Abstract
Alveolar echinococcosis (AE) in humans is a chronic disease characterized by slowly expanding liver lesions. Cellular immunity restricts the spreading of the extracellular pathogen, but functional contributions of CD4+ and CD8+ T cells are not defined. Here we studied ex vivo the phenotype and function of circulating T-cell subsets in AE patients by means of flow cytometry, T-cell receptor spectratyping, and lymphocyte proliferation. AE patients with parasitic lesions displayed a significant increase of activation of predominantly CD8+ T cells compared to healthy controls and AE patients without lesions. In vitro, proliferative T-cell responses to polyclonal stimulation with recall antigens and Echinococcus multilocularis vesicular fluid antigen were sustained during chronic persisting infection in all AE patients. Only in AE patients with parasitic lesions did T-cell receptor spectratyping reveal increased oligoclonality of CD8+ but not CD4+ T cells, suggesting a persistent antigenic drive for CD8+ T cells with subsequent proliferation of selected clonotypes. Thus, our data provide strong evidence for an active role of CD8+ T cells in AE.
Alveolar ecchinococcosis (AE) in humans is an aggressive chronic parasitic infection caused by the larval stage of the cestode Echinococcus multilocularis. The clinical manifestation is characterized by parasitic liver lesions with tumor-like growth, hardly inhibited by antihelminthic drugs (38). There is accumulating evidence that the presence of a specific antiparasitic cellular immune response restricts the growth of the parasite, as an accelerated course of the disease was demonstrated in T-cell-deficient mice and in cellularly immunocompromised patients (34, 42). Further evidence comes from human and rodent studies of protective immune mechanisms against larval cestodes (reviewed in reference 45) that, for E. multilocularis infection, might involve resistance to early postoncospheral development after primary infection or resistance to the proliferating germinative compartment.
In general, protective immunity against infection with an extracellular pathogen is considered to be essentially cell mediated, dependent on the interaction of macrophages with T lymphocytes (21, 37). However, the relative contributions of CD8+ and CD4+ T-cell subpopulations have not yet been clearly determined. Antigen-specific CD4+ T cells play an important role in IFN-γ-mediated monocyte-macrophage activation (4, 5). Specific cellular responses in AE were previously demonstrated by in vitro proliferation (8, 16). The role of CD8+ T cells in protective immunity against extracellular pathogens is less clear. However, the liver lesion in most patients with AE harbors an infiltrate composed mainly of activated CD8+ T cells (7). Recently, our group provided evidence for the presence of circulating antigen-specific effector CD8+ T cells in AE patients (23). In an effort to substantiate these data, we now studied the phenotype of peripheral T-cell subsets in patients chronically infected with E. multilocularis and otherwise free of overt diseases in comparison with that in age-matched healthy controls. CD4+ and CD8+ cells were analyzed for activated subpopulations by four-color fluorescence-activated cell sorter (FACS) analysis.
Moreover, in an attempt to further characterize the immune response during a chronic persisting infection, we performed T-cell receptor analysis and “spectratyping” for assessment of the clonality of CD4+ and CD8+ T-cell populations. Increased clonality is a marker for ongoing or previous vigorous immune responses (reviewed in detail in references 14 and 33). Based on this technique, we developed an algorithm to express the age-independent clonality of CD8+ T-cell populations.
Since general or antigen-specific hyporesponsiveness of effector T cells has been hypothesized to account for persistence of chronic infections, we tested the functional capacity of peripheral blood mononuclear cells (PBMC) to respond to antigenic stimuli (i.e., E. multilocularis vesicular fluid antigen and recall antigens) and polyclonal stimulation (i.e., phytohemagglutinin [PHA] and superantigens).
MATERIALS AND METHODS
Study participants, diagnostic routines, and criteria.
The study population was composed of 26 patients with AE, with a mean age of 53.4 years (range, 22 to 82 years). Diagnosis of AE was based either on histology, if applicable, or on a typical morphological appearance of lesions by ultrasound (US) imaging and computerized tomography (CT) or magnetic resonance imaging in conjunction with a serological marker. All patients had at least one well-defined hepatic lesion educible by US and CT. For assessment of progressive disease, imaging was repeated once every year for the last 2 years before entry into the study. In addition, 14F-deoxyglucose positron emission tomography was used to assess glucose utilization of liver lesions as described previously (39).
For immunodiagnosis a commercially available Em2+ enzyme-linked immunosorbent assay (Bordier Affinity Products SA, Crissier, Switzerland) with a high diagnostic sensitivity of 97% for the detection of E. multilocularis metacestode infection was used (15).
AE patients were classified into two groups, referred to as “progressive AE” and “nonprogressive AE” according to the level of evidence for parasite activity as assessed by changes of the parasitic mass, extent of calcification, and activity in a positron emission tomography scan (39). In addition, four patients (mean age, 51 years; range, 29 to 80 years) who had been diagnosed with AE previously and who had remained free of detectable parasitic masses for at least 3 years after complete surgical removal of liver lesions were recruited for the study. The control group consisted of 30 age-matched healthy individuals (mean age, 52.9 years; range, 22 to 82 years) without any history of chronic diseases. An overview of the study populations is given in Table 1. For the comparison of the clonal contingent within peripheral CD8+ T cells, a disease control group consisting of 28 patients with chronic active hepatitis C was formed. Informed consent was obtained from each patient and control subject. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki as reflected in approval by the local ethics committee.
TABLE 1.
Clinical characteristics of the three patient groups
| AE group | n | Gender (m/f) | Mean age (range) (yr) | Mean time after diagnosis (SE) (yr) |
|---|---|---|---|---|
| Progressive | 18 | 9/9 | 52.5 (22-82) | 8.0 (2.1) |
| Nonprogressive | 8 | 2/6 | 55.5 (34-64) | 5.9 (1.2) |
| Cured | 4 | 3/1 | 51.0 (29-80) | 4.1 (1.6) |
Isolation of lymphocytes and cell separation.
PBMC from every subject were separated by Ficoll-Hypaque (Pharmacia, Freiburg, Germany) density gradient centrifugation. CD4+ and CD8+ T cells were positively selected from PBMC by anti-CD4 or anti-CD8 antibody-coated paramagnetic beads (Dynal, Hamburg, Germany) according to the manufacturer's instructions. Bead-coated CD4+ and CD8+ cells were washed three times in phosphate-buffered saline (PBS)-0.5% bovine serum albumin prior to RNA isolation.
Flow cytometry analysis.
T-cell populations were quantified by four-color FACS analysis with a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany) using a panel of combinations of up to five monoclonal antibodies (BD Biosciences, Heidelberg, Germany). The major lymphocyte subsets were counted using antibodies to CD3 (clone SK7; fluorescein isothiocyanate [FITC] or peridinin-chlorophyll protein [PerCP] conjugated), CD4 (clone SK3; FITC conjugated), CD8 (clone SK1; FITC, phycoerythrin [PE], or allophycocyanin [APC] conjugated), CD45RA (clone L48; FITC conjugated), CD45RO (clone UCHL-1; PE conjugated), CD19 (clone SJ25C1; APC conjugated), T-cell receptor [TCR] γ/δ (clone 11F2; PE conjugated), CD16 plus CD56 (clones B73.1 and NCAM16.2; PE conjugated). Activation and differentiation markers on CD4+ and CD8+ T cells were measured with antibodies to CD25 (clone 2A3; FITC conjugated), CD28 (clone L293; PE conjugated), CD38 (clone HB7; PE conjugated), HLA-DR (clone L243; APC conjugated), and CD62L (clone SK11; PE conjugated). Isotypic immunoglobulin G1 (IgG1) and IgG2 served as background control. Fifty microliters of EDTA-anticoagulated blood was incubated with the appropriate mixture of antibodies for 15 min at room temperature. Following incubation for 10 min with 2 ml of FACS lysis solution cells were washed twice with PBS-0.3% bovine serum albumin and fixed with PBS-1% paraformaldehyde. At least 5,000 cells were analyzed on the flow cytometer after single gating on lymphoid cells for all monoclonal antibody combinations. CD4+ and CD8+ T-cell subsets were consecutively gated with a lymphocyte gate in a side scatter/forward scatter plot and a CD3+ gate. Subset analysis was performed using CellQuest software (Becton Dickinson).
Proliferation assays.
PBMC were adjusted to 106 cells/ml in RPMI 1640 medium (Life Technologies GmbH, Karlsruhe, Germany) supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin (Life Technologies GmbH)/ml, and 10% heat-inactivated fetal calf serum (Seromed, Biochrom KG, Berlin, Germany). Cells were stimulated with recall antigens tetanus toxoid (TT; Calbiochem, Bad Soden, Germany; 10 μg/ml) and protein purified derivative (PPD; Chiron Behring, Liederbach, Germany; 10 μg/ml). Control wells contained responder PBMC with medium alone. Polyclonal stimulation was also performed with phytohemagglutinin (PHA) (Sigma, Deisenhofen, Germany; 10 μg/ml) and superantigens staphylococcal enterotoxin A (SEA) and toxic shock syndrome toxin 1 (TSST-1) (Sigma-Aldrich, Deisenhofen, Germany) at 1 ng/ml for 5 days. Antigen-specific stimulation was performed with E. multilocularis vesicular fluid at 20 μg/ml for 5 days. The antigen preparation was obtained by aspiration of fluid from in vitro-cultivated E. multilocularis vesicular cysts (19) (kindly provided by B. Gottstein). PBMC (105) were cultured in round-bottom plates for 5 days as described above with and without antigenic stimulus and then pulsed with 0.5 μCi of [3H]thymidine/well and harvested after 18 h for processing according to standard liquid scintigraphy. Results are expressed in stimulation index (SI) units, which represent the mean proliferation in counts per minute in response to a given stimulus divided by the mean background proliferation. For polyclonal stimulation SIs >10 were considered positive.
TCRBV analysis.
The experimental strategy of quantitative analysis of rearranged TCR β-chain sequences (TCRBV) and CDR3 length imaging (spectratyping) was carried out as described previously (27). In brief, total RNA was extracted directly from 105 to 106 bead-coated cells by the guanidinium thiocyanate-phenol-chloroform method using RNAzol B (Biotecx Laboratories, Houston, Tex.) or TriStar (AGS, Heidelberg, Germany). First-strand cDNA was copied from 1 μg of total RNA with Moloney murine leukemia virus reverse transcriptase (PE Biosystems, Weiterstadt, Germany) following oligo(dT) priming. For estimation of the overall amount of completely rearranged TCR sequences in a given sample, competitive PCR with an exogenous semihomologous DNA competitor was performed. For CDR3 length imaging 26 aliquots of 0.2 to 1.0 μl of cDNA were subjected to 26 to 29 cycles of PCR coamplification with an exogenous standard and incorporation of a fluorescence-labeled constant-region primer. Three-microliter aliquots of the fluorescence-labeled PCR products were heat denatured in 50% formamide and loaded onto a 6% denaturing polyacrylamide gel. Electrophoresis was performed in a DNA sequencer (model 373A; PE Biosystems) at constant current. Quantitative determination of DNA fragments was performed by measurement of laser light-induced fluorescence. Typically, each of the 24 BV-specific amplicons contains transcripts of 7 to 10 different lengths. For data analysis, the GeneScan software (PE Biosystems) was used. The fluorescence intensity of an amplicon is expressed as the area under the curve (AUC) in relative fluorescence units. The sensitivity of clonal detection was tested by serial dilution of Jurkat cells in peripheral blood lymphocytes. The detection limit determined for the TCRBV amplification ranged between 0.5 and 0.1% clonal contingent (1 in 500 to 1,000 cells). The relative expression of sequences of one TCRBV-specific amplification in a T-cell population was calculated by normalization with the semihomologous standard. For assessment of the clonal contingent an algorithm similar to that described earlier by Gorochov and coworkers (14) was used. CDR3 lengths were regarded as relevant expansion if the AUC (in percent) exceeded the corresponding AUC from a reference panel by 2.5 standard deviations. This reference panel of CDR3 length profiles was derived from unstimulated peripheral CD4+ T cells from a mean of 14 (range, 12 to 16) healthy donors. CDR3 length profiles of each of the 22 TCRBV families with functional sequences were generated under semiquantitative PCR conditions as outlined above. The lengths of CDR3 regions were defined by the criteria described by Moss and Bell (29, 30). The mean CDR3 length for the TCRBV families ranged from 9 to 14 amino acids. For each subject, 150 to 220 individual CDR3 lengths were characterized by probability distributions.
Clonality of peripheral CD8+ T cells is known to increase with age (20, 36). Therefore, we analyzed the age dependency of clonality in peripheral CD8+ T cells in a cohort of 37 healthy individuals (age range, 19 to 82 years). We observed a linear correlation (correlation coefficient = 0.57; P = 0.0002; R2 = 0.33) between age and clonality. The excess clonal contingent was introduced for correction of age dependency and was defined as the clonal contingent of a given T-cell population exceeding the mean clonality observed in age-matched healthy subjects. A linear regression algorithm was used: EC = Cobs − Cexp and Cexp = 0.21 × age − 3.86, where EC is the excess clonal contingent, C is the clonal contingent, Cobs is the observed clonal contingent, and Cexp is the expected clonal contingent.
Sequence analysis of TCRBV transcripts.
For cloning of PCR products amplification of the same cDNA was performed with a nested constant-region primer (TCRBCcl; 5′-ATCTCTGCTTCTGATGGCTCA) and the same TCRBV sequence-specific primer under conditions outlined above. PCR products were separated on a 1.5% agarose gel. Bands of the expected sizes were excised, and DNA was eluted using a DNA purification device (Qiaquick; Qiagen, Hilden, Germany). The purified fragments were blunt ended with Klenow DNA polymerase and phosphorylated with T4 polynucleotide kinase (Boehringer GmbH, Mannheim, Germany) and subsequently ligated into an EcoRV-linearized Bluescript SK(−) vector (Stratagene, Heidelberg, Germany). Escherichia coli XLBlue cells were transformed and grown in Luria-Bertani agar plates. Selected colonies were grown overnight, and the plasmid DNA was purified and sequenced by the Taq cycle dye terminator reaction according to the protocol of the manufacturer (PE Biosystems).
Statistical analysis.
The Mann-Whitney U test and the standard chi-square test were used to compare groups with regard to continuous and discrete variables, respectively. Differences with a P value <0.05 were considered significant.
RESULTS
T-cell subsets and activation markers in AE.
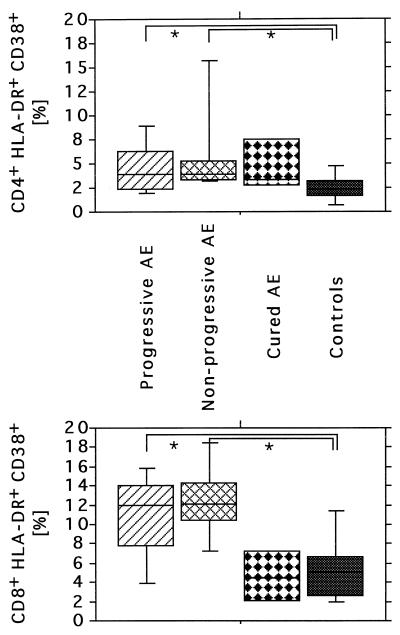
Analyzing the surface expression of multiple phenotypic markers allowed us to quantify activated T-cell subsets within both circulating CD4+ and CD8+ T-cell populations. Most notably, AE patients harbored a significantly higher number of HLA-DR+ peripheral CD8+ T cells than age-matched controls (18.8 versus 11.2%; P = 0.009) (Table 2). This difference was even more pronounced for T cells coexpressing both activation markers, HLA-DR and CD38 (11.5 versus 5.5%; P < 0.001). Concordantly, significantly greater numbers of HLA-DR+ CD4+ T cells were found in AE patients than in healthy controls (9.9 versus 5.6%; P = 0.001) (Fig. 1). No significant shift of the relative proportion of T cells, B cells, and NK cells was detectable in AE patients. Furthermore, no significant differences in the CD4+/CD8+ T-cell ratio, the CD45RA+/CD45RO+ ratio, and relative numbers of γ/δ T cells among study groups were found. Data are summarized in Table 2.
TABLE 2.
Summary of peripheral lymphocyte subpopulations in AE patients and age-matched control subjects as determined by four-color FACS analysis
| Group | Mean ratio (SEM) of:
|
Mean % (SEM) of cell typea:
|
||||||
|---|---|---|---|---|---|---|---|---|
| CD4/CD8 | CD45RA+/CD45RO+ (CD4+) | CD45RA+/CD45RO+ (CD8+) | CD4+ HLA-DR+ | CD8+ HLA-DR+ | CD4+ CD28+ | CD8+ CD28+ | NK | |
| Progressive AE | 2.33 (0.26) | 0.59 (0.13) | 1.24 (0.21) | 9.34 (1.53)∗ | 17.03 (3.84)∗ | 91.6 (3.49) | 68.73 (3.87) | 11.41 (2.83) |
| Nonprogressive AE | 2.16 (0.46) | 0.5 (0.16) | 2.02 (0.58) | 10.95 (2.91)∗ | 22.27 (3.84)∗ | 73.46 (15.13) | 69.87 (11.86) | 7.75 (1.66) |
| Cured AE | 2.10 (0.35) | 0.65 (0.27) | 1.24 (0.46) | 5.63 (0.86)∗ | 8.67 (0.15) | 97.80 (1.23) | 73.32 (3.59) | 6.58 (2.42) |
| Controls | 1.90 (0.19) | 0.72 (0.13) | 2.30 (0.66) | 5.71 (1.33) | 11.49 (2.22) | 97.89 (1.23) | 73.07 (4.05) | 9.99 (1.00) |
∗, P < 0.05.
FIG. 1.
Comparison of activated CD4+ (A) and CD8+ (B) T-cell subsets. Coexpression of activation and differentiation marker CD38 with HLA-DR on peripheral CD8+ T cells was used as activation marker. Whole blood from 30 AE patients at various disease stages and 30 age-matched controls was analyzed by four- color cytometry by staining with anti-CD3, anti-CD8, anti-CD38, and anti-HLA-DR antibodies. The activation marker expression was calculated as a percentage of the gated CD3+ CD8+ T cells. Results are given as box plots indicating the median and the 10th, 25th, 75th, and 90th percentiles within each subject group. ∗, P ≤ 0.05.
Clonal composition within the peripheral CD4+ and CD8+ T-cell subsets.
Acute or chronic activation of CD8+ T cells as demonstrated by the increased levels of activation markers is expected to result in clonal expansion of antigen-specific cells. Therefore, we monitored changes of the TCR repertoire of isolated CD8+ and CD4+ T cells in the patients with AE. Our attention focused on profound expansions within the CD8+ populations, exceeding the corresponding AUC from a reference panel by 2.5 standard deviations.
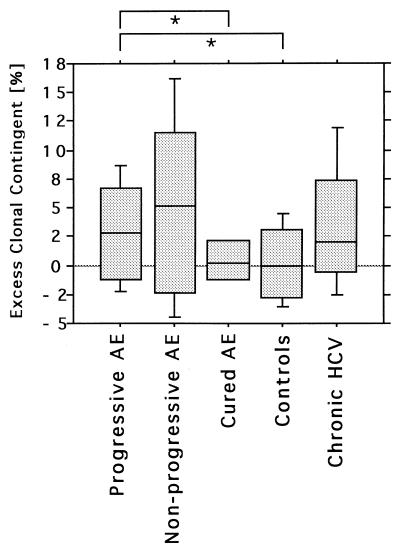
Statistical comparison of subject groups was performed after correction for the age-dependent expansion of clonality of peripheral CD8+ T cells, referred to as the excess clonal contingent. AE patients displayed a significantly higher clonal contingent of CD8+ T cells than the age-matched controls. A significant excess clonal contingent was detectable for progressive AE (P = 0.04). Nonprogressive AE was associated with an increased, but not significantly, excess clonal contingent compared to that of age-matched controls (P = 0.1). In contrast, no excess clonal contingent was detectable in the four cured patients without parasitic lesions (Fig. 2).
FIG. 2.
The excess clonal contingent of circulating CD8+ T cells was calculated for each study subject. AE patients with progressive disease display a significantly higher clonal contingent than age-matched healthy controls and cured AE patients without parasitic lesions (∗, P > 0.05). This excess clonality compares well to that in patients with chronic hepatitis C, a situation in which chronic antigenic challenge is also limited to the liver compartment.
For a better understanding of this phenomenon we performed the same analysis on patients with chronic hepatitis due to hepatitis C virus (HCV) infection, in which the infectious agent is also limited to the liver and a chronic antigen presentation during several years might challenge the same lymphocyte compartment. In contrast to the infection with extracellular pathogen E. multilocularis, HCV infection has a well-established route of activation of antigen-specific CD8+ T cells. Similar levels of excess clonal contingent were found in HCV and AE patients with progressive disease (Fig. 2). In contrast to the data for CD8+ T-cell subsets, clonal expansions in CD4+ T cells were rare in AE patients and were not increased in comparison to that for the age-matched control cohort (data not shown).
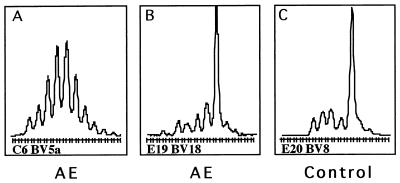
We analyzed expanded populations by means of cloning and sequencing in order to define the extent of clonality. Five selected TCRBV populations with expanded CDR3 sizes were analyzed in detail. All expansions revealed a dominant sequence with a frequency between 60 and 100% of all sequences with the same CDR3 length. As an example, the expanded BV18 sequences from patient E19 revealed a single dominant clone in 12 out of 25 sequences (48%) (Fig. 3B). The BV8 expansion in patient E20 was oligoclonal, revealing a dominant sequence in 9 out of 24 sequences (37.5%), and 3 other sequences were each detected three times (12.5%) (Fig. 3C). In contrast, all sequences derived from a nonskewed spectratype of a control individual, using the same primers and PCR conditions, were unique.
FIG. 3.
CDR3 length distribution. (A) The CDR3 length distributions of unskewed PBMC populations indicate a Gauss-like size distribution for the CDR3 region of TCRBV transcripts. Profound clonal or oligoclonal expansions of CD8+ T cells are markers of acute or previous vigorous immune responses or aging. The clonal contingent of a T-cell population can be assessed by the spectratyping method. (B and C) CDR3 size image showing an example of an oligoclonal expansion (B) and a monoclonal expansion (C), as determined by cloning and sequencing.
The phenotypes of expansions.
The phenotypes of 15 oligo- or monoclonally expanded CD8+ T-cell populations from three AE patients and three healthy controls were studied in detail by FACS analysis using TCRBV-specific antibodies. All TCRBV-gated subpopulations from either subject group harboring a clonal expansion as determined by spectratyping revealed an increased proportion of CD8+ CD45RO+ cells in comparison to the corresponding total cD8+ cell populations, but HLA-DR- and CD38 expressions were not elevated. Moreover, a significant inverse correlation between CD28 expression and the excess clonal contingent in AE patients was observed (P < 0.005). The phenotypes of these expansions are in agreement with previous reports of HLA-DR− HLA38− CD28− expansions of CD8+ T cells appearing with aging in healthy individuals (20, 28, 36).
Influence of clinical parameters on immunological alterations.
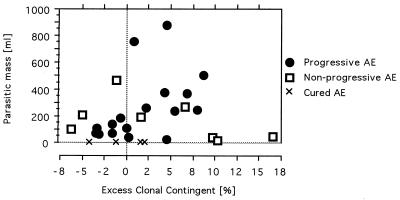
A summary of the combined group data comparing AE patient groups and controls is shown in Table 3. In view of the chronic infection and the relatively high median age of the patients, we wondered whether immunological alterations were related to the helminth infection per se or to age or gender. The excess clonal contingent correlated positively with the parasitic mass (P = 0.03) (Fig. 4). Accordingly, all four cured AE patients had clonal contingents of peripheral CD8+ T cells markedly lower than those of AE patients with parasitic masses (progressive and nonprogressive), although the stratum size was not sufficient to prove statistical significance. There was no statistically significant difference between progressive and nonprogressive patient groups with respect to age, gender, duration of the disease after the initial diagnosis, and localization and volume of the parasitic mass.
TABLE 3.
Prevalence of oligoclonality in peripheral CD8+ T cells of AE patients and controlsa
| Group | n | Mean clonal composition (SE [%]) | Mean excess clonal contingent (SE [%]) | Parasitic volume (ml) |
|---|---|---|---|---|
| Patients | ||||
| Progressive AE | 18 | 8.4 (1.3) | 1.5 (1.0) | 248 (58) |
| Nonprogressive AE | 8 | 13.1 (4.7) | 5.9 (4.1) | 166 (54) |
| Cured AE | 4 | 6.0 (1.5) | 0.3 (1.5) | 0 |
| Controls | 30 | 6.4 (0.9) | 0 (0.7) |
CD8+ T cells were positively selected from PBMC and subjected to TCRBV analysis and CDR3 length analysis. CDR3 lengths were regarded as relevant expansion if the AUC exceeded the corresponding AUC from a reference panel of unstimulated peripheral CD4+ T cells by 2.5 standard deviations. The level of clonality is expressed as excess clonal contingent to account for age dependence.
FIG. 4.
The extent of the parasitic mass in the liver displays a correlation with the clonality of peripheral CD8+ T cells in patients with progressive AE but not in patients with nonprogressive AE. The four subjects with previous AE that was cured after complete resection of the parasite display low levels of CD8+ T-cell clonality (asterisks).
Proliferative responses.
To assess the capability of peripheral T cells of AE patients for proliferative responses, we performed in vitro stimulation of PBMC with an E. multilocularis vesicular fluid antigen preparation, recall antigens, superantigens, and PHA. A marked proliferative response (SI > 10) to E. multilocularis vesicular fluid antigen was detected in 64% of AE patients and in 3% of the controls. Most notably, no significant difference among SIs for progressive (mean SI = 16.1), nonprogressive (mean SI = 19.4), and cured AE patients (mean SI = 15.5) was found. Compared to those from healthy controls, PBMC from AE patients from all three groups showed similar proliferative responses to mitogen PHA and superantigens SEA and TSST-1. In addition, the proliferative responses to recall antigens TT and PPD were not different among subject groups (data not shown).
DISCUSSION
AE in humans is a chronic disease characterized by a slowly expanding larval mass with tumor-like growth in the liver. Several observations provide evidence for an active role of the host's cellular immune system in control of parasite growth and spreading, although the role of individual cell subsets and effector mechanisms in this host defense is unknown. First, AE in immunocompromised hosts is associated with a greatly enhanced disease (42). Second, rodents serving as an adequate animal model for human AE display strain-dependent resistance and susceptibility to metacestode development (1, 31). A perilesional inflammation appeared to control larval mass growth in C.B-17 mice (35). Increased susceptibility to E. multilocularis was reported in SCID mice, which lack functional T and B lymphocytes (34).
While the immune response against primary infections with intracellular and extracellular pathogens has been studied extensively with the help of animal models, the mechanisms by which chronic persisting infections in humans are controlled by the cellular immune system are less well understood.
Here, we demonstrate two major findings for the immune response in a chronic persisting helminth infection. First, elevated numbers of activated CD8+ T cells were found in the peripheral blood of patients with chronic AE. Second, an increased clonality of CD8+ T cells is associated with the disease. What is the significance of an increased number of activated peripheral T cells in a chronic infection of the liver? CD8+ T lymphocytes have been found to be the predominant population of T cells in the livers of most AE patients (7). One can argue that the T-lymphocyte population in peripheral blood might not reflect the intrahepatic population. However, considering that the adult liver is an organ without constitutive lymphoid components, any intrahepatic T cell found in AE should have migrated into the liver after infection and might also reappear in the circulation. Most likely, the activated CD8+ T cells are the in vivo correlate of a dominant response of CD8+ T cells after in vitro stimulation of peripheral lymphocytes with E. multilocularis vesicular fluid antigen, as previously reported by our group (23). Increased activation of peripheral CD8+ as determined by HLA-DR and CD38 coexpression, was associated with the presence of a parasitic lesion. The fact that the four cured AE patients (i.e., complete surgical resection of parasite) had normal levels of activated CD8+ T cells within the range of healthy control subjects provides further strong evidence for the interpretation that activation of CD8+ T cells is indeed triggered by the parasite. Nevertheless, the activation of CD8+ T cells might be surprising in the setting of an extracellular antigen. In general, it is believed that proteinaceous antigens added exogenously elicit primarily CD4+ T-cell responses, which are major histocompatibility complex class II (MHC-II) restricted. However, recent reports have demonstrated cross-priming of CD8+ T cells in infectious diseases (11). APC can acquire exogenous antigens by phagocytosis and present them to CD8+ T cells in the context of MHC-I molecules (17, 40, 41). Some of these reports have proposed the existence of a subset of APC located throughout the lymphoid tissues with this specialized function. Dendritic cells were shown to be able to stimulate MHC-I-restricted T-cell responses by exogenous routes (24).
What is the nature of the immunodominant antigens in AE? So far, for human AE only very limited information on relevant E. multilocularis antigens and no information regarding T-cell epitopes are available. Although we cannot provide experimental evidence, our results point to proteins as immunodominant antigens. Activated CD8+ T cells are α/β T cells without involvement of γ/δ T cells or NK cells or NK T cells. Besides proteins E. multilocularis vesicular fluid contains lipids and carbohydrates, which might also exhibit antigenic properties. Very recently, experimental evidence for carbohydrates as inducers of a humoral response in rodents was presented (10). Nevertheless, no such data are available for the human host.
However, identification of immunodominant T-cell epitopes is a prerequisite for performing assays testing the antigen specificity of CD8+ T cells (i.e., cytotoxic T lymphocyte assays, peptide-based cytokine enzyme-linked immunospot [ELISPOT] assays, and MHC peptide tetramer assays). Therefore, we used an indirect approach by means of clonal composition analysis to study the antigen dependency of CD8+ T-cell activation in AE.
The analysis of the clonal composition of peripheral CD8+ T cells has become a widely used tool for studies of immune responses in various pathological conditions. Oligoclonal expansions are frequently derived from distinct T cells harboring TCR β-chains selected by the same MHC-peptide complex and therefore share CDR3 length and show amino acid conservation in the CDR3 loop. Monoclonal or oligoclonal expansions of TCRBV sequences suggest that cell proliferation was antigen driven, whereas polyclonal sequences suggest proliferation by superantigens or nonspecific activation.
By analyzing more than 10,000 CDR3 length profiles and calculating the age-adjusted clonal contingent we were able to show that AE patients with parasitic lesions had a significantly greater clonal composition of peripheral CD8+ T cells than healthy controls or patients cured of AE. Sequence analysis revealed oligoclonality or even monoclonality of the expansions. This finding strongly suggests a parasite-specific activation and proliferation of CD8+ T cells in AE patients. To our knowledge, this is the first report of an assessment of oligoclonal expansion of peripheral T cells in parasitic disease. Further evidence for this hypothesis is given by the observation that the magnitude of clonal composition correlates significantly with the parasitic mass independent of presumed metacestode activity. Antigens relevant for the cellular response might also be continuously released from inactive and regressed lesions. This interpretation is substantiated by the observation that the humoral response to the Em2+ antigen as used for routine diagnostic testing remains almost unchanged even in patients with completely calcified lesions exhibiting no parasite viability. Only the complete surgical removal of the lesions, and thus the removal of the immunostimulating source, resulted in the seroconversion to negativity with regard to anti-Em2 antibodies (16).
What is the origin of an increased level of clonality? Massive oligoclonal expansions within CD8+ T-cell populations were observed during acute infections with high antigenic burden. Experiments using MHC-peptide complexes or ELISPOT assays suggested that antigen-specific CD8+ T-cell frequencies can rise up to 40%, with clones present at frequencies as high as 10% of all CD8+ cells (9). In the setting of a chronic persisting infection the origin of increased oligoclonality is divergent, as oligoclonally expanded T cells display a different phenotype. TCRBV-gated FACS analysis of oligoclonally expanded CD8+ T-cell subpopulations revealed reduced CD28 expression on CD45RO+ memory T cells. This phenotype resembles that reported to occur during chronic viral infections, during bone marrow transplantation, and in the normal process of aging (2, 3, 12, 13, 22, 28, 46). Lowered expression of CD28 reflects terminal differentiation, shortening of the telomeres, and cellular senescence (3).
Activation of T cells leads to proliferation. Therefore, prolonged activation results in proliferation with marked telomeric shortening of clonotypes. This phenomenon in peripheral lymphocytes is referred to as replicative senescence and is associated with a shift in the functional profile. CD8+ T cells and, to a lesser extent, CD4+ T cells lose the expression of CD28 and gain independence from the regulatory control brought about by CD80 and CD86 (44).
What is the function of circulating activated CD8+ T cells in AE? Previous work of our group has shown IL-10 production of CD8+ T cells in response to E. multilocularis (23). This finding suggests a regulatory role for these cells. Although highly desirable, detection of other conceivable functions such as cytotoxicity is hampered by the lack of information on immunodominant T-cell epitopes in human AE. Our group currently pursues this topic.
Chronic infection can lead to antigen-specific hyporesponsiveness. This was demonstrated for parasitic diseases onchocerciasis and filiariasis (6, 18, 26, 32) as well as in a group of Alaskan patients severely infected with E. multilocularis. The Alaskan patients were shown to have a depressed proliferation rate by using vesicular fluid antigen (16). The phenomenon of hyporesponsiveness in chronically infected HCV individuals has been extensively studied (25, 43). It was convincingly shown that during the chronic state of the disease the lack of in vitro proliferative responses of CD4+ T cells to HCV antigens was paralleled by ex vivo (tetramer staining) and in vitro (ELISPOT and cytotoxicity assays) hyporesponsiveness of epitope-specific CD8+ T cells (43). By analogy, E. multilocularis-specific hyporesponsiveness in AE patients resulting in lack of proliferative response in vitro would not be consistent with our finding of activated CD8+ T cells in vivo. To assess a possible antigen-specific hyporesponsiveness of peripheral T cells in our AE patients, we tested the ability of PBMC to respond to vesicular fluid in vitro. In addition, generalized hyporesponsiveness was excluded by challenge with recall antigens TT and PPD of Mycobacterium tuberculosis as well as with PHA and superantigens TSST-1 and SEA. Our data demonstrate that, upon antigenic stimulation, the lymphoproliferative response of AE patients did not differ significantly from that of healthy controls irrespective of the disease status. The phenomenon of hyporesponsiveness is associated with a high antigenic burden during the chronic phase of untreated infection. The lack of hyporesponsiveness in our patient groups might therefore be due to benzimidazole treatment. This parasitostatic drug might reduce the release of antigens from the E. multilocularis lesion, resulting in a much lower antigenic load compared to that for the untreated Alaskan patients. This hypothesis is being tested by a prospective follow-up of untreated patients.
In conclusion, this study is the first to demonstrate activated peripheral CD8+ T cells in human chronic helminth disease. Our findings extend the descriptions of immunological alterations including CD8+ T cells in AE patients. Although our results clearly show that elevated numbers of activated cells are present in peripheral blood during the chronic state of the disease, the role of these cells in control of E. multilocularis infection requires further study. Most likely, chronic activation and proliferation of specific clonotypes resulted in increased clonality of the peripheral CD8+ T cells by a process known as proliferative senescence.
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft and IZKF Ulm to B.J.M. and P.K.
We thank all subjects who donated blood for this study and Marion Merkle, Kerstin Barth, and Angelika Kurkhaus for expert technical assistance. We gratefully acknowledge Bruno Gottstein (University of Bern) for supplying E. multilocularis vesicular fluid antigen.
REFERENCES
- 1.Alkarmi, T. O., and Z. Ali Khan. 1984. Chronic alveolar hydatidosis and secondary amyloidosis: pathological aspects of the disease in four strains of mice. Br. J. Exp. Pathol. 65:405-417. [PMC free article] [PubMed] [Google Scholar]
- 2.Batliwalla, F., J. Monteiro, D. Serrano, and P. K. Gregersen. 1996. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum. Immunol. 48:68-76. [DOI] [PubMed] [Google Scholar]
- 3.Batliwalla, F. M., N. Rufer, P. M. Lansdorp, and P. K. Gregersen. 2000. Oligoclonal expansions in the CD8(+)CD28(−) T cells largely explain the shorter telomeres detected in this subset: analysis by flow FISH. Hum. Immunol. 61:951-958. [DOI] [PubMed] [Google Scholar]
- 4.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424. [DOI] [PubMed] [Google Scholar]
- 6.Brattig, N. W., U. Rathjens, M. Ernst, F. Geisinger, A. Renz, and F. W. Tischendorf. 2000. Lipopolysaccharide-like molecules derived from wolbachia endobacteria of the filaria onchocerca volvulus are candidate mediators in the sequence of inflammatory and antiinflammatory responses of human monocytes. Microbes Infect. 2:1147-1157. [DOI] [PubMed] [Google Scholar]
- 7.Bresson Hadni, S., B. Monnot Jacquard, E. Racadot, D. Lenys, J. P. Miguet, and D. A. Vuitton. 1991. Soluble IL-2-receptor and CD8 in the serum and the periparasitic granuloma of patients with alveolar echinococcosis. Eur. Cytokine Netw. 2:339-344. [PubMed] [Google Scholar]
- 8.Bresson Hadni, S., D. A. Vuitton, D. Lenys, M. Liance, E. Racadot, and J. P. Miguet. 1989. Cellular immune response in Echinococcus multilocularis infection in humans. I. Lymphocyte reactivity to Echinococcus antigens in patients with alveolar echinococcosis. Clin. Exp. Immunol. 78:61-66. [PMC free article] [PubMed] [Google Scholar]
- 9.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai, W. J., A. Hemphill, A. Waldvogel, K. Ingold, P. Deplazes, H. Mossmann, and B. Gottstein. 2001. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of αβ+ CD4+ T cells. Infect. Immun. 69:6074-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Haan, J. M., and M. J. Bevan. 2001. Antigen presentation to CD8(+) T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13:437-441. [DOI] [PubMed] [Google Scholar]
- 12.Dolstra, H., E. Van de Wiel van Kemenade, T. De Witte, and F. Preijers. 1996. Clonal predominance of cytomegalovirus-specific CD8+ cytotoxic T lymphocytes in bone marrow recipients. Bone Marrow Transplant. 18:339-345. [PubMed] [Google Scholar]
- 13.Effros, R. B., R. Allsopp, C. P. Chiu, M. A. Hausner, K. Hirji, L. Wang, C. B. Harley, B. Villeponteau, M. D. West, and J. V. Giorgi. 1996. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 10:F17-F22. [DOI] [PubMed] [Google Scholar]
- 14.Gorochov, G., A. U. Neumann, A. Kereveur, C. Parizot, T. Li, C. Katlama, M. Karmochkine, G. Raguin, B. Autran, and P. Debre. 1998. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 4:215-221. [DOI] [PubMed] [Google Scholar]
- 15.Gottstein, B., P. Jacquier, S. Bresson-Hadni, and J. Eckert. 1993. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2plus antigen. J. Clin. Microbiol. 31:373-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottstein, B., B. Mesarina, I. Tanner, R. W. Ammann, J. F. Wilson, J. Eckert, and A. Lanier. 1991. Specific cellular and humoral immune responses in patients with different long-term courses of alveolar echinococcosis (infection with Echinococcus multilocularis). Am. J. Trop. Med. Hyg. 45:734-742. [DOI] [PubMed] [Google Scholar]
- 17.Grant, E. P., and K. L. Rock. 1992. MHC class I-restricted presentation of exogenous antigen by thymic antigen-presenting cells in vitro and in vivo. J. Immunol. 148:13-18. [PubMed] [Google Scholar]
- 18.Greene, B. M., M. M. Fanning, and J. J. Ellner. 1983. Non-specific suppression of antigen-induced lymphocyte blastogenesis in Onchocerca volvulus infection in man. Clin. Exp. Immunol. 52:259-265. [PMC free article] [PubMed] [Google Scholar]
- 19.Hemphill, A., and B. Gottstein. 1995. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestodes. Parasitol. Res. 81:605-614. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani, R., I. H. Choi, P. Akolkar, B. Gulwani Akolkar, R. Pergolizzi, J. Silver, and P. K. Gregersen. 1993. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J. Immunol. 151:5762-5769. [PubMed] [Google Scholar]
- 21.Kaufmann, S. H., and P. C. Doherty. 1997. Immunity to infection. Curr. Opin. Immunol. 9:453-455. [DOI] [PubMed] [Google Scholar]
- 22.Kern, F., E. Khatamzas, I. Surel, C. Frommel, P. Reinke, S. L. Waldrop, L. J. Picker, and H. D. Volk. 1999. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29:2908-2915. [DOI] [PubMed] [Google Scholar]
- 23.Kilwinski, J., L. Jenne, A. Jellen Ritter, P. Radloff, W. Flick, and P. Kern. 1999. T lymphocyte cytokine profile at a single cell level in alveolar Echinococcosis. Cytokine 11:373-381. [DOI] [PubMed] [Google Scholar]
- 24.Larsson, M., J. F. Fonteneau, and N. Bhardwaj. 2001. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 22:141-148. [DOI] [PubMed] [Google Scholar]
- 25.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahanty, S., and T. B. Nutman. 1995. Immunoregulation in human lymphatic filariasis: the role of interleukin 10. Parasite Immunol. 17:385-392. [DOI] [PubMed] [Google Scholar]
- 27.Manfras, B. J., W. A. Rudert, M. Trucco, and B. O. Boehm. 1997. Analysis of the alpha/beta T-cell receptor repertoire by competitive and quantitative family-specific PCR with exogenous standards and high resolution fluorescence based CDR3 size imaging. J. Immunol. Methods 210:235-249. [DOI] [PubMed] [Google Scholar]
- 28.Morley, J. K., F. M. Batliwalla, R. Hingorani, and P. K. Gregersen. 1995. Oligoclonal CD8+ T cells are preferentially expanded in the CD57+ subset. J. Immunol. 154:6182-6190. [PubMed] [Google Scholar]
- 29.Moss, P. A., and J. I. Bell. 1996. Comparative sequence analysis of the human T cell receptor TCRA and TCRB CDR3 regions. Hum. Immunol. 48:32-38. [DOI] [PubMed] [Google Scholar]
- 30.Moss, P. A., and J. I. Bell. 1995. Sequence analysis of the human alpha beta T-cell receptor CDR3 region. Immunogenetics 42:10-18. [DOI] [PubMed] [Google Scholar]
- 31.Nakaya, K., M. Nakao, and A. Ito. 1997. Echinococcus multilocularis: mouse strain difference in hydatid development. J. Helminthol. 71:53-56. [DOI] [PubMed] [Google Scholar]
- 32.Namangala, B., L. Brys, S. Magez, P. De Baetselier, and A. Beschin. 2000. Trypanosoma brucei brucei infection impairs MHC class II antigen presentation capacity of macrophages. Parasite Immunol. 22:361-370. [DOI] [PubMed] [Google Scholar]
- 33.Pannetier, C., J. Even, and P. Kourilsky. 1995. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol. Today 16:176-181. [DOI] [PubMed] [Google Scholar]
- 34.Playford, M. C., H. K. Ooi, M. Ito, and M. Kamiya. 1993. Lymphocyte engraftment conveys immunity and alters parasite development in scid mice infected with Echinococcus multilocularis. Parasitol. Res. 79:261-268. [DOI] [PubMed] [Google Scholar]
- 35.Playford, M. C., H. K. Ooi, Y. Oku, and M. Kamiya. 1992. Secondary Echinococcus multilocularis infection in severe combined immunodeficient (scid) mice: biphasic growth of the larval cyst mass. Int. J. Parasitol. 22:975-982. [DOI] [PubMed] [Google Scholar]
- 36.Posnett, D. N., R. Sinha, S. Kabak, and C. Russo. 1994. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy.” J. Exp. Med. 179:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimann, J., and S. H. Kaufmann. 1997. Alternative antigen processing pathways in anti-infective immunity. Curr. Opin. Immunol. 9:462-469. [DOI] [PubMed] [Google Scholar]
- 38.Reuter, S., B. Jensen, K. Buttenschoen, W. Kratzer, and P. Kern. 2000. Benzimidazoles in the treatment of alveolar echinococcosis: a comparative study and review of the literature. J. Antimicrob. Chemother. 46:451-456. [DOI] [PubMed] [Google Scholar]
- 39.Reuter, S., H. Schirrmeister, W. Kratzer, C. Dreweck, S. N. Reske, and P. Kern. 1999. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin. Infect. Dis. 29:1157-1163. [DOI] [PubMed] [Google Scholar]
- 40.Rock, K. L., S. Gamble, and L. Rothstein. 1990. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science 249:918-921. [DOI] [PubMed] [Google Scholar]
- 41.Rock, K. L., L. Rothstein, S. Gamble, and C. Fleischacker. 1993. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J. Immunol. 150:438-446. [PubMed] [Google Scholar]
- 42.Sailer, M., B. Soelder, F. Allerberger, D. Zaknun, H. Feichtinger, and B. Gottstein. 1997. Alveolar echinococcosis of the liver in a six-year-old girl with acquired immunodeficiency syndrome. J. Pediatr. 130:320-323. [DOI] [PubMed] [Google Scholar]
- 43.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6:578-582. [DOI] [PubMed] [Google Scholar]
- 44.Vallejo, A. N., A. R. Nestel, M. Schirmer, C. M. Weyand, and J. J. Goronzy. 1998. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J. Biol. Chem. 273:8119-8129. [DOI] [PubMed] [Google Scholar]
- 45.Wakelin, D. 1997. Immune response to Echinococcus infection: parasite avoidance and host protection. Parassitologia 39:355-358. [PubMed] [Google Scholar]
- 46.Wang, E. C., P. A. Moss, P. Frodsham, P. J. Lehner, J. I. Bell, and L. K. Borysiewicz. 1995. CD8highCD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J. Immunol. 155:5046-5056. [PubMed] [Google Scholar]