Abstract
Tropheryma whipplei was established as the agent of Whipple's disease in 2000, but the mechanisms by which it survives within host cells are still unknown. We show here that T. whipplei survives within HeLa cells by controlling the biogenesis of its phagosome. Indeed, T. whipplei colocalized with lysosome-associated membrane protein 1, a membrane marker of late endosomal and lysosomal compartments, but not with cathepsin D, a lysosomal hydrolase. This defect in phagosome maturation is specific to live organisms, since heat-killed bacilli colocalized with cathepsin D. In addition, T. whipplei survived within HeLa cells by adapting to acidic pH. The vacuoles containing T. whipplei were acidic (pH 4.7 ± 0.3) and acquired vacuolar ATPase, responsible for the acidic pH of late phagosomes. The treatment of HeLa cells with pH-neutralizing reagents, such as ammonium chloride, N-ethylmaleimide, bafilomycin A1, and chloroquine, increased the intravacuolar pH and promoted the killing of T. whipplei. The ability of T. whipplei to survive in an acidic environment and to interfere with phagosome-lysosome fusion is likely critical for its prolonged persistence in host cells during the course of Whipple's disease. Our results suggest that manipulating the intravacuolar pH may provide a new approach for the treatment of Whipple's disease.
Whipple's disease (WD), first described in 1907 (33), is a systemic bacterial infection (11). It is characterized by low-grade fever, weight loss, abdominal pain, diarrhea, polyarthralgia, and lymphadenopathy. Occasionally, hyperpigmentation of the skin, cardiac manifestations, and neurologic abnormalities are observed (30). The diagnosis of WD is usually based on duodenal biopsy, which shows the infiltration of the lamina propria by large, foamy macrophages containing periodic acid-Schiff (PAS) inclusions (16). It is well established that PAS granules represent intact and degenerated bacilli (26). Despite patient susceptibility to antibiotics, clinical relapses often occur after an antibiotic regimen (14). In 1991-1992, the agent responsible for WD was characterized as an actinomycete by PCR and sequencing of the 16S rRNA gene, and the name Tropheryma whippelii was proposed (23, 34). In 1997, it was reported that the WD agent survived in deactivated human macrophages, but the isolate could not be subcultured (25). Recently, our group succeeded in cultivating the WD agent (19, 21), and the name Tropheryma whipplei was made official (15).
As T. whipplei is detected in PAS-positive inclusions inside macrophages, its intracellular survival is likely related to subversion of phagosome maturation. Indeed, once internalized by host cells, microorganisms are engulfed in phagosomes, which interact with endocytic components through successive fusion events (8). Early phagosomes rapidly and transiently acquire markers of early endocytosis and then markers of late endocytosis, including the lysosome-associated membrane protein (Lamp-1) and the vacuolar proton ATPase (V-ATPase) responsible for acidic pH. Finally, phagosomes fuse with lysosomes and acquire hydrolases, such as cathepsin D (7), thus leading to the lysis of the microorganism. Intracellular bacteria avoid being destroyed in phagolysosomes by different methods. Listeria, Shigella, and Rickettsia escape from the nascent vacuole into the cytoplasm (9). Other bacteria, such as Legionella pneumophila, Brucella abortus, or Chlamydia, replicate in vacuoles that avoid the endocytic pathway (18). Other organisms interfere with phagosome maturation. Mycobacterium species interact with early endosomes but do not fuse with lysosomes (7); mycobacterial vacuoles exclude V-ATPase and consequently do not acidify (28). Salmonella enterica serovar Typhimurium replicates in vacuoles that recruit preexisting lysosomal membrane proteins but do not directly interact with lysosomes (27). Finally, Coxiella burnetii, the agent of Q fever, replicates within acidic phagosomes (3). Increasing the phagosomal pH with lysosomotropic agents such as chloroquine inhibits C. burnetii replication and restores the bactericidal activity of antibiotics in vitro, which is the foundation of the treatment of chronic Q fever, combining tetracycline and chloroquine (20).
In this paper, we show that T. whipplei survives in HeLa cells in acidic vacuoles that interact with late endocytic compartments but exclude cathepsin D, demonstrating that T. whipplei interferes with phagosome-lysosome fusion. The survival of T. whipplei requires acidic pH, since raising the vacuolar pH is detrimental to the bacteria. Thus, the defect of phagolysosome fusion and the acidic pH of T. whipplei vacuoles are likely critical for the persistence of T. whipplei during WD. Manipulating the intravacuolar pH may improve antibiotic efficiency and lead to curing WD.
MATERIALS AND METHODS
Culture of T. whipplei.
The strain Twist-Marseille of T. whipplei (Collection Nationale de Culture de Microorganismes de l’Institut Pasteur; no. I-2202) was cultured as described elsewhere (15). MRC5 cells were obtained from the European Collection of Animal Cell Cultures, Sofia-Antipolis, France (ECACC 84101801). They were cultured in Eagle minimal essential medium (MEM) containing 4% fetal bovine serum (FBS; Gibco-BRL, Life Technologies, Eragny, France) in 150-cm2 cell culture flasks. When the MRC5 cells were heavily infected by T. whipplei, they were detached from the plastic substrate with glass beads and sonicated. The homogenates were spun down at 300 × g for 10 min to remove unbroken cells, and the supernatants were centrifuged at 8,000 × g for 10 min. The collected bacteria were layered on sucrose gradients, and the gradients were spun down. The purified organisms were collected and washed in Hanks balanced salt solution (Gibco-BRL) before being stored at −80°C. The bacteria were counted by Gimenez staining and indirect immunofluorescence (see below). Heat-killed organisms were obtained by heating the bacteria at 100°C for 1 h and were stored at −80°C.
Intracellular survival of T. whipplei.
The intracellular survival and trafficking of T. whipplei was studied in HeLa cells, the reference model for traffic investigation. HeLa cells (ECACC 93021013) were grown in MEM containing 10% FBS and 2 mM l-glutamine. Cells (2.5 × 104 per assay) were seeded on 12-mm-diameter round coverslips in flat-bottom 24-well plates for 16 h. They were then incubated with T. whipplei at different ratios in antibiotic-free MEM containing 2% FBS for 4 h, washed to remove free bacteria, and incubated for additional times. Intracellular bacteria were counted by using rabbit antibodies (Abs) generated in the laboratory (1:2,000 dilution) and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin G (IgG) Abs (1:100 dilution; Beckman-Coulter, Roissy, France). The number of intracellular bacteria was expressed as an infection index, which is the product of the mean number of bacteria per infected cell and the percentage of infected cells × 100 (5). The bacterial viability was assessed using the LIVE/DEAD BacLight viability kit (Molecular Probes, Eugene, Oreg.) as recommended by the manufacturer. In brief, cells were lysed, the bacterial suspension was incubated with a combination of SYTO 9 stain and propidium iodide for 15 min, and the fluorescence of the organisms was observed. Only dead organisms were labeled by propidium iodide; viable organisms were labeled by SYTO 9. The results were expressed as the percentage of live bacteria. In some experiments, HeLa cells were first infected by T. whipplei (bacterium-to-cell ratio, 100:1) for 4 h and then incubated with pH-neutralizing reagents for 48 h. The infection index of HeLa cells and the viability of intracellular organisms were determined as described above.
Intracellular traffic of T. whipplei.
HeLa cells were incubated with T. whipplei (bacterium-to-cell ratio, 50:1) for different times. After being washed, the cells were fixed with 3% paraformaldehyde, and free aldehydes were blocked with 0.5 M ammonium chloride. The cells were permeabilized by 0.1% saponin in phosphate-buffered saline containing 10% horse serum for 30 min, washed, and incubated for 30 min with phosphate-buffered saline containing 0.1% saponin, 5% horse serum, a 1:2,000 dilution of rabbit Abs to T. whipplei, and monoclonal Abs specific for Lamp-1 (1:50 dilution; Transduction Laboratories, Lexington, Ky.), cathepsin D (1:500 dilution; Transduction Laboratories), or V-ATPase (1:250 dilution; Chemicon International, Inc., Temecula, Calif.). After being washed, the cells were incubated with 1:100 dilutions of Texas Red-conjugated anti-rabbit IgG Abs and FITC-conjugated anti-mouse IgG Abs (Beckman-Coulter) for 20 min. In some experiments, rabbit Abs specific for all forms of cathepsin D (a gift from S. Kornfeld, St. Louis, Mo.) were used at 1:1,000 dilution, and bacteria were revealed by using mouse anti-T. whipplei Abs at 1:50 dilution. Thereafter, the cells were washed, mounted with Mowiol (Calbiochem, San Diego, Calif.), and stored at 4°C until they were examined. The colocalization of organisms with intracellular markers was examined with a laser scanning confocal fluorescence microscope (TCS 4D; Leica, Heidelberg, Germany) equipped with a 100× oil immersion lens and suitable filters. Optical sections of images (1 μm thick) were analyzed using Adobe Photoshop version 5.5 software. Vacuoles containing T. whipplei were scored as positive for cathepsin D when fluorescence was observed in the phagosome lumen; for membrane markers such as Lamp-1, vacuoles were scored as positive when a fluorescence ring surrounded the organisms. About 30 vacuoles containing T. whipplei were scored per coverslip, and at least three distinct experiments were performed per condition. The results are expressed as the percentage of phagosomes that colocalized with intracellular markers.
Cathepsin D activity measurement.
HeLa cells were infected with live or heat-killed T. whipplei for 4 h. The cathepsin D-sensitive near-infrared fluorescence (NIRF) probe was prepared as previously described (31, 32). It was conjugated with FITC to monitor probe internalization and with Cy5.5 marker that became fluorescent in the near-infrared spectrum after cathepsin D activation. NIRF probe at 0.1 μM was added to the cells 1 h before the end of the infection time. The HeLa cells were then washed to remove noninternalized organisms and free NIRF probe and fixed with methanol at −20°C for 5 min. Bacteria were revealed by Texas Red immunofluorescence as described above. The colocalization of bacteria with NIRF probe was examined with a laser scanning confocal fluorescence microscope equipped with appropriate excitation and emission filters for FITC, Texas Red, and Cy5.5. Images were analyzed using Adobe Photoshop version 5.5 software.
Vacuolar pH.
The phagosome acidification was analyzed using DM-NERF dextran (Molecular Probes) (molecular mass, 10 kDa) (22). HeLa cells were incubated with T. whipplei (at a bacterium-to-cell ratio of 100:1) in the presence of 20 μg of DM-NERF dextran ml−1 for 24 h to allow sufficient dye internalization in the phagosomes. Bacteria were revealed by Texas Red immunofluorescence. The colocalization of the pH probe with intracellular bacteria was analyzed by merging the DM-NERF and Texas Red fluorescence images. The inhibition of V-ATPase by bafilomycin A1 (Sigma Chemicals, St. Louis, Mo.), a specific inhibitor of V-ATPase involved in phagosome acidification (10), was performed by incubating HeLa cells with 10 nM bafilomycin A1 for 2 h after infection with T. whipplei. The intraphagosomal pH was determined by ratiometric analysis of fluorescence intensities. Infected HeLa cells were incubated with buffer solutions with graded pHs (4.0, 5.0, 6.0, 7.0, and 7.5) in the absence or the presence of 10 μM monensin (Sigma Chemicals), which equilibrated the intravacuolar pH with the extracellular pH. After 30 min, fluorescence of DM-NERF dextran (490/440-nm excitation ratio; 530-nm emission) was recovered using a microplate fluorescent reader (Fisher Scientific, Elancourt, France). The mean pH value of the samples was calculated using a reference pH curve.
Data analysis.
Results are given as mean ± standard deviation (SD). The statistical analysis was conducted with paired Student's t tests. Differences were considered significant when P was <0.05.
RESULTS
T. whipplei survives in HeLa cells.
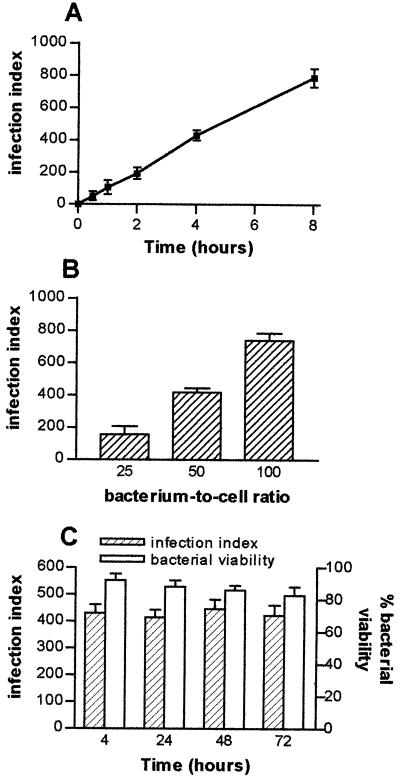
We wondered if T. whipplei is able to infect HeLa cells and to survive within them. T. whipplei organisms were incubated with HeLa cells, and their uptake was determined. For a bacterium-to-cell ratio of 50:1, bacterial phagocytosis was detected after 30 min and steadily increased thereafter. As after 4 h all HeLa cells phagocytosed four to five bacteria (Fig. 1A), the next experiments used 4 h as the phagocytosis time. T. whipplei phagocytosis depended on the bacterium-to-cell ratio; a ratio of 50:1 provided substantial and reproducible infection of HeLa cells (Fig. 1B) suitable for trafficking studies (see below). The uptake of heat-killed bacteria by HeLa cells was lower than that of viable bacteria: after 4 h of incubation with 50 bacteria per cell, the infection index was 150 ± 26 versus 430 ± 32 for viable bacteria. The intracellular survival of T. whipplei was studied by incubating HeLa cells with the organisms for 4 h, washing the cells to remove extracellular bacteria, and again incubating the cells for 72 h. The infection index remained constant for 72 h (Fig. 1C). The results were similar when the viability of intracellular bacteria was assessed. About 90% of the bacteria were viable after 4 h, and the percentage of living organisms did not vary during the experiment (Fig. 1C). These results clearly indicate that T. whipplei is able to survive in HeLa cells.
FIG. 1.
Intracellular survival of T. whipplei. (A) HeLa cells were infected with T. whipplei (bacterium-to-cell ratio, 50:1) for different periods. (B) Cells were infected with different concentrations of T. whipplei for 4 h. (C) Cells were infected with T. whipplei (bacterium-to-cell ratio, 50:1) for 4 h, washed to remove free bacteria, and incubated for different additional periods. Bacteria were revealed by indirect immunofluorescence, and the infection index was determined. The viability of intracellular bacteria was studied using a combination of SYTO 9 and propidium iodide; the results are expressed as percentages of viable bacteria. The results represent the mean ± SD of four experiments.
T. whipplei colocalizes with Lamp-1 but not with cathepsin D.
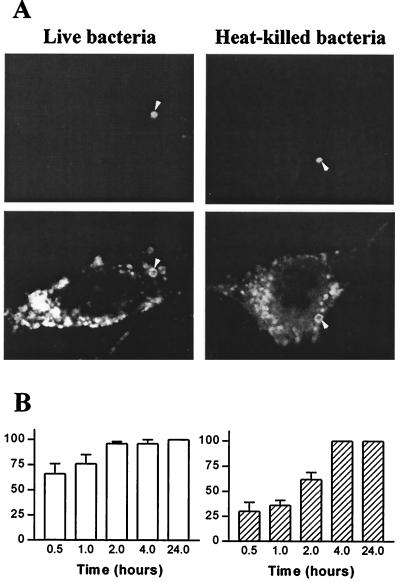
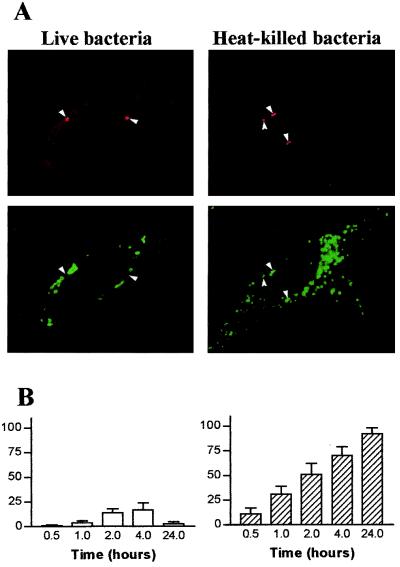
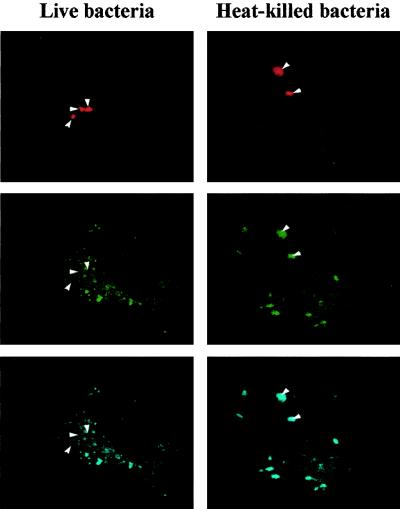
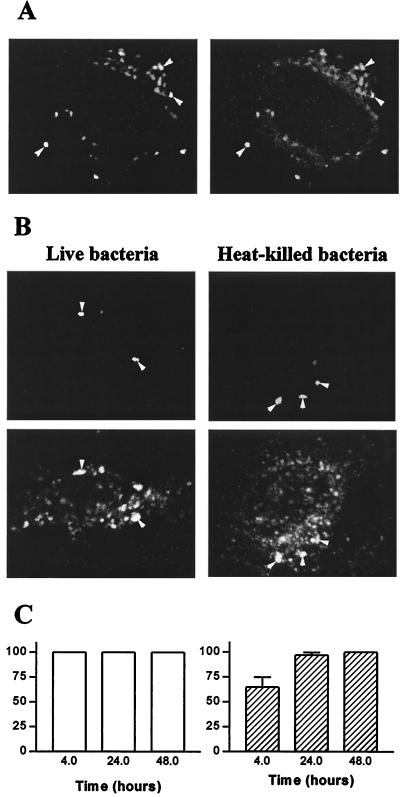
We wondered if the survival of T. whipplei in HeLa cells was associated with the alteration of intracellular trafficking. Cells were incubated with live or heat-killed T. whipplei, and the colocalization of the organisms with Lamp-1 was assessed by immunofluorescence and confocal microscopy. The Lamp-1 fluorescence was distributed in cytoplasmic patches and appeared as a bright ring surrounding organisms (Fig. 2A), indicating that Lamp-1 was localized in the phagosome membrane. At 30 min postinfection, 66% ± 10% of phagosomes containing live T. whipplei organisms colocalized with Lamp-1; this percentage increased thereafter and reached 100% at 4 h (Fig. 2B). The kinetics of Lamp-1 acquisition by phagosomes containing heat-killed organisms was delayed. At 30 min postinfection, only 30% ± 9% of phagosomes were colocalized with Lamp-1; at 2 h, 62% ± 7% were colocalized, and at 4 h 100% were colocalized (Fig. 2B). The delay in Lamp-1 acquisition by vacuoles containing heat-killed bacteria is likely related to the low uptake of heat-killed bacteria by HeLa cells. Hence, T. whipplei resides in vacuoles that acquire markers of late endosomes. The ability of vacuoles containing T. whipplei to fuse with lysosomes was investigated by determining the colocalization of cathepsin D with organisms. Using Abs recognizing all forms of cathepsin D, the fluorescence of cathepsin D appeared as vesicular, and it accumulated in the lumen of vacuoles containing heat-killed T. whipplei organisms (Fig. 3A). After 1 h of incubation with heat-killed bacteria, 31% ± 8% of phagosomes acquired cathepsin D; this percentage increased to 70%± 9% and 92% ± 6% after 4 and 24 h, respectively (Fig. 3B). In contrast, live T. whipplei organisms did not colocalize with cathepsin D (Fig. 3A). Indeed, accumulation of cathepsin D was detectable in 14% ± 4% and 17% ± 7% of vacuoles at 2 and 4 h post-infection, respectively (Fig. 3B). Similar results were obtained with monoclonal Abs directed against the 43-kDa processed form of cathepsin D (data not shown). To characterize more precisely the impaired colocalization of cathepsin D with T. whipplei, the activity of cathepsin D was assessed in situ (Fig. 4). NIRF probe accumulated in the lysosomal compartment and colocalized with heat-killed bacteria. Cathepsin D was active in vacuoles containing heat-killed bacteria, as shown by the distribution of Cy5.5 fluorescence. In contrast, NIRF probe did not colocalize with T. whipplei (Fig. 4), whatever the infection time (data not shown). Taken together, these data indicate that vacuoles containing live T. whipplei organisms are unable to fuse with lysosomes.
FIG. 2.
T. whipplei colocalizes with Lamp-1. (A) HeLa cells were incubated with live or heat-killed T. whipplei at a bacterium-to-cell ratio of 50:1. Bacteria (top) and Lamp-1 (bottom) were revealed by indirect immunofluorescence and examined by confocal microscopy. Arrowheads, colocalization of bacteria and Lamp-1. Paired photomicrographs show organisms after a 4-h incubation. (B) Cells were infected for different periods. About 100 phagosomes were enumerated under each experimental condition. The results are expressed as the percentage ± SD of phagosomes that expressed Lamp-1.
FIG. 3.
T. whipplei does not colocalize with cathepsin D. (A) HeLa cells were incubated with live or heat-killed T. whipplei at a bacterium-to-cell ratio of 50:1. Bacteria (top) and cathepsin D (bottom) were revealed by indirect immunofluorescence and examined by confocal microscopy. Paired photomicrographs show organisms after a 4-h incubation. Arrowheads, bacteria and phagosomes containing cathepsin D. (B) Cells were infected for different periods. About 100 phagosomes were enumerated under each experimental condition. The results are expressed as the percentage ± SD of phagosomes that expressed cathepsin D.
FIG. 4.
In situ measurement of cathepsin D activity. HeLa cells were incubated with live or heat-killed T. whipplei. Bacteria (top) were revealed by Texas Red immunofluorescence. The colocalization of bacteria with NIRF probe (FITC filter) was examined with a confocal microscope (middle). The Cy5.5 fluorescence corresponds to active cathepsin D (bottom). The figure is representative of three experiments. Arrowheads, bacteria and phagosomes containing active cathepsin D.
T. whipplei survives in acidic vacuoles.
As T. whipplei interfered with phagosome-lysosome fusion, we wondered if it also affected the vacuolar pH. HeLa cells were incubated with T. whipplei in the presence of DM-NERF dextran for 24 h (Fig. 5A). Using a ratiometric analysis of fluorescence intensities, the pH of vacuoles containing organisms was measured. The pH was 4.7± 0.3 for live organisms and 4.9 ± 0.2 for heat-killed organisms. Similar values were found at 48 h of culture (4.8 ± 0.2 and 5.0 ± 0.3, respectively). The acidic pH of T. whipplei vacuoles resulted from the acquisition of V-ATPase. All phagosomes containing T. whipplei organisms expressed V-ATPase at 4 h (Fig. 5B), and its accumulation persisted for at least 48 h (Fig. 5C). The vacuolar V-ATPase was functional, since vacuoles containing live and heat-killed organisms displayed pHs of 6.6 ± 0.2 and 6.5 ± 0.2, respectively, when HeLa cells infected in the presence of DM-NERF dextran were incubated with 10 nM bafilomycin A1 for 2 h. Taken together, these results show that T. whipplei resides in vacuoles acidified by V-ATPase.
FIG.5.
Colocalization of T. whipplei with DM-NERF dextran and V-ATPase. (A) HeLa cells were incubated with live T. whipplei (bacterium-to-cell ratio, 100:1) in the presence of DM-NERF dextran. Paired photomicrographs show organisms (left) and DM-NERF dextran (right) after a 24-h incubation. Arrowheads, colocalization of bacteria and DM-NERF dextran. (B and C) HeLa cells were incubated with live or heat-killed T. whipplei at a bacterium-to-cell ratio of 50:1. (B) Bacteria (top) and V-ATPase (bottom) were revealed by indirect immunofluorescence and were examined by confocal microscopy. Paired photomicrographs show organisms after a 4-h incubation. Arrowheads, bacteria and phagosomes containing V-ATPase. (C) Cells were infected for different periods. About 100 phagosomes were enumerated under each experimental condition. The results are expressed as the percentage ± SD of phagosomes that contained V-ATPase.
T. whipplei survival requires acidic pH.
To assess the role of acidic pH in the survival of T. whipplei, we investigated the effect of pH-neutralizing reagents on T. whipplei infection and viability. The agents were used at the lowest concentrations that neutralized the intracellular pH without affecting the viability of HeLa cells. Indeed, the vacuolar pH was significantly increased by 100 nM ammonium chloride, 25 mM N-ethylmaleimide, 0.1 nM bafilomycin A1, and 2 mM chloroquine. These concentrations did not affect the viability of HeLa cells after a 48-h incubation (Table 1). HeLa cells were infected by T. whipplei and then treated with pH-neutralizing agents for 48 h, and the infection index was determined. In untreated cells, the infection index was high (696 ± 32) after 48 h of culture. In cells treated with ammonium chloride, N-ethylmaleimide, bafilomycin A1, or chloroquine, the infection index was significantly (P < 0.01) lower (85 to 95% inhibition) than in untreated cells (Table 1). In addition, neutralizing the vacuolar pH did not modify intracellular traffic of T. whipplei. Indeed, after 48 h, when bacterial killing was maximal in cells treated with pH-neutralizing agents, cathepsin D was not colocalized with vacuoles containing T. whipplei (data not shown). These results indicate that the survival of T. whipplei in HeLa cells depends on vacuolar acidic pH.
TABLE 1.
T. whipplei survival requires acidic pHa
| Agent | Vacuolar pH | Cell viability (%) | Cell infection |
|---|---|---|---|
| Control | 4.7 ± 0.3 | 98 ± 2 | 696 ± 32 |
| NH4Cl (100 nM) | 6.45 ± 0.15 | 85 ± 6 | 70 ± 12∗ |
| N-Ethylmaleimide (25 mM) | 6.35 ± 0.2 | 87 ± 5 | 108 ± 15∗ |
| Bafilomycin A1 (0.1 nM) | 6.5 ± 0.15 | 90 ± 4 | 81 ± 11∗ |
| Chloroquine (2 mM) | 6.35 ± 0.2 | 89 ± 6 | 37 ± 8∗ |
HeLa cells were incubated with T. whipplei (bacterium-to-cell ratio, 100:1) for 4 h and then with pH-neutralizing drugs for 48 h. The vacuolar pH was measured by ratiometric analysis of DM-NERF fluorescence intensities, and mean pH values were calculated using a pH reference curve. The cell viability was determined by trypan blue exclusion. Intracellular bacteria were revealed by indirect immunofluorescence, and their viability was assessed by using a combination of SYTO 9 and propidium iodide. The infection by viable organisms was expressed as the mean index (absolute value of infection index) ± SD of three experiments. ∗, P < 0.01.
DISCUSSION
The results show that T. whipplei survives in HeLa cells in membrane-bound vacuoles. These vacuoles rapidly acquired Lamp-1 and V-ATPase, two markers associated with late endocytic organelles (8). However, the vacuoles do not fuse with lysosomes, as shown by the lack of colocalization of cathepsin D with organisms. This finding is not related to the specificity of Abs directed against cathepsin D, since the same defect was observed with Abs recognizing all forms of cathepsin D or only its processed form. In addition, vacuoles containing T. whipplei organisms were devoid of cathepsin D probe, which indicates enzyme activity. The lack of colocalization is specific to viable bacteria, since cathepsin D clearly colocalized with vacuoles containing heat-killed bacteria, and this cathepsin D was active. This alteration of vacuole trafficking has been shown in Salmonella infection, in which bacterial vacuoles acquire prelysosomal and lysosomal markers, including Lamp-1, but are devoid of lysosomal enzymes (27). We provide evidence that the blockade of phagosome maturation by T. whipplei does not interfere with vacuole acidification. Indeed, T. whipplei vacuoles were markedly acidic and accumulated V-ATPase, which can be inhibited by bafilomycin A1. This ability to reside in acidic vacuoles is shared with pathogens, including parasites such as Leishmania (2) and bacteria such as C. burnetii (3) and Francisella tularensis (12). Hence, T. whipplei inhabits an acidic compartment that is unable to fuse with lysosomes.
Acidic pH is required for the intracellular survival of T. whipplei. The treatment of HeLa cells with ammonium chloride, N-ethylmaleimide, and chloroquine increased the vacuolar pH and promoted the killing of T. whipplei, suggesting that bacterial survival depends on the intraphagosomal pH. In addition, bafilomycin A1, which specifically inhibits V-ATPase (10) and raised the pH of T. whipplei vacuoles, also induced the killing of T. whipplei. Nevertheless, these reagents may affect other mechanisms that might control the intracellular survival of T. whipplei. Bafilomycin A1 has been reported to affect endosome maturation (6); it is unlikely that the killing of T. whipplei results from pH-dependent alteration of intracellular traffic, since T. whipplei remained in vacuoles that did not fuse with lysosomes in cells treated with pH-neutralizing reagents (data not shown). Bafilomycin A1 and chloroquine decrease inflammatory response (29), and bafilomycin A1 may affect microbicidal activity through changes in cytosolic pH (4). Finally, pH-neutralizing agents may impair the membrane potential necessary for amino acid uptake (6, 22). Such a diversity of side effects of pH-neutralizing reagents could not account for their shared ability to kill T. whipplei. Their ability to kill T. whipplei through increased vacuolar pH is more likely. The metabolic requirement of T. whipplei for an acidic pH remain to be elucidated. Acid-resistant bacteria may exploit the acidic vacuoles as a primary source of carbon and energy. Hence, acidic pH is required for transport of glucose in C. burnetii (13). Phagosome acidification may induce the expression of the two-component regulatory system essential for Salmonella survival (1). Localization in an acidic environment may facilitate the availability of iron essential for the growth of F. tularensis via its dissociation from transferrin (12).
The survival of T. whipplei in acidic nonphagolysosomal vacuoles may have major pathophysiological consequences. WD is characterized by massive infiltration by large foamy macrophages positive for PAS stain within the lamina propria. PAS-positive macrophages are also found in intestinal Mycobacterium avium infection (24), which is characterized by an alteration of the interaction of bacterial phagosomes with late endosomes and a defect of phagosome acidification (7, 28). The characterization of the intracellular compartment that contains organisms may discriminate between the two causes. On the other hand, the high frequency of relapses in WD despite prolonged antibiotic treatment (14) may be related to the low activity of antibiotics in an acidic environment (17). We suggest that combining antibiotics and chloroquine may provide a promising therapeutic approach to WD.
Acknowledgments
This work was supported by the Programme de Recherche en Microbiologie Fondamentale et Maladies Infectieuses et Parasitaires.
REFERENCES
- 1.Alpuche Aranda, C. M., J. A. Swanson, W. P. Loomis, and S. I. Miller. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA 89:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine, J. C., E. Prina, C. Jouanne, and P. Bongrand. 1990. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect. Immun. 58:779-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baca, O. G., Y. P. Li, and H. Kumar. 1994. Survival of the Q fever agent Coxiella burnetii in the phagolysosome. Trends Microbiol. 2:476-480. [DOI] [PubMed] [Google Scholar]
- 4.Bidani, A., B. S. Reisner, A. K. Haque, J. Wen, R. E. Helmer, D. M. Tuazon, and T. A. Heming. 2000. Bactericidal activity of alveolar macrophages is suppressed by V-ATPase inhibition. Lung 178:91-104. [DOI] [PubMed] [Google Scholar]
- 5.Capo, C., F. P. Lindberg, S. Meconi, Y. Zaffran, G. Tardei, E. J. Brown, D. Raoult, and J. L. Mege. 1999. Subversion of monocyte functions by Coxiella burnetii: impairment of the cross-talk between αvβ3 integrin and CR3. J. Immunol. 163:6078-6085. [PubMed] [Google Scholar]
- 6.Clague, M. J., S. Urbe, F. Aniento, and J. Gruenberg. 1994. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269:21-24. [PubMed] [Google Scholar]
- 7.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardins, M., L. A. Huber, R. G. Parton, and G. Griffiths. 1994. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124:677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dramsi, S., and P. Cossart. 1998. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14:137-166. [DOI] [PubMed] [Google Scholar]
- 10.Drose, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Fenollar, F., and D. Raoult. 2001. Whipple's disease. Clin. Diagn. Lab. Immunol. 8:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortier, A. H., D. A. Leiby, R. B. Narayanan, E. Asafoadjei, R. M. Crawford, C. A. Nacy, and M. S. Meltzer. 1995. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect. Immun. 63:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackstadt, T., and J. C. Williams. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA 78:3240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keinath, R. D., D. E. Merrell, R. Vlietstra, and W. O. D. Dobbins. 1985. Antibiotic treatment and relapse in Whipple's disease. Long-term follow-up of 88 patients. Gastroenterology 88:1867-1873. [DOI] [PubMed] [Google Scholar]
- 15.La Scola, B., F. Fenollar, P. E. Fournier, M. Altwegg, M. N. Mallet, and D. Raoult. 2001. Description of Tropheryma whipplei gen. nov. sp. nov., the Whipple's disease bacillus. Int. J. Syst. E vol. Microbiol. 51:1471-1479. [DOI] [PubMed] [Google Scholar]
- 16.Marth, T., and W. Strober. 1996. Whipple's disease. Semin. Gastrointest. Dis. 7:41-48. [PubMed] [Google Scholar]
- 17.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J. Infect. Dis. 166:1097-1102. [DOI] [PubMed] [Google Scholar]
- 18.Méresse, S., O. Steele-Mortimer, E. Moreno, M. Desjardins, B. Finlay, and J. P. Gorvel. 1999. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1:E183-E188. [DOI] [PubMed] [Google Scholar]
- 19.Raoult, D., M. L. Birg, B. La Scola, P. E. Fournier, M. Enea, H. Lepidi, V. Roux, J. C. Piette, F. Vandenesch, D. Vital-Durand, and T. J. Marrie. 2000. Cultivation of the bacillus of Whipple's disease. N. Engl. J. Med. 342:620-625. [DOI] [PubMed] [Google Scholar]
- 20.Raoult, D., P. Houpikian, H. Tissot Dupont, J. M. Riss, J. Arditi-Djiane, and P. Brouqui. 1999. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch. Intern. Med. 159:167-173. [DOI] [PubMed] [Google Scholar]
- 21.Raoult, D., B. La Scola, P. Lecocq, H. Lepidi, and P. E. Fournier. 2001. Culture and immunological detection of Tropheryma whippelii from the duodenum of a patient with Whipple disease. JAMA 285:1039-1043. [DOI] [PubMed] [Google Scholar]
- 22.Rathman, M., M. D. Sjaastad, and S. Falkow. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Relman, D. A., T. M. Schmidt, R. P. MacDermott, and S. Falkow. 1992. Identification of the uncultured bacillus of Whipple's disease. N. Engl. J. Med. 327:293-301. [DOI] [PubMed] [Google Scholar]
- 24.Roth, R. I., R. L. Owen, D. F. Keren, and P. A. Volberding. 1985. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS). Histological and clinical comparison with Whipple's disease. Dig. Dis. Sci. 30:497-504. [DOI] [PubMed] [Google Scholar]
- 25.Schoedon, G., D. Goldenberger, R. Forrer, A. Gunz, F. Dutly, M. Hochli, M. Altwegg, and A. Schaffner. 1997. Deactivation of macrophages with interleukin-4 is the key to the isolation of Tropheryma whippelii. J. Infect. Dis. 176:672-677. [DOI] [PubMed] [Google Scholar]
- 26.Silva, M. T., P. M. Macedo, and J. F. Moura Nunes. 1985. Ultrastructure of bacilli and the bacillary origin of the macrophagic inclusions in Whipple's disease. J. Gen. Microbiol. 131:1001-1013. [DOI] [PubMed] [Google Scholar]
- 27.Steele-Mortimer, O., S. Méresse, B. H. Toh, J. P. Gorvel, and B. B. Finlay. 1999. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1:33-50. [DOI] [PubMed] [Google Scholar]
- 28.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, K. Nakayama, M. Yanai, Y. Numazaki, and H. Sasaki. 2001. Bafilomycin A1 inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am. J. Physiol. Lung Cell Mol. Physiol. 280:L1115-L1127. [DOI] [PubMed] [Google Scholar]
- 30.Swartz, M. N. 2000. Whipple's disease: past, present, and future. N. Engl. J. Med. 342:648-650. [DOI] [PubMed] [Google Scholar]
- 31.Tung, C. H., S. Bredow, U. Mahmood, and R. Weissleder. 1999. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug. Chem. 10:892-896. [DOI] [PubMed] [Google Scholar]
- 32.Tung, C. H., U. Mahmood, S. Bredow, and R. Weissleder. 2000. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 60:4953-4958. [PubMed] [Google Scholar]
- 33.Whipple, G. H. 1907. A hitherto undescribed disease characterized anatomvically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull. Johns Hopkins Hosp. 18:382-391.
- 34.Wilson, K. H., R. Blitchington, R. Frothingham, and J. A. Wilson. 1991. Phylogeny of the Whipple's-disease-associated bacterium. Lancet 338:474-475. [DOI] [PubMed] [Google Scholar]