Abstract
We investigated the role of osteopontin (OPN) in interleukin-12 (IL-12) production from peripheral blood mononuclear cells (PBMCs) stimulated with Penicillium marneffei. Kinetic studies showed that OPN synthesis preceded that of IL-12 at both mRNA and protein levels when PBMCs were cocultured with P. marneffei. Treatment with anti-OPN monoclonal antibodies (MAb) significantly suppressed IL-12 secretion. Furthermore, native OPN induced a profound level of synthesis of IL-12 from noninfected PBMCs. The major cellular source of OPN was monocytes, because depletion of CD14+ cells resulted in the abrogation of such production. We also examined the regulatory role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in OPN secretion from P. marneffei-stimulated PBMCs. Neutralizing anti-GM-CSF MAb significantly reduced OPN secretion, and treatment with this cytokine induced OPN production from both infected and noninfected PBMCs. Finally, antagonists against the mannose receptor but not the β-glucan receptor almost completely abrogated the production of OPN. Our results demonstrated that OPN secreted from monocytes is involved in the production of IL-12 from PBMCs after stimulation with P. marneffei and that OPN production is regulated by GM-CSF. Our results also indicated the possible involvement of the mannose receptor as a signal-transducing receptor for triggering the secretion of OPN by P. marneffei-stimulated PBMCs.
Penicillium marneffei, an opportunistic fungal pathogen, causes a life-threatening deep-seated systemic infection in patients with AIDS in Southeast Asian countries. The incidence of P. marneffei infection has been increasing among AIDS patients in Thailand since a decade ago (8, 12, 40). As predicted by the high incidence in these patients, cellular immunity is a central mechanism that mediates host resistance against infection with P. marneffei (18), and CD4+ T cells play an important role in eradicating the microorganisms from infected sites (19). Interleukin-12 (IL-12) protects mice against this infection (17). Furthermore, gamma interferon, generated during the progression of cellular immunity, renders murine macrophages highly active in killing P. marneffei yeast cells, a form usually detected in biological samples from infected patients, by promoting the release of nitric oxide (20).
Osteopontin (OPN), a phosphoprotein originally isolated from bone, contains an RGD sequence similar to that of other extracellular matrix and serum proteins, including fibronectin, vitronectin, collagen, thrombospondin, and fibrinogen (7, 30, 42). OPN promotes integrin- and CD44-mediated cell adhesion and chemotaxis of osteoclasts, smooth muscle cells, macrophages, and T cells (30). OPN production is elevated in various pathophysiological conditions, such as atherosclerosis, nephritis, malignancy, pulmonary fibrosis, wound healing, and bone remodeling (6, 9, 13, 21, 28, 35, 41). OPN is also abundantly produced by T cells and macrophages during the formation of granulomatous lesions in sarcoidosis and tuberculosis (4, 25, 27, 29).
Recently, a new aspect of OPN was reported by Ashkar and coworkers (1). OPN-deficient mice showed severe impairment in developing a Th1 response to herpes simplex virus type 1 and Listeria monocytogenes and granuloma formation caused by polyvinylpyrrolidone. The production of IL-12 and gamma interferon was diminished while the synthesis of IL-10 was elevated in these mice, and OPN treatment restored the reduced production of IL-12 from their macrophages. These findings suggested that OPN may polarize the Th1 cytokine response and contribute to host defense against infectious pathogens. In early studies, the OPN gene was proposed to relate to the susceptibility of mice against Orientia tsutsugamushi infection (31). Recently, Nau et al. (26) revealed the reduced clearance of Mycobacterium bovis BCG and the increased formation of granulomas in OPN-deficient mice. Thus, accumulating evidence supports the above hypothesis for a murine system. However, to our knowledge, no reports have demonstrated such an aspect of OPN for human cells.
In the present study, we elucidated the role of OPN in IL-12 production from peripheral blood mononuclear cells (PBMCs) stimulated with P. marneffei. For this purpose, we examined the kinetics of mRNA expression and protein secretion for OPN and IL-12 during coculturing of PBMCs with P. marneffei. Next, we tested the effect of anti-OPN monoclonal antibodies (MAb) on the secretion of IL-12 and the ability of native OPN to induce IL-12 from PBMCs. Furthermore, we investigated the involvement of granulocyte-macrophage colony-stimulating factor (GM-CSF) in the production of OPN from P. marneffei-stimulated PBMCs. Finally, the effects of various antagonists against the mannose or β-glucan receptor were examined to define the mechanism of PBMC recognition of the fungal organism leading to OPN synthesis.
MATERIALS AND METHODS
Culture media and reagents.
RPMI 1640 medium was purchased from GIBCO Laboratories (Grand Island, N.Y.); fetal calf serum was obtained from Cansera (Rexdale, Ontario, Canada); native OPN was obtained from Hokudo Co. (Sapporo, Japan); recombinant human GM-CSF was obtained from Pepro Tec EC (London, United Kingdom); antihuman GM-CSF MAb (mouse immunoglobulin G1 [IgG1]) was obtained from Endogen, Inc. (Cambridge, Mass.); antihuman IL-1 receptor type I (IL-1RI) MAb, antihuman tumor necrosis factor alpha (TNF-α) MAb, and isotype control mouse IgG1 antibody were obtained from Genzyme-Techne (Minneapolis, Minn.); laminarin was obtained from Sigma (St. Louis, Mo.); laminariheptaose was obtained from Seikagaku Co. (Tokyo, Japan); and α-mannan and ovalbumin were obtained from Sigma. Recombinant human OPN and anti-OPN MAb (mouse IgG1) were kindly provided by Immuno-Biological Laboratories Co., Ltd. (Gunma, Japan).
Preparation of PBMCs.
PBMCs were isolated from heparinized blood of healthy adult volunteers by standard density gradient centrifugation over Ficoll-Paque PLUS (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). Interface PBMCs were pelleted, washed twice, and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μM 2-mercaptoethanol.
Microorganisms and culture conditions.
A clinical strain of P. marneffei, designated H1140 and registered by the American Type Culture Collection (ATCC 201013), was isolated from a blood sample of a patient with AIDS at Chiang Mai University, Chiang Mai, Thailand, and kindly provided by Parasit Tharvichitkul from the same university. Methods for preparation of the yeast cell suspension were described in detail previously by Kudeken et al. (20). PBMCs were cocultured at 5 × 106/ml with P. marneffei (2.5 × 106/ml) for different times.
Extraction of RNA and reverse transcription-PCR.
Total cellular RNA was extracted from PBMCs cultured with P. marneffei after various incubation periods with Isogen (Wako, Osaka, Japan), followed by reverse transcription (14). The obtained cDNA was then amplified in an automatic DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) with specific primers: 5′-CCACATTCCTACTTCTC-3′ (sense) and 5′-GTCTATTCCGTTGTGTC-3′ (antisense) for IL-12p40, 5′-CCAAGTAAGTCCAACGAAAG-3′ (sense) and 5′-GGTGATGTCCTCGTCTGTA-3′ (antisense) for OPN, and 5′-ACCACCATGGAGAAGGCTGG-3′ (sense) and 5′-CTCAGTGTAGCCCAGGATGC-3′ (antisense) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). We added 1.0 μl of the sample cDNA solution to 49 μl of the reaction mixture, which contained the following components: 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 10 μg of gelatin/ml, deoxynucleoside triphosphates (each at a concentration of 200 μM), 1.0 μM each sense and antisense primers, and 1.25 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus). PCR conditions of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min, and extension at 72°C for 2 min were used for IL-12p40. The sequence of amplification for OPN involved initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 0.5 min, annealing at 55°C for 2 min, and extension at 72°C for 2 min. Cycling conditions for GAPDH were 94°C for 0.5 min, 55°C for 0.5 min, and 72°C for 2 min for 26 cycles. The PCR products were electrophoresed on 2% agarose gels, stained with 0.5 μg of ethidium bromide/ml, and observed with a UV transilluminator. The obtained bands of amplified DNA were quantitated using the NIH image analysis software application, and the level of expression of OPN or IL-12p40 mRNA was quantitated relative to that of GAPDH mRNA.
Measurement of cytokines.
The concentration of OPN in PBMC culture supernatants was measured by an antigen capture enzyme-linked immunosorbent assay (ELISA) as previously described (15). The IL-12p40 concentration was measured with a commercial ELISA kit (Biosource International, Inc., Camarillo, Calif.). The sensitivities of these assays were 5 ng/ml and 8.2 pg/ml for the respective cytokines.
Depletion of cell subsets from PBMCs.
CD3+, CD14+, or CD56+ cells were depleted from PBMCs with a magnetic cell separation system (MACS) under standard conditions. Briefly, 2 × 107 to 3 × 107 PBMCs were incubated with anti-CD3, -CD14, or -CD56 MAb-conjugated MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany), and the labeled cells were separated on a MACS column. Depleted cell suspensions contained less than 0.5, 0.5, or 0.6% contaminating CD3+, CD14+, or CD56+ cells, respectively.
Preparation of human T cells, NK cells, and monocytes.
T cells, NK cells, and monocytes were separated on the basis of CD3, CD56, and CD14 expression by magnetic cell sorting with the MACS, respectively. Briefly, PBMCs (2 × 107 cells/80 μl) in MACS buffer (Ca2+- and Mg2+-free phosphate-buffered saline supplemented with 1% bovine serum albumin and 10 mmol of EDTA/liter) were labeled with the respective MAb conjugated with MACS superparamagnetic microbeads for 20 min at a concentration of 20 μl/107 cells at 6 to 12°C. After two washes in MACS buffer, cells were separated on a magnetic stainless steel wool column according to the protocol provided by the manufacturer. Cells positive for respective cell surface markers attached to the magnetized matrix were obtained after removal of the column from the magnet and were flushed with MACS buffer into another tube. Flow cytometric analysis of the collected cells showed 99% CD3, 98% CD14, and 99% CD56 positivity.
Statistical analysis.
Statistical analysis was performed with Statview II software (Abacus Concept, Inc., Berkeley, Calif.) on a Macintosh computer. Data are expressed as mean and standard deviation (SD). Differences between groups were examined with the analysis of variance test and post hoc analysis (Fisher’s protected least significant difference test). A P value of less than 0.05 was considered significant.
RESULTS
Contribution of OPN to IL-12 synthesis by PBMCs upon stimulation with P. marneffei.
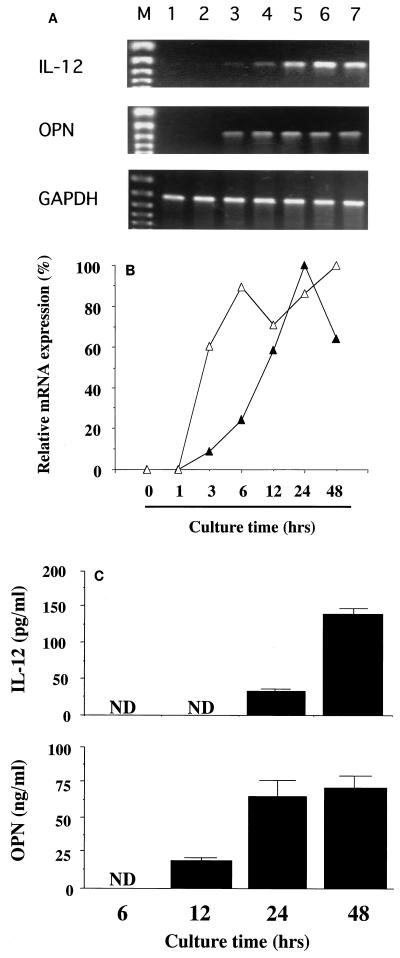
To elucidate the role of OPN in the synthesis of IL-12 by PBMCs, we initially compared the kinetics of production of these two cytokines during culturing with live P. marneffei at both mRNA and protein levels. Representative data from RT-PCR analysis are shown in Fig. 1A, and the mRNA expression of each cytokine relative to that of GAPDH is indicated in Fig. 1B. The mRNA of OPN was expressed at 3 h and reached a plateau at 6 h, while the commencement of IL-12 mRNA expression was delayed for 3 h, with a peak level at 24 h. Similarly, OPN protein secretion commenced at 12 h, and the synthesis of IL-12 commenced at 24 h (Fig. 1C).
FIG. 1.
Kinetics of OPN and IL-12p40 mRNA expression in P. marneffei-stimulated PBMCs. (A) PBMCs were cocultured with P. marneffei for various times, and total RNA was extracted from the cells. Subsequently, reverse transcription-PCR was carried out for OPN and IL-12p40. GAPDH was used as an internal control. Lanes: M, DNA size marker; 1, 0 h; 2, 1 h; 3, 3 h; 4, 6 h; 5, 12 h; 6, 24 h; 7, 48 h. (B) The levels of expression of IL-12p40 (closed triangles) and OPN (open triangles) mRNAs were quantitated relative to that of GAPDH mRNA. The results are expressed as a percentage relative to the peak level for each cytokine. Each symbol represents the mean for three independent cultures. (C) For some cultures, supernatants were collected at various times, and the concentration of each cytokine was measured. Each bar represents the mean and SD for three independent cultures. ND, not detected.
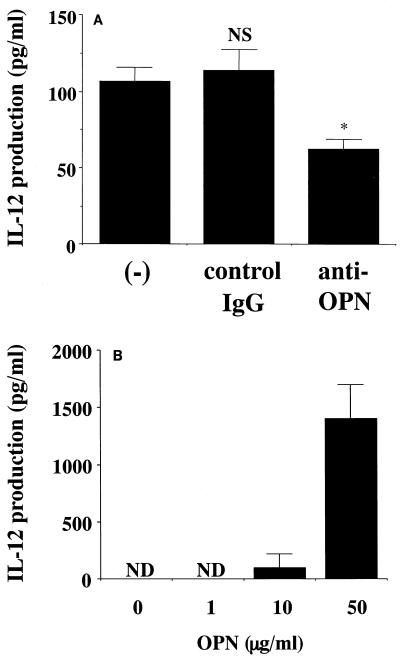
The sequential production of these cytokines suggested that OPN may be positively involved in the synthesis of IL-12, as previously indicated in a murine system (1). To test this possibility, we conducted two separate experiments. First, we examined the effect of neutralizing anti-OPN MAb on the secretion of IL-12 by P. marneffei-stimulated PBMCs. As shown in Fig. 2A, this treatment significantly but partially reduced IL-12 production, while control IgG did not have such an effect. In the next experiment, we examined the effect of native OPN on the production of IL-12 by PBMCs in the absence of P. marneffei. As shown in Fig. 2B, the administration of native OPN induced IL-12 production in a dose-dependent manner, with an optimal concentration of 50 μg/ml.
FIG. 2.
Involvement of OPN in the production of IL-12 from PBMCs stimulated with P. marneffei. (A) PBMCs were cocultured at 5 × 106/ml with P. marneffei (2.5 × 106/ml) in the presence or absence of 1 μg of anti-OPN MAb or control IgG/ml for 48 h. (B) PBMCs were cultured at 5 × 106/ml in the presence or absence of increasing doses of native OPN for 48 h. The concentrations of IL-12p40 in culture supernatants were measured. Each bar represents the mean and SD for three independent cultures. NS, not significant; an asterisk indicates a P value of <0.01 in a comparison with IL-12 production by P. marneffei-stimulated PBMCs in the absence of antibody (−). ND, not detected.
Monocytes are a major source of P. marneffei-driven OPN production.
To define the cellular source of OPN, we depleted CD3+, CD14+, or CD56+ cells from PBMCs as described in Materials and Methods and examined the effect of such treatment on the production of OPN upon stimulation with P. marneffei. As shown in Fig. 3, the lack of CD14+ cells resulted in a dramatic reduction in the synthesis of OPN, while such reduction was not found following the depletion of CD3+ or CD56+ cells. These results suggested that CD14+ cells predominantly secreted OPN upon stimulation with P. marneffei.
FIG. 3.
Removal of CD14+ cells abrogates OPN production. PBMCs or CD3-, CD14-, or CD56-depleted PBMCs at 5 × 106/ml were cocultured and stimulated with P. marneffei (2.5 × 106/ml) for 48 h. The concentrations of OPN in culture supernatants were measured. Each bar represents the mean and SD for three independent cultures. Open bars, undepleted; closed bars, depleted. NS, not significant; a single asterisk and double asterisks indicate P values of <0.05 and<0.01, respectively, in comparisons with OPN production by undepleted PBMCs.
To further confirm this possibility, CD3+, CD14+, or CD56+ cells were purified from PBMCs by magnetic sorting, and the cells obtained were stimulated with various doses of P. marneffei. As shown in Table 1, CD14+ cells but not CD3+ or CD56+ cells produced a considerable amount of OPN upon stimulation with P. marneffei. These results indicated that CD14+ monocytes are the major source of OPN among cultured PBMCs.
TABLE 1.
Monocytes are the major source of OPN productiona
| Donor | OPN production (ng/ml) in the following cells:
|
||
|---|---|---|---|
| CD3+ | CD14+ | CD56+ | |
| 1 | <5 | 63.5 ± 8.8 | <5 |
| 2 | 8.0 ± 2.3 | 150.2 ± 2.4 | 14.1 ± 1.1 |
| 3 | 13.6 ± 2.2 | 106.4 ± 5.2 | 27.7 ± 2.0 |
CD3+, CD14+, or CD56+ cells purified from PBMCs were cultured at 106/ml with P. marneffei (5 × 105/ml) for 48 h. The concentration of OPN in the culture supernatants was measured. Results are reported as the mean and SD for three independent cultures.<5, below the detection limit.
GM-CSF is involved in P. marneffei-induced production of OPN by PBMCs.
OPN production is known to be induced by proinflammatory cytokines such as TNF-α and IL-1β in nonimmune cells (31, 36). Therefore, to elucidate the contribution of these cytokines to OPN synthesis by PBMCs, we examined the effects of neutralizing MAb against TNF-α and IL-1RI on such production caused by P. marneffei. Unexpectedly, none of these antibodies had an inhibitory effect on OPN production by PBMCs stimulated with P. marneffei (Fig. 4). Other studies have shown that OPN production is induced by GM-CSF in mouse bone marrow cells (22). To examine the involvement of this cytokine, a neutralizing anti-GM-CSF MAb was added to cultures of PBMCs stimulated with P. marneffei at a concentration of 1 μg/ml. As shown in Fig. 5A, this treatment significantly reduced the synthesis of OPN, while control IgG did not have such an effect. These results suggested that GM-CSF might be involved in the synthesis of OPN by PBMCs. In order to confirm this possibility, we tested whether recombinant GM-CSF (rGM-CSF) directly caused the secretion of OPN. As shown in Fig. 5B, the administration of rGM-CSF stimulated such production by PBMCs in a dose-dependent manner, with an optimal dose of 10 ng/ml. Furthermore, the same treatment enhanced the synthesis of OPN by P. marneffei-stimulated PBMCs (Fig. 5C).
FIG. 4.
Failure of anti-TNF-α and -IL-1RI MAb to inhibit OPN production. PBMCs were cocultured at 5 × 106/ml with P. marneffei (2.5 × 106/ml) in the presence or absence of 1 μg of anti-TNF-α or -IL-1RI MAb or control IgG/ml for 48 h. The concentrations of OPN in culture supernatants were measured. Each bar represents the mean and SD for three independent cultures. NS, not significant in comparisons with OPN production by P. marneffei-stimulated PBMCs in the absence of antibody (−).
FIG. 5.
Involvement of GM-CSF in the production of OPN from PBMCs stimulated with P. marneffei. (A). PBMCs were cocultured at 5 × 106/ml with P. marneffei (Pm) (2.5 × 106/ml) in the presence or absence of 1 μg of anti-GM-CSF MAb or control IgG/ml for 48 h. (B and C) In other experiments, PBMCs were cultured at 5 × 106/ml with increasing doses of rGM-CSF in the absence (B) or presence (C) of P. marneffei (2.5 × 106/ml) for 48 h. The concentrations of OPN in culture supernatants were measured. Each bar represents the mean and SD for three independent cultures. NS, not significant; an asterisk indicates a P value of <0.01 in a comparison with OPN production by P. marneffei-stimulated PBMCs in the absence of antibody.
Molecular mechanism of OPN production by P. marneffei-stimulated PBMCs.
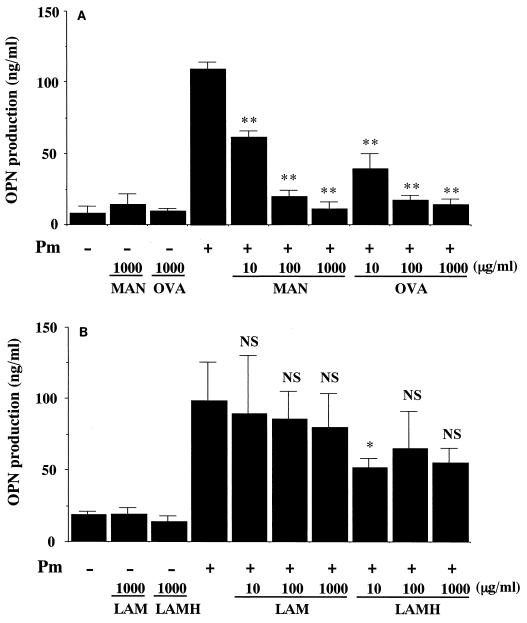
Finally, we determined the molecules responsible for the recognition of P. marneffei by PBMCs, which leads to the production of OPN. For this purpose, the effects of various antagonists to the mannose or β-glucan receptor were examined. Incubation of PBMCs in the presence of either yeast mannan or ovalbumin, each of which interferes with ligand binding to the mannose receptor, almost completely abrogated OPN secretion (Fig. 6A). In contrast, laminarin and laminariheptaose, soluble carbohydrate antagonists to the β-glucan receptor, caused no or only marginal suppression of OPN release induced by P. marneffei-stimulated PBMCs (Fig. 6B). These results suggested the important role of the mannose receptor in the fungus-induced synthesis of OPN by PBMCs.
FIG. 6.
The mannose receptor but not the β-glucan receptor mediates OPN production from P. marneffei-stimulated PBMCs. PBMCs were cocultured at 5 × 106/ml with or without P. marneffei (Pm) (2.5 × 106/ml) in the presence or absence of the indicated doses of mannose receptor antagonists (A) or β-glucan receptor antagonists (B) for 48 h. The concentrations of OPN in culture supernatants were measured. Each bar represents the mean and SD for three independent cultures. NS, not significant; a single asterisk and double asterisks indicate P values of <0.05 and <0.01, respectively, in comparisons with OPN production by P. marneffei-stimulated PBMCs in the absence of antagonists. MAN,α-mannan; OVA, ovalbumin; LAM, laminarin; LAMH, laminariheptaose.
DISCUSSION
Recently, Ashkar et al. (1) proposed the hypothesis that OPN polarizes the immune response toward a Th1-dominant rather than a Th2-dominant condition through the induction of IL-12 production by macrophages in mice. In the present study, we investigated whether such a hypothesis could be applied to humans by examining the expression of OPN and IL-12 by PBMCs stimulated with P. marneffei. Based on three different lines of evidence, our study indicated that OPN is involved in the production of IL-12 from PBMCs. First, in the kinetic analysis, the production of OPN preceded that of IL-12 at both mRNA and protein levels. Second, neutralizing anti-OPN MAb exerted significant suppression of IL-12 production by P. marneffei-infected PBMCs. Finally, administration of native OPN strikingly induced the release of IL-12 by uninfected PBMCs. In the second experiment, the effect of anti-OPN MAb was partial, although the MAb was administered at a dose not higher than 1μg/ml. IL-12 production was increased in a nonspecific manner by not only anti-OPN MAb but also control IgG when used at a higher dose. In addition, similar results were observed even with the F(ab′)2 fragment of the antibody (data not shown). At present, the mechanism of this effect remains unknown. To define the precise contribution of OPN to fungus-induced IL-12 production, further studies with other strategies, such as dominant-negative suppression, are necessary.
OPN exists in multiple forms, depending on the posttranslational modification. In addition to sulfation (24), glycosylation (38), and transglutamination (2), it undergoes extensive phosphorylation. The highly phosphorylated form has been isolated from mineralized extracellular matrix of bone tissue (34) and is synthesized by osteoblasts (10, 11). Breast milk has also been shown to contain highly phosphorylated OPN (37). OPN is usually secreted in both nonphosphorylated and phosphorylated forms (5, 16, 35, 37). In the present study, we did not elucidate which form of OPN was secreted from P. marneffei-stimulated PBMCs, because the ELISA used recognizes both. Interestingly, a native form of OPN obtained from human breast milk induced considerable production of IL-12, while a recombinant form derived from human OPN gene-transfected CHO cells failed to do so (data not shown). These findings are consistent with earlier reports demonstrating that dephosphorylation of native OPN abolished IL-12 stimulatory activity and, in contrast, that phosphorylation of recombinant OPN at specific sites restored such activity (1).
The OPN gene has been independently identified as an early T-cell activation gene (Eta-1), and its product is the most abundant early RNA transcript in concanavalin A-activated murine T cells (31). Recently, Nagai and coworkers (23) reported that OPN mRNA was preferentially expressed in activated human Th1 cells. On the other hand, Nau et al. (25) demonstrated that the OPN gene was the most prominent early gene which was specifically upregulated by peritoneal and alveolar macrophages after in vitro infection with Mycobacterium tuberculosis. The OPN protein is also an early protein expressed by natural killer cells (33). In the present study, we extended these early findings by identifying peripheral blood monocytes as an additional and major producer of OPN in PBMCs stimulated with P. marneffei. Furthermore, our results suggested that OPN enhanced IL-12 production via autocrine and paracrine mechanisms.
OPN production by nonimmune cells is regulated by proinflammatory cytokines, such as TNF-α and IL-1β (31, 36). In contrast, we did not observe any inhibitory effect of neutralizing anti-TNF-α or -IL-1RI MAb on OPN production by P. marneffei-stimulated PBMCs. However, neutralization of GM-CSF bioactivity by a specific antibody resulted in a significant reduction of such synthesis. In addition, the administration of GM-CSF caused abundant production of OPN by uninfected PBMCs. Our data suggested that GM-CSF mediated a critical regulatory mechanism for OPN. This hypothesis is compatible with a previous report by Lin et al. (22) indicating that OPN released as a result of IL-3 or GM-CSF signaling contributed to the survival-promoting activities of these two cytokines.
Finally, we demonstrated that antagonists against the mannose receptor but not the β-glucan receptor almost completely abrogated the production of OPN from P. marneffei-stimulated PBMCs. A set of observations suggests that the mannose receptor, when engaged by microorganisms or particles, may be a signal-transducing receptor for triggering the secretion of cytokines (39). Cao et al. (3) recently cloned the Mp1 gene, which encodes an abundant antigenic cell wall mannoprotein (Mp1p), a 462-amino-acid protein, from P. marneffei. They showed that Mp1p was located specifically in the cell wall of the yeast form of P. marneffei and was evenly spread throughout the entire thickness of the yeast cell wall. Furthermore, Pitzurra and coworkers (32) revealed that mannoprotein, a cryptococcal envelope antigen, was responsible for the early production of IL-12 by human peripheral blood monocytes. For mannoprotein-induced cytokine secretion, two different processes are required: (i) recognition of this protein by a specific receptor on monocytes and (ii) its internalization via the endocytic pathway. Taken together, our results suggested the possible involvement of such a mechanism mediated by mannoprotein in the production of OPN by monocytes upon stimulation by P. marneffei.
In conclusion, we have provided sufficient evidence in the present study indicating that OPN promotes a Th1 response through the induction of IL-12 synthesis by human macrophages, similar to earlier findings described for mice. We have also demonstrated that OPN production by monocytes is regulated by GM-CSF. These findings provide another option for investigating the mechanism of Th1-cell development under various pathological conditions, including infectious diseases, in humans.
Acknowledgments
This work was supported in part by grants-in-aid for science research (09670292 and 12670261) from the Ministry of Education, Science and Culture and by grants from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Ashkar, S., G. F. Weber, V. Panoutsakopoulou, M. E. Sanchirico, M. Jansson, S. Zawaideh, S. R. Rittling, D. T. Denhardt, M. J. Glimcher, and H. Cantor. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860-864. [DOI] [PubMed] [Google Scholar]
- 2.Beninati, S., D. R. Senger, E. Cordella-Miele, A. B. Mukherjee, I, Chackalaparampil, V. Shanmugam, K. Singh, and B. B. Mukherjee. 1994. Osteopontin: its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J. Biochem. (Tokyo) 115:675-682. [DOI] [PubMed] [Google Scholar]
- 3.Cao, L., C. M. Chan, C. Lee, S. S. Wong, and K. Y. Yuen. 1998. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 66:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson, I., K. Tognazzi, E. J. Manseau, H. F. Dvorak, and L. F. Brown. 1997. Osteopontin is strongly expressed by histiocytes in granulomas of diverse etiology. Lab. Investig. 77:103-108. [PubMed] [Google Scholar]
- 5.Chambers, A. F., E. I. Behrend, S. M. Wilson, and D. T. Denhardt. 1992. Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic, ras-transformed NIH 3T3 cells. Anticancer Res. 12:43-47. [PubMed] [Google Scholar]
- 6.Chiba, S., M. M. Rashid, H. Okamoto, H. Shiraiwa, S. Kon, M. Maeda, M. Murakami, M. Inobe, A. Kitabatake, A. F. Chambers, and T. Uede. 2000. The role of osteopontin in the development of granulomatous lesions in lung. Microbiol. Immunol. 44:319-332. [DOI] [PubMed] [Google Scholar]
- 7.Denhardt, D. T., and X. Guo. 1993. Osteopontin: a protein with diverse functions. FASEB J. 7:1475-1482. [PubMed] [Google Scholar]
- 8.Duong, T. A. 1996. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin. Infect. Dis. 23:125-130. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick, L. A., A. Severson, W. D. Edwards, and R. T. Ingram. 1994. Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J. Clin. Investig. 94:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerstenfeld, L. C., J. B. Lian, Y. Gotoh, D. D. Lee, W. J. Landis, M. D. McKee, A. Nanci, and M. J. Glimcher. 1989. Use of cultured embryonic chicken osteoblasts as a model of cellular differentiation and bone mineralization. Connect. Tissue Res. 21:215-223. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh, Y., L. C. Gerstenfeld, and M. J. Glimcher. 1990. Identification and characterization of the major chicken bone phosphoprotein. Analysis of its synthesis by cultured embryonic chick osteoblasts. Eur. J. Biochem. 187:49-58. [DOI] [PubMed] [Google Scholar]
- 12.Hilmarsdottir, I., J. L. Meynard, O. Rogeaux, G. Guermonprez, A. Datry, C. Katlama, G. Brucker, A. Coutellier, M. Danis, and M. Gentilini. 1993. Disseminated Penicillium marneffei infection associated with human immunodeficiency virus: a report of two cases and review of 35 published cases. J. Acquir. Immune Defic. Syndr. 6:466-471. [PubMed] [Google Scholar]
- 13.Katagiri, Y. U., J. Sleeman, H. Fujii, P. Herrlich, H. Hotta, K. Tanaka, S. Chikuma, H. Yagita, K. Okumura, M. Murakami, I. Saiki, A. F. Chambers, and T. Uede. 1999. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 59:219-226. [PubMed] [Google Scholar]
- 14.Kawakami, K., M. Tohyama, X. Qifeng, and A. Saito. 1997. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect. Immun. 65:1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kon, S., M. Maeda, T. Segawa, Y. Hagiwara, Y. Horikoshi, S. Chikuma, K. Tanaka, M. M. Rashid, M. Inobe, A. F. Chambers, and T. Uede. 2000. Antibodies to different peptides in osteopontin reveal complexities in the various secreted forms. J. Cell. Biochem. 77:487-498. [PubMed] [Google Scholar]
- 16.Kubota, T., Q. Zhang, J. L. Wrana, R. Ber, J. E. Aubin, W. T. Butler, and J. Sodek. 1989. Multiple forms of SppI (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochem. Biophys. Res. Commun. 162:1453-1459. [DOI] [PubMed] [Google Scholar]
- 17.Kudeken, N., K. Kawakami, M. Tohyama, N. Kusano, and A. Saito. 1996. Therapeutic effect of interleukin-12 in the murine model of pulmonary infection with Penicillium marneffei. Kansenshogaku Zasshi. 70:842-843. (In Japanese.) [DOI] [PubMed]
- 18.Kudeken, N., K. Kawakami, N. Kusano, and A. Saito. 1996. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J. Med. Vet. Mycol. 34:371-378. [DOI] [PubMed] [Google Scholar]
- 19.Kudeken, N., K. Kawakami, and A. Saito. 1997. CD4+ T cell-mediated fatal hyperinflammatory reactions in mice infected with Penicillium marneffei. Clin. Exp. Immunol. 107:468-473. [DOI] [PubMed] [Google Scholar]
- 20.Kudeken, N., K. Kawakami, and A. Saito. 1998. Different susceptibilities of yeasts and conidia of Penicillium marneffei to nitric oxide-mediated fungicidal activity of murine macrophages. Clin. Exp. Immunol. 112:287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liaw, L., D. E. Birk, C. B. Ballas, J. S. Whitsitt, J. M. Davidson, and B. L. Hogan. 1998. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 101:1468-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, Y. H., C. J. Huang, J. R. Chao, S. T. Chen, S. F. Lee, J. J. Yen, and H. F. Yang-Yen. 2000. Coupling of osteopontin and its cell surface receptor, CD44, to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol. Cell. Biol. 20:2734-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai, S., S. Hashimoto, T. Yamashita, N. Toyoda, T. Satoh, T. Suzuki, and K. Matsushima. 2001. Comprehensive gene expression profile of human activated Th1- and Th2-polarized cells. Int. Immunol. 13:367-376. [DOI] [PubMed] [Google Scholar]
- 24.Nagata, T., R. Todescan, H. A. Goldberg, Q. Zhang, and J. Sodek. 1989. Sulphation of secreted phosphoprotein I (SPPI, osteopontin) is associated with mineralized tissue formation. Biochem. Biophys. Res. Commun. 165:234-240. [DOI] [PubMed] [Google Scholar]
- 25.Nau, G. J., P. Guilfoile, G. L. Chupp, J. S. Berman, S. J. Kim, H. Kornfeld, and R. A. Young. 1997. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. USA 94:6414-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nau, G. J., L. Liaw, G. L. Chupp, J. S. Berman, B. L. Hogan, and R. A. Young. 1999. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 67:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nau, G. J., G. L. Chupp, J. F. Emile, E. Jouanguy, J. S. Berman, J. L. Casanova, and R. A. Young. 2000. Osteopontin expression correlates with clinical outcome in patients with mycobacterial infection. Am. J. Pathol. 157:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ophascharoensuk, V., C. M. Giachelli, K. Gordon, J. Hughes, R. Pichler, P. Brown, L. Liaw, R. Schmidt, S. J. Shankland, C. E. Alpers, W. G. Couser, and R. J. Johnson. 1999. Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 56:571-580. [DOI] [PubMed] [Google Scholar]
- 29.O'Regan, A. W., G. L. Chupp, J. A. Lowry, M. Goetschkes, N. Mulligan, and J. S. Berman. 1999. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J. Immunol. 162:1024-1031. [PubMed] [Google Scholar]
- 30.O'Regan, A. W., G. J. Nau, G. L. Chupp, and J. S. Berman. 2000. Osteopontin (Eta-1) in cell-mediated immunity: teaching an old dog new tricks. Immunol. Today 21:475-478. [DOI] [PubMed] [Google Scholar]
- 31.Patarca, R., R. A. Saavedra, and H. Cantor. 1993. Molecular and cellular basis of genetic resistance to bacterial infection: role of early T-lymphocyte activation-1/osteopontin gene. Crit. Rev. Immunol. 13:225-246. [PubMed] [Google Scholar]
- 32.Pitzurra, L., R. Cherniak, M. Giammarioli, S. Perito, F. Bistoni, and A. Vecchiarelli. 2000. Early induction of interleukin-12 by human monocytes exposed to Cryptococcus neoformans mannoproteins. Infect. Immun. 68:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack, S. B., P. A. Linnemeyer, and S. Gill. 1994. Induction of osteopontin mRNA expression during activation of murine NK cells. J. Leukoc. Biol. 55:398-400. [DOI] [PubMed] [Google Scholar]
- 34.Prince, C. W., T. Oosawa, W. T. Butler, M. Tomana, A. S. Bhown, M. Bhown, and R. E. Schrohenloher. 1987. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J. Biol. Chem. 262:2900-2907. [PubMed] [Google Scholar]
- 35.Reinholt, F. P., K. Hultenby, A. Oldberg, and D. Heinegard. 1990. Osteopontin—a possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 87:4473-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittling, S., and D. T. Denhardt. 1999. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp. Nephrol. 7:103-113. [DOI] [PubMed] [Google Scholar]
- 37.Sorensen, E. S., and T. E. Petersen. 1994. Identification of two phosphorylation motifs in bovine osteopontin. Biochem. Biophys. Res. Commun. 198:200-205. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen, E. S., P. Hojrup, and T. E. Petersen. 1995. Posttranslational modifications of bovine osteopontin: identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 4:2040-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl, P. D., and R. A. Ezekowitz. 1998. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10:50-55. [DOI] [PubMed] [Google Scholar]
- 40.Supparatpinyo, K., C. Khamwan, V. Baosoung, K. E. Nelson, and T. Sirisanthana. 1994. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet 344:110-113. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, F., K. Takahashi, T. Okazaki, K. Maeda, H. Ienaga, M. Maeda, S. Kon, T. Uede, and Y. Fukuchi. 2001. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis Am. J. Respir. Cell Mol. Biol. 24:264-271. [DOI] [PubMed] [Google Scholar]
- 42.Uede, T., Y. Katagiri, J. Iizuka, and M. Murakami. 1997. Osteopontin, a coordinator of host defense system: a cytokine or an extracellular adhesive protein? Microbiol. Immunol. 41:641-648. [DOI] [PubMed] [Google Scholar]