Abstract
The cytokine secretion of the Salmonella-permissive, HLA-B27-positive U937 cells was examined, as it was previously shown that these cells kill Salmonella less efficiently than controls. Salmonella-permissive U937 cells showed upregulated production of interleukin 10 and to a lesser extent tumor necrosis factor alpha. HLA-B27-associated modulation of cytokine responses may have importance in the pathogenesis of reactive arthritis.
Cytokines are key mediators of several physiological and pathophysiological host responses during inflammation and infection. During infection with intracellular bacteria, a T helper type 1 (Th1) response (e.g., gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]) triggering cell-mediated immunity is normally needed to effectively eliminate the infectious agent (3, 27). TNF-α can induce the formation of many important proinflammatory mediators as well as antagonizing cytokines. The downregulation of immune responses via macrophage deactivation by anti-inflammatory cytokines (e.g., interleukin 10 [IL-10] or transforming growth factor beta) is important as well (4). Activated monocytes and macrophages form the major source of TNF-α and IL-10. IL-10 inhibits cytokine synthesis by activated T cells, natural killer cells, and monocytes/macrophages (10). Importantly, it also inhibits the antimicrobial functions of monocytes by downregulating major histocompatibility complex (MHC) class II expression and suppressing the production of proinflammatory cytokines (9). IL-10 thus acts as a powerful anti-inflammatory agent during host defense and may turn to be harmful to the host, especially during infection with intracellular microbes. Thus, the balance between synthesis of pro- and anti-inflammatory cytokines is essential for the ability of the host to efficiently kill and eliminate intracellular microbes with minimal tissue damage.
Reactive arthritis (ReA) is a quite commonly seen postinfectious complication triggered by certain obligatory or facultative intracellular microbes, including Salmonella. ReA most frequently develops in susceptible HLA-B27-positive persons, although the reason(s) for this firm association has remained unclear (22). Recently we have demonstrated the enhanced survival of Salmonella within HLA-B27-transfected human monocytic cells (21) and mouse fibroblasts (29) in vitro. These observations suggest that in addition to previously known functions of MHC class I molecules in antigen presentation, HLA-B27 may have a role in directly modulating host-microbe interactions during Salmonella infection. Interestingly, Hahn et al. have reported that MHC class I molecules may participate in modulating viral replication by mechanisms unrelated to the antigen presentation (15). Our previous findings prompted us to study the production of some pivotal pro- and anti-inflammatory cytokines of HLA-B27-transfected (termed Salmonella permissive) and HLA-A2-transfected (Salmonella nonpermissive) human monocytic U937 cells during Salmonella infection. We hypothesized that failure to mount an appropriate cytokine response could lead to an impaired capacity to kill Salmonella.
Salmonella permissive U937 cells synthesize more IL-10 than Salmonella-nonpermissive cells during Salmonella infection
To study the capacity of transfected U937 cells to synthesize certain pivotal cytokines during Salmonella infection, we measured the protein and mRNA synthesis of TNF-α and IL-10 using the same experimental setup described earlier (21). U937 cells were transfected with either DNA containing the gene or cDNA of HLA-B*2705 or DNA containing the HLA-A2 gene, as described earlier (21). Cells were cultured in tissue culture flasks (Greiner, Frickenhausen, Germany) under lipopolysaccharide (LPS)-free conditions (11) in VLE RPMI 1640 medium (Seromed; Biochrom KG, Berlin, Germany) containing 10% heat-inactivated fetal calf serum, 1.8 mM l-glutamine (both from Biological Industries, Kibbutz Beit Herennek, Israel) and 0.75 mg of Geneticin (Sigma, St. Louis, Mo.)/ml at 37°C in humidified 5% CO2-95% air. Precautions were taken to prevent endotoxin contamination during experimental procedures. The culture medium was filtrated through an Ultrafilter 2000 column (Gambro medizintechnik, Munich, Germany) after the addition of supplements other than fetal calf serum. Differentiation of premonocytic U937 cells toward more mature macrophage-like cells was induced by incubating the cells with phorbol myristate acetate (PMA) (Sigma) before infection or LPS stimulation as described earlier (21). In brief, cells were seeded into 250-ml culture flasks (Greiner), six-well plates (Corning, Acton, Mass.), or 24-well plates (Greiner) in a concentration of 1 million cells/ml and incubated with 10 ng of PMA/ml at 37°C for 24 h. The infection of PMA-stimulated U937 cells with Salmonella was performed as described earlier (21). Briefly, the Salmonella enterica serovar Enteritidis strain that was originally isolated from a patient with Salmonella-triggered ReA was grown in Luria-Bertani broth at 37°C to the logarithmic phase of growth. After the 24-h preincubation with PMA, the adherent cells were washed once with filtrated phosphate-buffered saline (PBS) and then overlaid with fresh VLE RPMI 1640 medium containing 10% human AB serum (Finnish Red Cross, Helsinki, Finland). PMA-stimulated U937 cells were cocultured with Salmonella at a 1:5-to-10 cell/bacterium ratio for 1 h at 37°C. After three careful washes with PBS, the cells were overlaid with fresh incubation medium containing 50 μg of gentamicin (Biological Industries)/ml and incubated at 37°C. After the defined periods of time, supernatants were collected, cells were harvested, and viable and dead cells were counted after staining with Trypan blue. To determine the number of viable intracellular bacteria, cells were lysed with 1% Triton X-100, resuspended in PBS, and cultured in Luria-Bertani broth plates after serial dilutions. In 24-well plates, the cells were counted and lysed without detachment, and the number of bacteria was reported as CFU recovered/well. Cytokine secretion was measured from cell supernatants using commercially available enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and IL-10 (Amersham International plc, Buckinghamshire, United Kingdom) following the instructions of manufacturers. Similar results were obtained with sandwich ELISA using antibody pairs for measurement of TNF-α and IL-10 (mAb1 and mAb11 for TNF-α; JES3-9D7 and JES3-12G8 for IL-10; Pharmingen, San Diego, Calif.). The absorbances were detected with a Victor Multilabel Counter (Wallac Oy, Turku, Finland) at a wavelength of 450 nm (ELISA kits) or at 405 nm (sandwich ELISA). According to the manufacturers, the assays used do not show any cross-reactivity with a variety of other cytokines. Statistical comparison was performed using either unpaired two-tailed Student's t test or the Mann-Whitney U test, and the appropriate test was chosen according to the characteristics of the data.
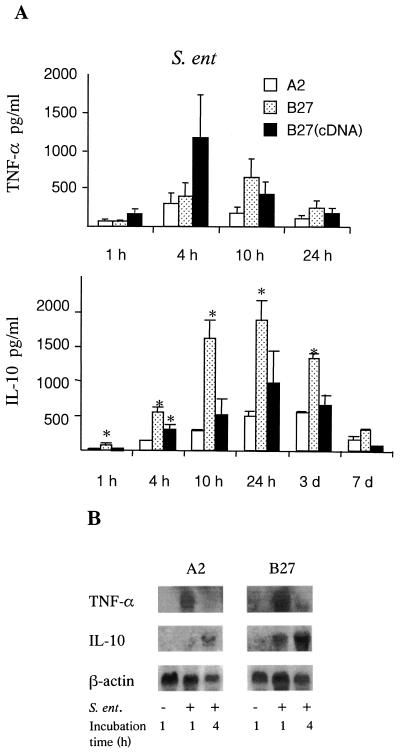
Our study revealed that the infection of PMA-stimulated U937 cells with Salmonella induced a rapid secretion of TNF-α followed by the synthesis of IL-10 (Fig. 1A). The production of TNF-α was rapidly induced, and maximal secretion occurred 4 to 10 h postinfection. Salmonella-permissive (HLA-B27) U937 cells tended to secrete more TNF-α than Salmonella-nonpermissive (HLA-A2) cells during the first 10 h after the infection; however, the difference was not statistically significant. IL-10 secretion was detected at 4 h postinfection, and maximal secretion occurred at 24 h after the infection. Interestingly, Salmonella-permissive U937 cells produced more IL-10 during the first 3 days postinfection than the Salmonella-nonpermissive cells. However, independent HLA-B27-transfectants had slightly different cytokine profiles during infection, although they were identical in their bacterial load. Notably, HLA-A2 transfectants secreted constantly the smallest amount of cytokines. Moreover, the enhanced secretion of cytokines by Salmonella-permissive U937 cells seemed to be specific for infection, since all transfectants showed similar secretion of TNF-α and IL-10 after the pretreatment with PMA or the internalization of Latex beads (data not shown). Nonstimulated cells secreted no IL-10 or TNF.
FIG. 1.
The production of TNF-α and IL-10 protein (A) and mRNA (B) by Salmonella-infected U937 cells. (A) The secretion of TNF-α and IL-10 was detected in the supernatants of transfected U937 cells during the infection of PMA-pretreated cells with Salmonella. U937 cells were transfected with either DNA containing the gene (B27) or cDNA [B27 (cDNA)] of HLA-B27 or DNA of the HLA-A2 gene (A2). Values are means ± standard deviations for three independent experiments. ∗, P < 0.05 when HLA-B27 transfectants were compared with HLA-A2. (B) The expression of TNF-α and IL-10 mRNA in transfected U937 cells after the stimulation of PMA and during the infection of PMA-pretreated cells with Salmonella was studied by the Northern blotting technique.
Cytokine mRNA expression was studied using the Northern hybridization technique to see whether the differences in the secretion of TNF-α and IL-10 originated with different regulation on the transcriptional level. Up to 10 million cells were collected, quickly resuspended in Lysis buffer RLT (Qiagen GmbH, Hilden, Germany) and frozen at −70°C. Total RNA was extracted using an Rneasy kit (Qiagen GmbH) following the manufacturer's instructions, resuspended in diethyl pyrocarbonate-treated water, and stored at −70°C until used. Twenty micrograms of RNA per lane was loaded on a 1.2% agarose gel containing 6.6% formaldehyde. After gel electrophoresis, RNA was transferred in a positively charged Hybond N+ nylon membrane (Amersham) and bound in UV light with the Spectrolinker (Spectronics Corporation, Westbury, N.Y.). Prehybridization was performed in a hybridization buffer containing 0.25 M Na2HPO4, 1 mM EDTA, and 20% sodium dodecyl sulfate at 68°C for 1 h. Hybridization was performed using 2.5 to 3 ng of digoxigenin-labeled TNF-α, IL-10, or β-actin probes/ml at 68°C overnight. A 600-bp HindIII/XhoI fragment from exon 4 of the TNF-α gene (kindly provided by Elisabeth Weiss) (14), a purified PCR-generated 204-bp cDNA fragment of IL-10 (23), or a 465-bp cDNA fragment of β-actin (6) were used as probes. IL-10 and β-actin probes were generated from high yields of IL-10- and β-actin-specific cDNAs synthesized by reverse transcription-PCR using specific primers (6, 23). cDNA was purified by agarose gel electrophoresis and using the QIAquick Gel Extraction kit (Qiagen) following the manufacturer's instructions. Probes were labeled with digoxigenin-dUTP (Boehringer Mannheim GmbH, Mannheim, Germany) with a random priming method. After hybridization, the membranes were washed with a buffer containing 20 mM Na2HPO4, 1 mM EDTA, and 1% sodium dodecyl sulfate three times at 65°C. Nonspecific binding was blocked with 0.5% blocking reagent (Boehringer Mannheim) before the detection of successful hybridization using Fab fragments of anti-digoxigenin-AP antibodies (Boehringer Mannheim). Chemiluminescence signals derived from hybridized probes were detected on X-ray films (Kodak), and the results were analyzed using a computer-based image analysis system.
Nonstimulated U937 cells showed negligible expression of TNF-α or IL-10 mRNA (data not shown). Salmonella infection rapidly induced the transcription of TNF-α and IL-10 mRNA (Fig. 1B) Maximal TNF-α mRNA expression was detected 1 to 4 h postinfection and was thereafter rapidly downregulated. Salmonella infection had already induced the expression of IL-10 mRNA 1 h postinfection, and maximal expression was observed at 4 h after the infection. Salmonella-permissive U937 cells expressed clearly more IL-10 mRNA than Salmonella-nonpermissive cells. By using a highly sensitive reverse transcription-PCR method, we could observe a difference in IL-10 mRNA expression at 4 days postinfection (data not shown). Thus, mRNA and protein results correlated well to each other concerning IL-10. On the basis of several experiments, there also was a tendency for Salmonella-permissive U937 cells infected with Salmonella to express more TNF-α mRNA than nonpermissive cells, but the difference was not as clear as in the case of IL-10.
The modulation of cytokine levels does not effect the killing capacity of transfected U937 cells
To examine the role of IL-10 and TNF-α in the survival of Salmonella in U937 cells, 10 ng of recombinant human IL-10 (rh-IL-10) (R&D Systems Inc., Minneapolis, Minn.)/ml, 5 μg of neutralizing antihuman MAb against IL-10 (IL-10 MAb) (R&D Systems Inc.)/ml, or 0.1 to 1 μg of neutralizing antihuman MAb against TNF-α (anti-TNF-α MAb) (Genzyme Diagnostics, Cambridge, Mass.)/ml was added to the fresh incubation medium after a 1-h infection period.
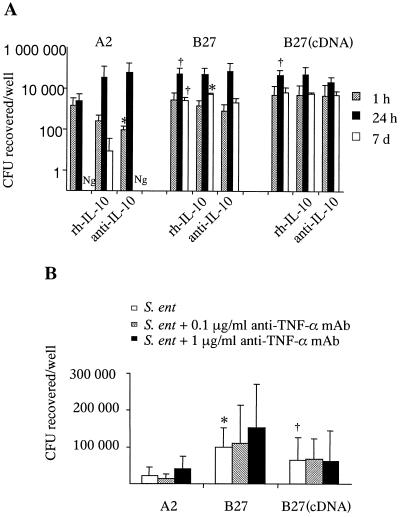
The administration of rh-IL-10 yielded the high and extended accumulation of IL-10, while anti-IL-10 MAb clearly decreased, but did not abrogate, the IL-10 levels in the supernatants of transfected Salmonella-infected U937 cells (data not shown). Surprisingly, despite the remarkable differences in IL-10 concentrations caused by rh-IL-10 or anti-IL-10 MAb, no profound differences in the survival of Salmonella were detected (Fig. 2A). Salmonella-permissive U937 cells contained more viable bacteria than Salmonella-nonpermissive cells, as expected. In addition, the incubation of U937 cells with rh-IL-10 dramatically abrogated the Salmonella-induced TNF-α secretion at 24 h postinfection, while anti-IL-10 MAb had no constant effect (data not shown). The administration of rh-IL-10 slightly decreased the number of viable Salmonella-infected U937 cells, while anti-IL-10 MAb did not influence the cell viability (data not shown).
FIG. 2.
Modification of the survival of Salmonella by IL-10 and TNF-α. The effect of the administration of rh-IL-10 and anti-human IL-10 MAb (A) or anti-human TNF-α MAb (B) on the survival of Salmonella by transfected U937 cells was studied. U937 cells transfected with either DNA containing the gene (B27) or cDNA [B27 (cDNA)] of HLA-B27 or DNA containing the HLA-A2 gene (A2) were infected with S. enteritidis and thereafter treated with either 10 ng of rh-IL-10/ml, 5 μg of anti-IL-10 MAb/ml, or 0.1 to 1 μg of anti-TNF-α MAb/ml or left untreated. Values are mean numbers ± standard deviations from three independent experiments with triplicate samples. (A) The number of viable bacteria recovered per well is shown on a log scale. ∗, P ≤ 0.05 when untreated cells were compared with rh-IL-10 or anti-IL-10 MAb-treated cells; †, P = 0.05 when untreated HLA-B27 transfectants were compared with HLA-A2 cells. Ng, no growth. (B) Cells were collected at 24 h postinfection for the measurement of the number of intracellular bacteria. ∗, P < 0.05, and †, P = 0.07, when untreated HLA-B27 transfectants were compared with HLA-A2.
To conclude, the better survival of Salmonella within HLA-B27-positive cells in vitro seemed not to be a direct consequence of the enhanced production of endogenous anti-inflammatory IL-10. However, due to the incomplete neutralization of endogenous IL-10 by anti-IL-10 MAb, we cannot exclude that IL-10 may still have a modifying effect on the microbial survival. Previously, Arai et al. reported that the neutralization of IL-10 by MAb led to enhanced host defense against Salmonella enterica serovar Choleraesuis in vivo (1) but did not directly affect the killing activity of mouse macrophages in vitro (2). Furthermore, Pie et al. demonstrated the link between the genetic susceptibility to Salmonella in mice and a high level of IL-10 expression, but the administration of anti-IL-10 MAb did not modify the course of infection (24) Nevertheless, there are controversial reports concerning the genetic susceptibility to Salmonella and cytokine responses. As a conclusion, according to previous reports and the present data, it seems that the high level of production of IL-10 during infection with Salmonella correlates to a more severe disease outcome in vivo, while the correlation in vitro stays unclear.
There was no secreted TNF-α detectable in cell supernatants of the Salmonella-infected U937 cells after the use of neutralizing anti-TNF-α MAb (data not shown). The neutralization of secreted TNF-α did not remarkably influence the intracellular survival of Salmonella (Fig. 2B). The viability of cells was not affected by the use of anti-TNF-α MAb (data not shown). Cell-mediated immunity and the involvement of TNF-α have been shown to be important for the clearance of Salmonella in mice (27). In a recent study, intracellular TNF-α was able to signal via the p55 receptor to target NADPH phagocyte oxidase to the Salmonella-containing phagosome, which appeared to be crucial in the killing of Salmonella for mice in vivo and by murine macrophages in vitro (28). The present data suggest that autocrine TNF-α production is not important in the capacity of U937 cells of human origin to clear Salmonella in vitro. This conclusion is based on the following observations: (i) the Salmonella-permissive cells showed an elevated secretion of TNF-α compared to the Salmonella-nonpermissive cells; (ii) the use of neutralizing MAbs against TNF-α did not clearly affect the survival of Salmonella; and (iii) the administration of rh-IL-10 strongly downregulated endogenous secretion of TNF-α with only slight effects on the clearance. Evidently, the requirement for TNF-α for the capacity of macrophages to kill Salmonella is different in vitro than in the in vivo environment and perhaps also in humans compared to mice.
LPS induces upregulated synthesis of TNF-α and IL-10 for Salmonella-permissive U937 cells
To address the question of whether viable Salmonella bacteria, or merely microbial antigens, were needed to induce the upregulated production of TNF-α or IL-10 by Salmonella-permissive U937 cells, the PMA-stimulated cells were incubated with 10 to 100 ng of S. enteritidis LPS (Sigma)/ml for 7 days. The LPS dose (50 ng/ml) was selected for further experiments because it could induce cytokine production in all transfectants without affecting the cell viability. Cytokine mRNA and protein measurements were performed as described above, with the exception that only 10 μg of mRNA was loaded on the gel for Northern hybridization. Compared to the Salmonella-nonpermissive U937 cells, the permissive cells generated more TNF-α and IL-10 on both the mRNA level and the protein level after stimulation with LPS (Fig. 3A and B). The expression of TNF-α mRNA was induced at 1 h after the incubation of PMA-stimulated U937 cells with LPS, followed by protein secretion after 4 h of incubation. In contrast to the case of infection with Salmonella, TNF-α secretion continued for 3 days during LPS stimulation. IL-10 secretion was induced 4 h after the beginning of incubation and continued steadily for 7 days despite downregulation in the mRNA level after 24 h (data not shown). These results indicate that the production of TNF-α and IL-10 during LPS stimulation is regulated both by transcriptional and posttranscriptional mechanisms. The expression of putative LPS receptors (CD11b, CD11c, CD14, and CD18) on transfected U937 cells before and after PMA pretreatment was also studied, but no differences between transfectants were found (data not shown). Thus, the stronger response of Salmonella-permissive U937 cells to Salmonella or its LPS was not due to differences in the expression of these putative LPS receptors. The expression of the novel LPS receptor, Toll-like 4, was not investigated due to the lack of available antibodies, but it would be of interest in further studies.
FIG. 3.
Induction of TNF-α and IL-10 production by Salmonella LPS. U937 cells transfected with either DNA containing the gene (B27) or cDNA [B27 (cDNA)] of HLA-B27 or DNA containing the HLA-A2 gene (A2) were pretreated with PMA for 24 h before LPS stimulation. (A) Expression of TNF-α and IL-10 mRNA was studied 4 h after the incubation with LPS. (B) Secretion of TNF-α and IL-10. Values are means ± standard deviations for duplicate samples from a representative experiment out of three with similar results. ∗, P ≤ 0.05, and †, P < 0.07, when HLA-B27 transfectants were compared to HLA-A2 transfectants.
Although viable bacteria have not been detected in joints by culturing, there is increasing evidence that bacterial antigens, especially LPS, and nucleic acids can occur in the joints and peripheral blood of patients with ReA (12, 13, 26). Despite several different hypotheses, it is currently unknown how bacteria interact with HLA-B27 and modify the immune system to give rise to the onset of HLA-B27-associated arthritides (18). It would most likely to be of importance if HLA-B27 molecules could modulate the cytokine responses in a complex in vivo system. In the present report we demonstrated that the presence of HLA-B27 was associated with the high production of IL-10, and to a lesser extent TNF-α, by U937 cells during the infection with Salmonella. The differences in the cytokine production were measured also at the early time points when the intracellular bacterial numbers were still equivalent. However, the different cytokine profiles produced did not provide any direct explanation for the killing deficiency of HLA-B27-positive cells. Interestingly, Salmonella LPS as such induced remarkable differences in cytokine synthesis between the Salmonella-permissive and Salmonella-nonpermissive U937 cells. Previously, the expression of HLA-B27 was shown to promote the induction of c-fos and monocyte chemoattractant molecule in HeLa cells after the invasion of S. enterica serovar Typhimurium (17). It may be that in addition to previously known functions of MHC class I molecules in antigen presentation, HLA-B27 has a role in directly modulating host-microbe interactions by modifying cytokine synthesis during Salmonella infection. In fact, the MHC genotype has been suggested to affect cytokine production in mice and humans by non-antigen-specific mechanisms (7). More importantly, HLA-B27 was recently shown to have a tendency to misfold, and the HLA-B27 misfolding could generate a cellular stress response and induce nuclear κB activation, followed by the production of cytokines (8).
On the basis of the present data, the secretion of TNF-α seemed not to be crucial in the killing of Salmonella in this model. In addition, the administration of rh-IL-10 or anti-human IL-10 MAb affected the killing capacity only slightly or not at all. Generally, cell-mediated immunity, or the Th1-type immune response, is required for the clearance of ReA-triggering microbes, including Salmonella (27). However, evidence exists for the predominance of the Th2-type response in the synovial fluid cells of patients with ReA. The IL-10/IFN-γ and IL-10/TNF-α ratios were especially high after the stimulation of synovial fluid cells derived from ReA patients with causative bacteria in vitro (30). Besides, IL-10 and IFN-γ mRNA were relatively abundant in synovial tissue samples in Chlamydia-associated arthritis (20), and the expression of several cytokines, including IL-10, was detected in peripheral blood mononuclear cells of patients with Salmonella-triggered ReA (19). The role of TNF-α in the course of ReA is still not clear. The capacity to induce a high level of production of TNF-α has been proposed to associate with HLA-B27 positivity and ReA (25). In contrast, low secretion of TNF-α by peripheral blood mononuclear cells derived from ReA patients was found to correlate to the HLA-B27 positivity and chronicity of the disease in another study (5). Allelic variation in the TNF-α promoter region seemed to influence susceptibility of HLA-B27-positive individuals to develop ankylosing spondylitis (16). Taken together, assuming that the persistence of arthritis-triggering microbes is important in the development of arthritis, the failure to mount the most appropriate cytokine response could contribute to the ineffective clearance of microbes and finally to the pathogenesis of ReA.
Acknowledgments
Elisabeth Weiss and Bernhard Frankenberger (Institut fυr Antropologie und Humangenetik, University of Munich, Munich, Germany) are acknowledged for providing the TNF-α probe and advice for Northern blot assays. Tiina Lähde, Tuija Turjas, and Erkki Nieminen are warmly thanked for their skillful technical assistance.
This work was supported by grants from the Academy of Finland, the European Commission Biomed 2 Programme (BMH4-CT96-1056), the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Turku University Foundation.
REFERENCES
- 1.Arai, T., K. Hiromatsu, H. Nishimura, Y. Kimura, N. Kobayashi, H. Ishida, Y. Nimura, and Y. Yoshikai. 1995. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology 85:381-388. [PMC free article] [PubMed]
- 2.Arai, T., K. Hiromatsu, H. Nishimura, Y. Kimura, N. Kobayashi, H. Ishida, Y. Nimura, and Y. Yoshikai. 1995. Endogenous interleukin 10 prevents apoptosis in macrophages during Salmonella infection. Biochem. Biophys. Res. Commun. 213:600-607. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., M. Beer, E. Bohn, S. H. E. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, J., Z. Yin, I. Spiller, S. Siegert, M. Rudwaleit, L. Liu, A. Radbruch, and J. Sieper. 1999. Low secretion of tumor necrosis factor α, but no other Th1 or Th2 cytokines, by peripheral blood mononuclear cells correlates with chronicity in reactive arthritis. Arthritis Rheum. 42:2039-2044. [DOI] [PubMed] [Google Scholar]
- 6.Cáceres-Dittmar, G., F. J. Tapia, M. A. Sánchez, M. Yamamura, K. Uyemura, R. L. Modlin, B. R. Bloom, and J. Convit. 1993. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin. Exp. Immunol. 91:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso, C., G. Candore, M. A. Modica, C. T. Bonanno, G. Sireci, F. Dieli, and A. Salerno. 1996. Major histocompatibility complex regulation of cytokine production. J. Interferon Cytokine Res. 16:983-988. [DOI] [PubMed] [Google Scholar]
- 8.Colbert, R. A. 2000. HLA-B27 misfolding and spondyloarthropathies: not so groovy after all? J. Rheumatol. 27:1107-1109. [PubMed] [Google Scholar]
- 9.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Waal Malefyt, R., H. Yssel, M.-G. Roncarolo, H. Spits, and J. E. de Vries. 1992. Interleukin-10. Curr. Opin. Immunol. 4:314-320. [DOI] [PubMed] [Google Scholar]
- 11.Frankenberger, M., H. Pechumer, and H. W. L. Ziegler-Heitbrock. 1995. Interleukin-10 is upregulated in LPS tolerance. J. Inflamm. 45:56-63. [PubMed] [Google Scholar]
- 12.Gaston, J. S. H., C. Cox, and K. Granfors. 1999. Clinical and experimental evidence for persistent Yersinia infection in reactive arthritis. Arthritis Rheum. 42:2239-2242. [DOI] [PubMed] [Google Scholar]
- 13.Granfors, K., R. Merilahti-Palo, R. Luukkainen, T. Möttönen, R. Lahesmaa, P. Probst, E. Hermann, and P. Toivanen. 1998b. Persistence of Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O:3 infection with or without reactive arthritis. Arthritis Rheum. 41:855-862. [DOI] [PubMed]
- 14.Haas, J. G., P. A. Baeuerle, G. Riethmüller, and H. W. L. Ziegler-Heitbrock. 1990. Molecular mechanisms in down-regulation of tumor necrosis factor expression. Proc. Natl. Acad. Sci. USA 87:9563-9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn, Y. S., A. Guanzon, C. M. Rice, and C. S. Hahn. 1999. Class I MHC molecule-mediated inhibition of Sindbis virus replication. J. Immunol. 162:69-77. [PubMed] [Google Scholar]
- 16.Höhler, T., T. Schäper, P. M. Schneider, K.-H. Meyer zum Büschenfelde, and E. Märker-Hermann. 1998. Association of different tumor necrosis factor α promoter allele frequencies with ankylosing spondylitis in HLA-B27 positive individuals. Arthritis Rheum. 41:1489-1492. [DOI] [PubMed] [Google Scholar]
- 17.Ikawa, T., M. Ikeda, A. Yamaguchi, W. C. Tsai, N. Tamura, N. Seta, M. Trucksess, R. B. Raybourne, and D. T. Y. Yu. 1998. Expression of arthritis-causing HLA-B27 on Hela cells promotes induction of c-fos in response to in vitro invasion by Salmonella typhimurium. J. Clin. Investig. 101:263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khare, S. D., H. S. Luthra, and C. S. David. 1998. HLA-B27 and other predisposing factors in spondyloarthropathies. Curr. Opin. Rheumatol. 10:282-291. [DOI] [PubMed] [Google Scholar]
- 19.Kirveskari, J., Q. He, T. Holmström, M. Leirisalo-Repo, M. Wuorela, J. Mertsola, and K. Granfors. 1999. Modulation of peripheral blood mononuclear cell activation status during Salmonella-triggered reactive arthritis. Arthritis Rheum. 42:2045-2054. [DOI] [PubMed] [Google Scholar]
- 20.Kotake, S., H. R. Schumacher, Jr., T. K. Arayssi, H. C. Gérard, P. J. Branigan, A. P. Hudson, C. H. Yarboro, J. H. Klippel, and R. L. Wilder. 1999. Gamma interferon and interleukin-10 gene expression in synovial tissues from patients with early stages of Chlamydia-associated arthritis and undifferentiated oligoarthritis and from healthy volunteers. Infect. Immun. 67:2682-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laitio, P., M. Virtala, M. Salmi, L. J. Pelliniemi, D. T. Y. Yu, and K. Granfors. 1997. HLA-B27 modulates intracellular survival of Salmonella enteritidis in human monocytic cells. Eur. J. Immunol. 27:1331-1338. [DOI] [PubMed] [Google Scholar]
- 22.López de Castro, J. A. 1998. The pathogenetic role of HLA-B27 in chronic arthritis. Curr. Opin. Immunol. 10:59-66. [DOI] [PubMed] [Google Scholar]
- 23.Melby, P. C., B. J. Darnell, and V. V. Tryon. 1993. Quantitative measurement of human cytokine gene expression by polymerase chain reaction. J. Immunol. Methods 159:235-244. [DOI] [PubMed] [Google Scholar]
- 24.Pie, S., P. Matsiota-Bernard, P. Truffa-Bachi, and C. Nauciel. 1996. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect. Immun. 64:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Repo, H., M. Jäättelä, M. Leirisalo-Repo, and M. Hurme. 1988. Production of tumour necrosis factor and interleukin 1 by monocytes of patients with previous Yersinia arthritis. Clin. Exp. Immunol. 72:410-414. [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor-Robinson, D., C. B. Gilroy, B. J. Thomas, and A. C. S. Keat. 1992. Detection of Chlamydia trachomatis DNA in joints of reactive arthritis patients by polymerase chain reaction. Lancet 340:81-82. [DOI] [PubMed] [Google Scholar]
- 27.Tite, J. P., G. Dougan, and S. N. Chatfield. 1991. The involvement of tumor necrosis factor in immunity to Salmonella infection. J. Immunol. 147:3161-3164. [PubMed] [Google Scholar]
- 28.Vázquez-Torres, A., G. Fantuzzi, C. K. Edvards III, C. A. Dinarello, and F. C. Fang. 2001. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc. Natl. Acad. Sci. USA 98:2561-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virtala, M., J. Kirveskari, and K. Granfors. 1997. HLA-B27 modulates the survival of Salmonella enteritidis in transfected L cells, possibly by impaired nitric oxide production. Infect. Immun. 65:4236-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin, Z., J. Braun, L. Neure, P. Wu, L. Liu, U. Eggens, and J. Sieper. 1997. Crucial role of interleukin-10/interleukin-12 balance in the regulation of the type 2 T helper cytokine response in reactive arthritis. Arthritis Rheum. 40:1788-1797. [DOI] [PubMed] [Google Scholar]