Abstract
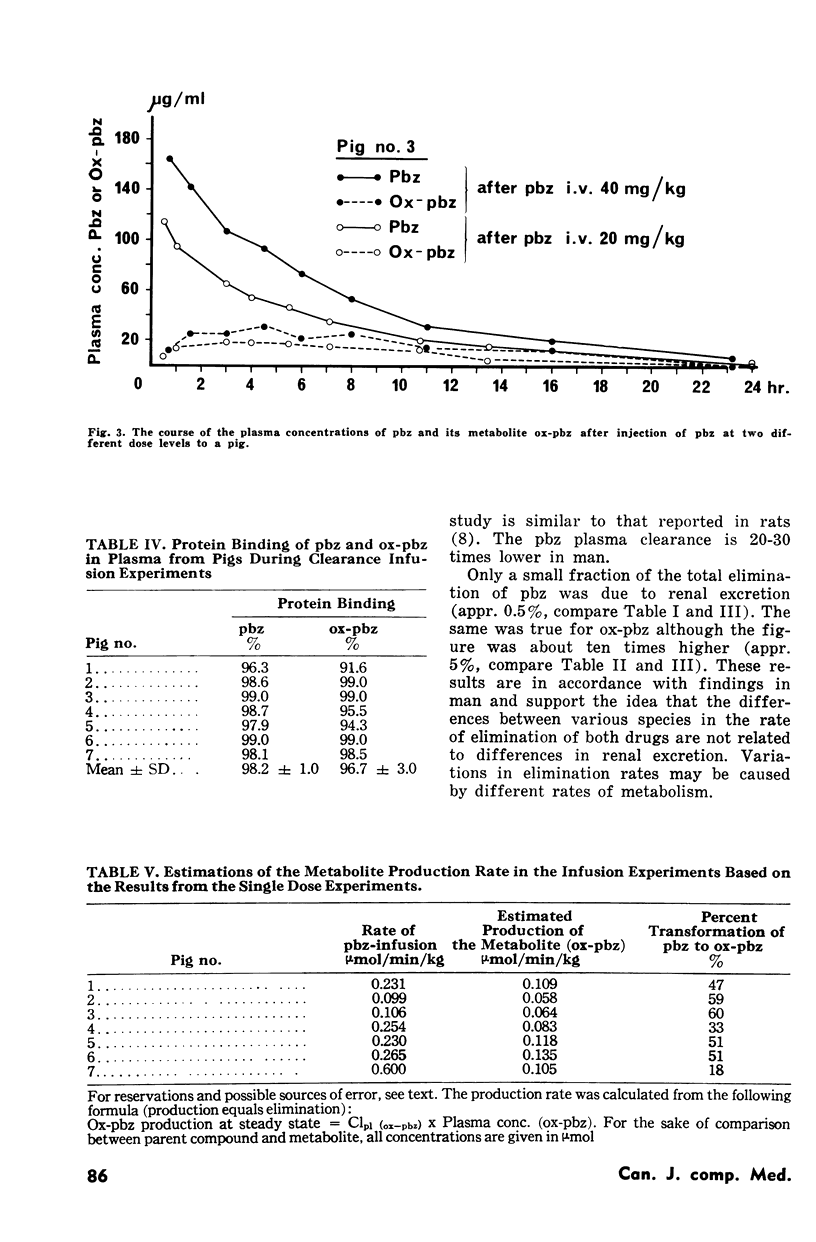
The kinetics of phenylbutazone (pbz) and its main metabolite oxyphenbutazone (oxpbz) were investigated in seven pigs in order to determine if this animal might serve as a model for human investigations. The study was performed in two stages, one as single dose experiments using intravenous injection, the second as infusion experiments. The rate of elimination for both compounds was demonstrated to be much faster than in man. Similar results have been observed in several other animal species. The degree of protein binding (pbz 98%, ox-pbz 97%), the apparent specific volume of distribution (pbz 0.18 1/kg, ox-pbz 0.28 1/kg) and the renal clearance were found to be similar to results obtained in man and rat. Ox-pbz had a higher renal clearance (0.13 ml/min/kg) than pbz (0.003 ml/min/kg) but still the renal excretion constituted only a small fraction of the total elimination (pbz 0.5%, ox-pbz 5%). Provided real steady state conditions were obtained during the infusion experiments calculations based on the results from the single dose experiments in the same animal revealed that between 20 and 60% of the injected pbz was metabolized to the ring-hydroxylation product (ox-pbz). It is concluded that the pig model is not superior to other animal models for studies of pbz/ox-pbz. The rapid elimination pattern is the main problem in relation to human investigations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS J. J., ROSE R. K., CHENKIN T., GOLDMAN A., SCHULERT A., BRODIE B. B. The physiological disposition of phenylbutazone (butazolidin) in man and a method for its estimation in biological material. J Pharmacol Exp Ther. 1953 Nov;109(3):346–357. [PubMed] [Google Scholar]
- Fuchs W. Blutspiegelbestimmung von Butazolidin und Tanderil aus einem Tropfen Kapillarblut. Munch Med Wochenschr. 1965 Jun 18;107(25):1267–1270. [PubMed] [Google Scholar]
- Gyrd-Hansen N. Renal clearances in pigs. Inulin, endogenous creatinine, urea, para-amino-hippuric acid, sodium, potassium, and chloride. Acta Vet Scand. 1968;9(3):183–198. doi: 10.1186/BF03547866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg E. F., Andreasen P. B., Ranek L. Plasma half-life of phenylbutazone in patients with impaired liver function. Clin Pharmacol Ther. 1974 Feb;15(2):171–177. doi: 10.1002/cpt1974152171. [DOI] [PubMed] [Google Scholar]
- Hvidberg E. F., Dayton P. G., Read J. M., 3rd, Wilson C. H. Studies of the interaction of phenylbutazone, oxyphenbutazone and methandrostenolone in man. Proc Soc Exp Biol Med. 1968 Nov;129(2):438–443. doi: 10.3181/00379727-129-33338. [DOI] [PubMed] [Google Scholar]
- Jähnchen E., Levy G. Inhibition of phenylbutazone elimination by its metabolite oxyphenbutazone. Proc Soc Exp Biol Med. 1972 Dec;141(3):963–965. doi: 10.3181/00379727-141-36911. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Sherlock S., Walker D. Phenylbutazone and isoniazid metabolism in patients with liver disease in relation to previous drug therapy. Lancet. 1968 Jun 15;1(7555):1275–1279. doi: 10.1016/s0140-6736(68)92292-7. [DOI] [PubMed] [Google Scholar]
- Nielsen C. K., Hogsbro E., Frey H. H. Versuche zur Dosierung von Phenylbutazon beim Hund. Dtsch Tierarztl Wochenschr. 1969 Jul 15;76(14):378–381. [PubMed] [Google Scholar]
- PEREL J. M., SNELL M. M., CHEN W., DAYTON P. G. A STUDY OF STRUCTURE--ACTIVITY RELATIONSHIPS IN REGARD TO SPECIES DIFFERENCE IN THE PHENYLBUTAZONE SERIES. Biochem Pharmacol. 1964 Sep;13:1305–1317. doi: 10.1016/0006-2952(64)90231-x. [DOI] [PubMed] [Google Scholar]
- Piperno E., Ellis D. J., Getty S. M., Brody T. M. Plasma and urine levels of phenylbutazone in the horse. J Am Vet Med Assoc. 1968 Jul 15;153(2):195–198. [PubMed] [Google Scholar]
- Rowland M. Application of clearance concepts to some literature data on drug metabolism in the isolated perfused liver preparation and in vivo. Eur J Pharmacol. 1972 Mar;17(3):352–356. doi: 10.1016/0014-2999(72)90115-x. [DOI] [PubMed] [Google Scholar]


