines, migration of DCs to sites of pathogen localization (14, 35, 39, 48). TNF-α also costimulates IL-6 and IL-12 synthesis by APC, the latter favoring IFN-γ production (8, 27), all of which we show here to be important for an optimal Ig response to R36A. NK cell production of IFN-γ is also promoted by TNF-α (77). TNF-α may play an additional role in recruiting T cells to immunologic sites, through upregulation of adhesion molecules on endothelial cells (50), as well as directly induce T cell activation (62).
In addition to the role played by TNF-α, we observed a similar, transient R36A-mediated induction of other proinflammatory cytokines in the spleen. Within 2 h, IL-1, IL-6, IL-12, and IFN-γ were also elevated, with levels returning to baseline by 24 h and remaining at baseline up to 72 h. Defects in humoral responses to both soluble antigens and pathogens have previously been demonstrated in some but not all studies using IL-6−/− mice (13, 42, 43, 46, 64, 66). The presence or absence of IL-6 dependence for Ig responses may relate to the nature of the immunogen, the presence or absence of an adjuvant, and/or the route of immunization. These differences may be understood by considering the relative complement dependence of a particular Ig response. Thus, endogenous IL-6 may stimulate humoral responses, primarily through induction of C3 synthesis by local macrophages, as well as through direct induction of B-cell maturation (43). Antigen-bound C3 is important for CD21-dependent B-cell antigen receptor signaling, as well as retention of antigen by follicular DCs (25). Although numerous cells, including DCs, macrophages, T cells, B cells, fibroblasts, and endothelial cells can potentially elaborate IL-6, one study implicated DCs as a primary source of IL-6 during a T-cell-dependent Ig response in vivo (43). In this regard we have observed that DCs obtained from IL-6−/− mice are defective, relative to wild-type DCs, in eliciting in vivo Ig responses when pulsed in vitro with S. pneumoniae and then adoptively transferred into naive mice (18a).
The requirement for IL-12 for Ig induction is observed only when relatively low doses of R36A are used, doses which induce a suboptimal humoral response. The stimulatory effect of IL-12 was Ig isotype nonselective. IL-12 may regulate isotype switching through induction of IFN-γ (40) with enhancements in IgG2a and possibly IgG3, and suppression of IgG1 and IgE (18, 71, 72). However, as is more likely here, IL-12 can also stimulate Ig secretion by postswitched B cells, in an Ig isotype-nonselective and IFN-γ-independent manner, perhaps through direct binding to activated B cells and through inhibition of B1-cell outgrowth (52). B1 cells have been reported to downregulate the function of conventional (B2) B cells (65). In this regard, although anti-PC responses often segregate with B1 cells, IL-12 had no significant effect on anti-PC responses to R36A. This may be due to the ability of conventional B2 cells to also elaborate anti-PC Ig, albeit of the non-T15 idiotype (52).
Although IL-12 plays a major role in stimulating IFN-γ release from T cells and NK cells, we observe a requirement for IFN-γ for maximal Ig responses even at optimal R36A doses, conditions under which the absence of IL-12 has no effect. These data suggest a functional role for IFN-γ that is IL-12 independent, and which has been previously described in studies using other model systems (52). Although IFN-γ is a switch factor for IgG2a and IgG3 and inhibits IgG1 and IgG2b (71, 72), IFN-γ−/− mice immunized with R36A showed reductions in all anti-PspA isotypes. Nevertheless, IgG2a anti-PspA was more strongly reduced than other anti-PspA isotypes, and of the anti-PC isotypes, only IgG2a was significantly reduced in IFN-γ−/− mice relative to controls. The Ig isotype-nonselective, stimulatory effect of IFN-γ on the humoral response to R36A could be due in part to its ability to sensitize DCs and macrophages for enhanced activation in response to microbial activators or to CD40-ligand (29, 74, 78). In this regard, induction of IL-12, IL-18, and TNF-α is reduced in LPS-injected IFN-γ−/− mice relative to controls. In addition, neutralization of endogenous IL-12 or IFN-γ can enhance transforming growth factor β production by DCs (49); transforming growth factor β has been implicated in tolerance induction (80).
In contrast to our observations on the Ig-inductive properties of endogenous proinflammatory cytokines, IL-10−/− mice elicited substantially higher primary and secondary anti-PspA responses and primary anti-PC responses to R36A than those observed for wild-type controls. The suppressive effect of endogenous IL-10 was isotype nonselective. In contrast, IL-4−/− mice, relative to wild-type controls, exhibited an Ig isotype-selective response to R36A, with endogenous IL-4 enhancing IgG1, but suppressing IgG2b and/or IgG2a anti-PspA and anti-PC responses. These data suggest that IL-10 has a broadly suppressive effect on the humoral response to R36A, whereas IL-4 regulates Ig isotype switching.
R36A induces IL-10, within several hours after immunization, from both splenic non-B and non-T cells, as well as B and T cells. B cells, T cells, DCs, macrophages, and NK cells are all potential sources of IL-10 in direct response to constituents expressed by R36A, including peptidoglycan, LTA, bacterial DNA, and even PC (34, 58, 79). IL-10 exhibits a number of immunosuppressive effects that may affect both the innate and the adaptive phases of the immune response to R36A. Early, during the innate phase of the response, IL-10 could inhibit antigen presentation, expression of antigen-presenting and costimulatory molecules, and maturation of DCs (6, 22, 44). In addition, IL-10 may suppress IL-12 synthesis by DCs (54, 76), with resultant inhibition of IFN-γ production, shown here to be important in stimulating the humoral response to R36A. Similarly, IL-10 can suppress proinflammatory cytokine production by macrophages (54). Finally, IL-10 may inhibit the early action of proinflammatory chemokines which in part mediate recruitment of DCs to pathogen-infected tissue sites (21). Indeed, we show here that endogenous IL-10 strikingly downregulates the induction of IL-6, TNF-α, IL-12, and IFN-γ from spleen cells activated with R36A in vitro. In addition to inhibiting cytokines that induce inflammatory chemokines, IL-10 mediates downregulation of chemokine receptors which then become uncoupled and fail to elicit migration. In addition, these frozen receptors trap free chemokines, removing them from the microenvironment. IL-10 may also act later during the adaptive phase of the humoral response to R36A by inhibiting CD28 signaling, through a direct effect on T cells (38). Further, IL-10 can act directly on B cells to stimulate switching to IgG3 (70), but this effect was not observed in response to R36A.
Like IL-10, IL-4 is released both by splenic non-B and non-T cells and by B and T cells within several hours after R36A immunization. Potential sources of this early IL-4 release include mast cells (61) and NK-T cells (4). IL-4 is also released in response to a number of other extracellular and intracellular bacteria (19, 56, 68). Like IL-10, IL-4 may be anti-inflammatory. Thus, IL-4 can inhibit macrophage production of proinflammatory cytokines (24, 56), shown here to stimulate Ig responses to R36A. In contrast, IL-4 can promote DC maturation, thus potentially enhancing antigen presentation (45). Further, IL-4 is known to have a number of stimulatory effects on B cells, including an increase in redistribution of B cells to the spleen and increased B-cell life span in vivo (55, 59), all of which could serve to augment Ig synthesis. Our observation that IL-4−/− mice exhibit reduced IgG1 but enhanced IgG2b and IgG2a responses to R36A relative to wild-type controls suggests that IL-4 is primarily regulating Ig class switching in response to R36A, and not, like IL-10, having a general immunosuppressive effect. IL-4 can directly regulate Ig class switching in B cells (18, 36, 71), as well as indirectly, through downregulation of IL-12 and IL-12 receptor expression by APCs with a resultant reduction in type 1 cytokines, including IFN-γ (32).
Collectively, these data indicate a stimulatory effect of endogenous proinflammatory cytokines on the in vivo humoral response to an extracellular bacteria, whereas endogenous anti-inflammatory cytokines are suppressive, with the exception of IL-4 induction of IgG1. Thus, the strength and quality of the humoral response to R36A is balanced by the concomitant release of a set of cytokines that have opposing actions. The observation that these cytokines have independent, but only partial effects on the R36A-mediated Ig response suggests that they manifest both unique and overlapping functions. These data also support the notion that cytokines classified as type 1 (e.g., IL-12, IFN-γ, and TNF-α) and type 2 (e.g., IL-4 and IL-10) may either inhibit or enhance Ig responses depending upon the particular immunologic milieu associated with distinct pathogens or immunogens. Thus, type 2 cytokines may enhance Ig responses in the setting of allergic hypersensitivity or nematode worm infections but inhibit Ig induction in response to extracellular bacteria. Finally, these data suggest that the relative requirement for endogenous cytokines for induction of protein-specific Ig may be greater than that observed for polysaccharide-specific Ig responses. This may reflect, in part, the greater degree of membrane Ig-mediated activation of B cells that specifically bind multivalent polysaccharides, relative to B cells binding monovalent or paucivalent proteins (53).
FIG. 8.
Role of cytokines in Ig isotype response to S. pneumoniae.
Acknowledgments
We thank Andrew Lees and Luba Grinberg (Biosynexus, Inc., Rockville, Md.) for providing PC-BSA/PC-KLH and recombinant PspA, respectively, and James C. Paton (Adelaide University, Adelaide, SA, Australia) for providing recombinant PsaA.
This work was supported by National Institutes of Health grants 1R01 AI46551 (C.M.S.) and 1R01 AI49192 (C.M.S.).
Editor: E. I. Tuomanen
REFERENCES
- 1.Abrams, J. S., M. G. Roncarolo, H. Yssel. U. Andersson, G. J. Gleich, and J. E. Silver. 1992. Strategies for anticytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol. Rev. 127:5-24. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2000. Toll-like receptors: lessons from knockout mice. Biochem. Soc. Trans. 28:551-556. [DOI] [PubMed] [Google Scholar]
- 3.AlonsoDeVelasco, E., A. F. M. Verheul, J. Verhoef, and H. Snippe. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase, H., N. Arase, K.-I. Nakagawa, R. A. Good, and K. Onoe. 1993. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 23:307-310. [DOI] [PubMed] [Google Scholar]
- 5.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-146. [DOI] [PMC free article] [PubMed]
- 6.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 8.Becher, B., M. Blain, P. S. Giacomini, and J. P. Antel. 1999. Inhibition Th1 polarization by soluble TNF receptor is dependent on antigen-presenting cell-derived IL-12. J. Immunol. 162:684-688. [PubMed] [Google Scholar]
- 9.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles, D. E., C. Forman, J. C. Horowitz, J. E. Volanakis, W. H. J. Benjamin, L. S. McDaniel, J. Eldridge, and J. Brooks. 1989. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect. Immun. 57:1457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles, D. E., C. Forman, and M. Crain. 1992. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect. Immun. 60:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromander, A. K., L. Ekman, M. Kopf, J. G. Nedrud, and N. Y. Lycke. 1996. IL-6-deficient mice exhibit normal mucosal IgA responses to local immunizations and Helicobacter felis infection. J. Immunol. 156:4290-4297. [PubMed] [Google Scholar]
- 14.Brunner, C., J. Seiderer, A. Schlamp, M. Bidlingmaier, A. Eigler, W. Haimerl, H. A. Lehr, A. M. Krieg, G. Hartmann, and S. Endres. 2000. Enhanced dendritic cell maturation by TNF-alpha or cytidine-phosphate-guanosine DNA drives T cell activation. In vitro and therapeutic anti-tumor immune responses in vivo. J. Immunol. 165:6278-6286. [DOI] [PubMed] [Google Scholar]
- 15.Caux, C., C. Massacrier, B. Vanbervliet, C. Barthelmy, Y.-J. Liu, and J. Banchereau. 1994. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int. Immunol. 6:1177-1185. [DOI] [PubMed] [Google Scholar]
- 16.Cherwinski, H. M., J. H. Schumacher, K. D. Brown, and T. R. Mosmann. 1987. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J. Exp. Med. 166:1229-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claflin, J. L., R. Lieberman, and J. M. Davie. 1974. Clonal nature of the immune response to phosphorycholine. I. Specificity, class, and idiotype of phosphorycholine-binding receptors on lymphoid cells. J. Exp. Med. 139:58-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffman, R. L., and J. Carty. 1986. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J. Immunol. 136:949-954. [PubMed] [Google Scholar]
- 18a.Colino, J., Y. Shen, and C. M. Snapper. 2002. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 195:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins, H., I. E. Flesch, M. Emoto, and S. H. Kaufmann. 1998. Interleukin-4 production in response to infection with intracellular bacteria. Adv. Exp. Med. Biol. 452:75-83. [DOI] [PubMed] [Google Scholar]
- 20.Cotran, R. S., V. Kumar, and T. Collins. 1999. Pathologic basis of disease, 6th ed., p. 50-88. W. B. Saunders Co., Philadelphia, Pa.
- 21.D'Amico, G., G. Frascaroli, G. Bianchi, P. Transidico, A. Doni, A. Vecchi, S. Sozzani, P. Allavena, and A. Mantovani. 2000. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat. Immunol. 1:387-391. [DOI] [PubMed] [Google Scholar]
- 22.Ding, L., P. S. Linsley, L. Y. Huang, R. N. Germain, and E. M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 23.Enk, A. H., V. L. Angeloni, M. C. Udey, and S. I. Katz. 1993. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J. Immunol. 151:2390-2398. [PubMed] [Google Scholar]
- 24.Essner, R., K. Rhoades, W. H. McBride, D. L. Morton, and J. S. Economou. 1989. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J. Immunol. 142:3857-3861. [PubMed] [Google Scholar]
- 25.Fearon, D. T., and M. C. Carroll. 2000. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 18:393-422. [DOI] [PubMed] [Google Scholar]
- 26.Fischer, R. T., D. L. Longo, and J. J. Kenny. 1995. A novel phosphocholine antigen protects both normal and X-linked immune deficient mice against Streptococcus pneumoniae. J. Immunol. 154:3373-3382. [PubMed] [Google Scholar]
- 27.Fong, Y., K. J. Tracey, L. L. Moldawer, D. G. Hesse, K. B. Manogue, J. S. Kenney, A. T. Lee, G. C. Kuo, A. C. Allison, S. F. Lowry, and A. Cerami. 1989. Antibodies to cachectin/tumor necrosis factor reduce interleukin1β and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 170:1627-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart, P. H., G. F. Vitti, D. R. Burgess, G. A. Whitty, D. S. Piccoli, and J. A. Hamilton. 1989. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor α, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 86:3803-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Held, T. K., X. Weihua, L. Yuan, D. V. Kalvakolanu, and A. S. Cross. 1999. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 67:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson, B., and M. Wilson. 1996. Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine 8:269-282. [DOI] [PubMed] [Google Scholar]
- 31.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Himmelrich, H., P. Launois, I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Rocken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. [DOI] [PubMed] [Google Scholar]
- 33.Hof, D. G., J. E. Repine, G. S. Giebink, and J. R. Hoidal. 1981. Productions of opsonins that facilitate phagocytosis of Streptococcus pneumoniae by human alveolar macrophages or neutrophils after vaccination with pneumococcal polysaccharide. Am. Rev. Respir. Dis. 124:193-195. [DOI] [PubMed] [Google Scholar]
- 34.Huang, L.-Y., A. M. Krieg, N. Eller, and D. E. Scott. 1999. Induction and regulation of Th1-inducing cytokines by bacterial DNA, lipopolysaccharide, and heat-inactivated bacteria. Infect. Immun. 67:6257-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. Reis e Sousa, R. N. Germain, I. Mellman, and R. M. Steinman. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isakson, P. C., E. Pure, E. S. Vitetta, and P. H. Krammer. 1982. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J. Exp. Med. 155:734-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston, R. B., Jr. 1991. Pathogenesis of pneumococcal pneumonia. Rev. Infect. Dis. 13(Suppl. 6):S509-S517. [DOI] [PubMed] [Google Scholar]
- 38.Joss, A., M. Akdis, A. Faith, K. Blaser, and C. A. Akdis. 2000. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur. J. Immunol. 30:1683-1690. [DOI] [PubMed] [Google Scholar]
- 39.Kimber, I., and M. Cumberbatch. 1992. Stimulation of Langerhans cell migration by tumor necrosis factor alpha (TNF-alpha). J. Investig. Dermatol. 99:48S-50S. [DOI] [PubMed]
- 40.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170:827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339-342. [DOI] [PubMed] [Google Scholar]
- 43.Kopf, M., S. Herren, M. V. Wiles, M. B. Pepys, and M. H. Kosco-Vilbois. 1998. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J. Exp. Med. 188:1895-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koppelman, B., J. J. Neefjes, J. E. de Vries, and R. de Waal Malefyt. 1997. Interleukin-10 down-regulates MHC class II ab peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7:861-871. [DOI] [PubMed] [Google Scholar]
- 45.Labeur, M. S., B. Roters, B. Pers, A. Mehling, T. A. Luger, T. Schwarz, and S. Grabbe. 1999. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J. Immunol. 162:168-175. [PubMed] [Google Scholar]
- 46.Ladel, C. H., C. Blum. A. Dreher, K. Reifenburg, M. Kopf, and S. H. E. Kaufmann. 1997. Lethal tuberculosis in interleukin-6 deficient mice. Infect. Immun. 65:4843-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapinet, J. A., P. Scapini, F. Calzetti, O. Perez, and M. A. Cassatella. 2000. Gene expression and production of tumor necrosis factor alpha, interleukin-1β (IL-1β), IL-8, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect. Immun. 68:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyakh, L. A., G. K. Koski, W. Telford, R. E. Gress, P. A. Cohen, and N. R. Rice. 2000. Bacterial lipopolysaccharide, TNF-alpha, and calcium ionophore under serum-free conditions promote rapid dendritic cell-like differentiation in CD14+ monocytes through distinct pathways that activate NF-kappaB. J. Immunol. 165:3647-3655. [DOI] [PubMed] [Google Scholar]
- 49.Marth, T., W. Strober, R. A. Seder, and B. L. Kelsall. 1997. Regulation of transforming growth factor-β production by interleukin-12. Eur. J. Immunol. 27:1213-1220. [DOI] [PubMed] [Google Scholar]
- 50.McHale, J. F., O. A. Harari, D. Marshall, and D. O. Haskard. 1999. TNF-alpha and IL-1 sequentially induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone mice. J. Immunol. 163:3993-4000. [PubMed] [Google Scholar]
- 51.Medzhitow, R., and C. A. J. Janeway. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 52.Metzger, D. W., J. M. Buchanan, J. T. Collins, T. L. Lester, K. S. Murray, V. H. V. Cleave, L. A. Vogel, and W. A. Dunnick. 1996. Enhancement of humoral immunity by interleukin-12. Ann. N. Y. Acad. Sci. 795:100-115. [DOI] [PubMed] [Google Scholar]
- 53.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 54.Moore, K. W., A. O'Garra, R. D. W. Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 55.Mori, M., S. C. Morris, T. Orekhova, M. Marinaro, E. Giannini, and F. D. Finkelman. 2000. IL-4 promotes the migration of circulating B cells to the spleen and increases splenic B cell survival. J. Immunol. 164:5704-5712. [DOI] [PubMed] [Google Scholar]
- 56.Newton, C., S. McHugh, R. Widen, N. Nakachi, T. Klein, and H. Friedman. 2000. Induction of interleukin-4 (IL-4) by Legionella pneumophila infection in BALB/c mice and regulation of tumor necrosis factor alpha, IL-6, and IL-1β. Infect. Immun. 68:5234-5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palanivel, V., C. Posey, A. Horauf, W. Solbach, W. F. Piessens, and D. A. Harn. 1996. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp. Parasitol. 84:168-177. [DOI] [PubMed] [Google Scholar]
- 59.Paul, W. E. 1987. Interleukin 4/B cell stimulatory factor 1: one lymphokine, many functions. FASEB J. 1:456-461. [DOI] [PubMed] [Google Scholar]
- 60.Pilling, P. A., M. C. Lawrence, A. M. Berry, A. D. Ogunniyi, R. A. Lock, and J. C. Paton. 1998. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of Streptococcus pneumoniae. Acta Crystallogr. Sect. D Biol. Crystallogr. 54:1464-1466. [DOI] [PubMed] [Google Scholar]
- 61.Plaut, M., J. H. Pierce, C. Watson, J. Hanley-Hyde, R. P. Nordan, and W. E. Paul. 1989. Mast cell lines produce lymphokines in response to cross-linkage of FcɛRI or to calcium ionophores. Nature 339:64-67. [DOI] [PubMed] [Google Scholar]
- 62.Prehn, J. L., C. J. Landers, and S. R. Targan. 1999. A soluble factor produced by lamina propria mononuclear cells is required for TNF-α enhancement of IFN-γ production by T cells. J. Immunol. 163:4277-4283. [PubMed] [Google Scholar]
- 63.Ramachandra, L., R. S. Chu, D. Askew, E. H. Noss, D. H. Canaday, N. S. Potter, A. Johnsen, A. M. Krieg, J. G. Nedrud, W. H. Boom, and C. V. Harding. 1999. Phagocytic antigen processing and effects of microbial products on antigen processing and T-cell responses. Immunol. Rev. 268:217-239. [DOI] [PubMed] [Google Scholar]
- 64.Ramsay, A. J., A. J. Gusband, I. A. Ramshaw, S. Bao, K. I. Matthaei, G. Koehler, and M. Kopf. 1994. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 264:561-563. [DOI] [PubMed] [Google Scholar]
- 65.Riggs, J. E., R. S. Stowers, and D. E. Mosier. 1990. The immunoglobulin allotype contributed by peritoneal cavity B cells dominates in SCID mice reconstituted with allotype-disparate mixtures of splenic and peritoneal cavity B cells. J. Exp. Med. 172:475-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romani, L., A. Menacci, E. Cenci, R. Spaccapelo, C. Toniatti, P. Puccetti, F. Bistoni, and V. Poli. 1996. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 68.Sasaki, S., S. Nishikawa, T. Miura, M. Mizuki, K. Yamada, H. Madarame, Y. I. Tagawa, Y. Iwakura, and A. Nakane. 2000. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect. Immun. 68:2424-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segura, M., J. Stankova, and M. Gottschalk. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 67:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shparago, N., P. Zelazowski, L. Jin, T. M. McIntyre, E. Stuber, L. M. Pecanha, M. R. Kehry, J. J. Mond, E. E. Max, and C. M. Snapper. 1996. IL-10 selectively regulates murine Ig isotype switching. Int. Immunol. 8:781-790. [DOI] [PubMed] [Google Scholar]
- 71.Snapper, C. M., and W. E. Paul. 1987. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 72.Snapper, C. M., T. M. McIntyre, R. Mandler, L. M. T. Pȩcanha, F. D. Finkelman, A. Lees, and J. J. Mond. 1992. Induction of IgG3 secretion by interferon γ: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 175:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snapper, C. M., P. Zelazowski, F. R. Rosas, M. R. Kehry, M. Tian, D. Baltimore, and W. C. Sha. 1996. B cells from p50/NF-κB knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 156:183-191. [PubMed] [Google Scholar]
- 74.Snijders, A., P. Kalinski, C. M. U. Hilkens, and M. L. Kapsenberg. 1998. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10:1593-1598. [DOI] [PubMed] [Google Scholar]
- 75.Timmerman, C. P., E. Mattsson, L. Martinez-Martinez, L. De Graaf, J. A. G. Van Strijp, H. A. Verbrugh, J. Verhoef, and A. Fleer. 1993. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect. Immun. 61:4167-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 77.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listerosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuji, H., N. Mukaida, A. Harada, S. Kaneko, E. Matsushita, Y. Nakanuma, H. Tsutsui, H. Okamura, K. Nakanishi, Y. Tagawa, Y. Iwakura, K. Kobayashi, and K. Matsushima. 1999. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-γ-deficient mice by a concomitant reduction of TNF-α, IL-12, and IL-18 production. J. Immunol. 162:1049-1055. [PubMed] [Google Scholar]
- 79.Wang, J. E., P. F. Jorgensen, M. Almlof, C. Thiemermann, S. J. Foster, A. O. Aasen, and R. Solberg. 2000. Peptidoglycan and lipoteichoic acid from staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect. Immun. 68:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiner, H. L., A. Friedman, A. Miller, S. J. Khoury, A. Al-Sabbagh, L. Santos, M. Sayegh, R. B. Nussenblatt, D. E. Trentham, D. A. Hafler. 1994. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu. Rev. Immunol. 12:809-837. [DOI] [PubMed] [Google Scholar]
- 81.Wortham, C., L. Grinberg, D. C. Kaslow, D. E. Briles, L. S. McDaniel, A. Lees, M. Flora, C. M. Snapper, and J. J. Mond. 1998. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect. Immun. 66:1513-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu, Z. Q., Q. Vos, Y. Shen, A. Lees, S. R. Wilson, D. E. Briles, W. C. Gause, J. J. Mond, and C. M. Snapper. 1999. In vivo polysaccharide-specific IgG isotype responses to intact Streptococcus pneumoniae are T cell dependent and require CD40- and B7-ligand interactions. J. Immunol. 163:659-667. [PubMed] [Google Scholar]
- 83.Wu, Z. Q., A. Q. Khan, Y. Shen, J. Schartman, R. Peach, A. Lees, J. J. Mond, W. C. Gause, and C. M. Snapper. 2000. B7 requirements for primary and secondary protein- and polysaccharide-specific Ig isotype responses to Streptococcus pneumoniae. J. Immunol. 165:6840-6848. [DOI] [PubMed] [Google Scholar]