Abstract
Langerhans cells (LC) take up Leishmania major and are critical for the induction of the parasite-specific T-cell response. Their functional activities are regulated by cytokines. We analyzed whether infection of LC with L. major modulates the expression of their cytokine receptors. The expression of the interleukin 4 (IL-4) receptor was increased on infected LC from susceptible mice but not on those from resistant mice. Moreover, IL-4 treatment strongly decreased the lipopolysaccharide-induced IL-12 response of infected LC from susceptible mice. This modulation of IL-4 receptor expression and IL-12 production by infection of LC with Leishmania may contribute to the development of Th2 cells and to susceptibility to infection.
Infection of mice with Leishmania major provides a useful model for analysis of the immune response to these parasites. Such studies have established that the outcome of infection is determined by the type of the developing T-helper (Th) cells (15). Th1 cells producing gamma interferon (IFN-γ) predominate in resistant C57BL/6 mice, whereas Th2 cells producing interleukin 4 (IL-4), IL-5, IL-10, and IL-13 are associated with the susceptibility of BALB/c mice. The development of Th1 cells in leishmaniasis is primarily directed by IL-12 (12, 17), and the differentiation of Th2 cells is driven by IL-4 (9, 10).
In the mammalian host, Leishmania parasites are obligatorily intracellular and reside within macrophages and dendritic cells (DC). Langerhans cells (LC), the DC of the skin, are critical for the initial triggering of the parasite-specific T-cell response (13). Within a few days of infection, they transport L. major from the newly infected skin to the draining lymph nodes (14) while differentiating into mature DC with potent antigen presentation functions and the capacity to activate resting T cells. Furthermore, parasite infection stimulates DC to produce IL-12 (4, 5, 8, 20). In fact, since macrophage IL-12 production is impaired by Leishmania infection (1, 5, 16, 22), DC are likely to be the sole source of IL-12.
The migration and concomitant differentiation of LC are tightly regulated by cytokines. The different functional states can be analyzed with LC cultured in the presence of growth factors in vitro. By use of this model, it has been shown that differential expression of cytokine receptors controls DC responsiveness and thus affects their activities (7, 21). However, it is not known whether infection of DC with microbial pathogens modulates their expression of cytokine receptors. To elucidate the molecular consequences of DC interaction with infectious agents and their potential impact on the ensuing immune response, we analyzed whether infection of LC with L. major induces changes in their expression of cytokine receptors.
Parasites and infection of LC.
Amastigotes of the L. major isolate MHOM/IL/81/FE/BNI were obtained from skin lesions as described previously (3). Single-cell suspensions of epidermal cells were prepared from the ear skin of BALB/c or C57BL/6 mice by trypsinization procedures (4, 18). These preparations contained 3 to 5% LC that constitutively express major histocompatibility complex class II (MHC-II) as well as MHC-II-negative keratinocytes, a source of cytokines that are essential for LC differentiation. For infection of LC with L. major, 3 × 106 epidermal cells were incubated with amastigotes at a ratio of three parasites per cell in 2 ml of culture medium (RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 10 mM HEPES buffer, 60 μg of penicillin/ml, 20 μg of gentamicin/ml, 17 mM NaHCO3, and 0.05 mM 2-mercaptoethanol). The nonadherent population, 30 to 50% of which was LC, was harvested after 1 to 6 days of culture.
Analysis of LC cytokine receptor expression by flow cytometry.
LC were identified by labeling 1 × 106 to 2 × 106 cells in 100 μl with biotin-conjugated mouse anti-I-Ad or anti-I-Ab monoclonal antibody (MAb) (PharMingen, Hamburg, Germany) and streptavidin-conjugated CyChrome (PharMingen). The expression of the cytokine receptors of LC was analyzed by staining with rat MAb directed against the IL-1 receptor (IL-1R) of type I or type II (PharMingen), the IL-4R (R&D, Wiesbaden, Germany), the tumor necrosis factor receptor of type I or type II (Genzyme, Rüsselsheim, Germany), or the IFN-γ receptor (IFN-γR) (PharMingen) or with isotype-matched control antibodies and phycoerythrin-conjugated donkey anti-rat antibodies (Dianova, Hamburg, Germany). Primary MAbs were used at 10 μg/ml, and secondary antibodies were used at 5 μg/ml diluted in phosphate-buffered saline containing 2% heat-inactivated fetal calf serum (30 min at 4°C). Two-color flow cytometry was performed on a FACSCalibur (Becton Dickinson, Heidelberg, Germany) by using Cell Quest software.
ELISA for detection of IL-12 production by LC.
LC were infected with L. major on day 0. In some experiments, LC cultures were supplemented with anti-IL-4 MAb (from hybridoma 11B11) until day 3. From day 3 to day 6 of culture, the cells were incubated with lipopolysaccharide (LPS) (100 ng/ml; Roth, Karlsruhe, Germany) and/or IL-4 (5 ng/ml; R&D). Kinetic studies demonstrated that this protocol resulted in higher activities than did shorter periods of LC culture (1 to 4 days). On day 6, culture supernatants were harvested for the determination of IL-12 p40 by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (4). Tris buffer (50 mM; pH 7.6) containing 0.05% Tween 20 was used for the washing and blocking steps. Rat MAbs against IL-12 p40 were used as capture antibodies, and biotinylated rat anti-IL-12 p40 MAbs were used as detection antibodies (both from PharMingen). Standard curves were generated using serial dilutions of murine recombinant IL-12 p40 (PharMingen). The detection threshold was 2.4 pg of IL-12 p40 per ml.
L. major modulates IL-4R expression by LC.
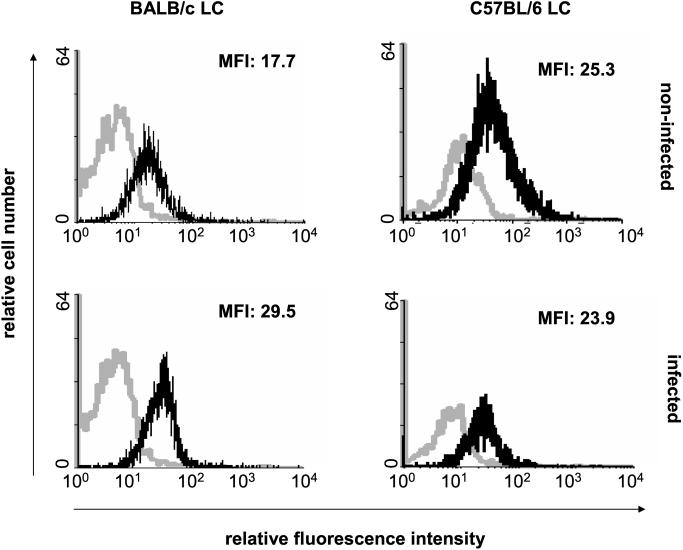
Differences in patterns of cytokine receptor expression by LC regulate their responsiveness to the corresponding factors and their functional activities (7, 21). However, there has been no information on the receptor profile of LC from L. major-resistant mice compared to that of LC from susceptible mice. We therefore analyzed the expression of receptors for cytokines relevant to LC growth and differentiation or to the activities of Th1 or Th2 cells. Flow cytometric analysis of gated MHC-II+ LC showed that the patterns of expression of IL-1R (type I and type II), tumor necrosis factor receptor (type I and type II), and IFN-γR by LC from resistant C57BL/6 and susceptible BALB/c mice were comparable and were not modulated by infection with L. major parasites (data not shown). In contrast, the expression of IL-4R was significantly upregulated by infection of LC from BALB/c but not from C57BL/6 mice (Fig. 1). Thus, infection with L. major modulated IL-4R expression by LC in a mouse strain-dependent manner. Interestingly, although not all LC take up L. major (2), exposure of LC to parasites did not result in a bimodal profile of IL-4R expression, suggesting that IL-4R upregulation in LC from BALB/c mice is not confined to LC infected with parasites.
FIG. 1.
Effect of L. major infection on the expression of IL-4R by LC from BALB/c or C57BL/6 mice. LC were infected with L. major on day 0 and were subjected to two-color fluorescence staining (anti-MHC-II and anti-IL-4R) followed by flow cytometric analysis on day 5. The histograms show gated MHC-II+ LC. Black lines, staining with anti-IL-4R MAb; grey lines, treatment with isotype-matched control antibodies. MFI, mean fluorescence intensity of IL-4R-stained cells. Similar results were obtained in five independent experiments.
Effect on IL-12 production by LC.
The IL-4 response mounted by L. major-susceptible BALB/c mice has been shown to be associated with disease exacerbation (10). Our finding that L. major infection induced a modulation of IL-4R expression by LC from BALB/c mice but not from C57BL/6 mice suggested that the alteration in receptor expression may control the responsiveness of LC from BALB/c mice and modify their activities. Since LC serve as host cells for L. major parasites (2), we first determined whether treatment with IL-4 affects the rate of LC infection. However, no effect was detected (data not shown). Another important feature of LC is their ability to produce IL-12 in response to Leishmania infection (4, 5, 8, 20). Therefore, we next evaluated whether the increased sensitivity to IL-4 of LC from susceptible BALB/c mice affects their IL-12 expression. The production of the inducible chain IL-12 p40 by LC from BALB/c mice was impaired by infection with L. major (Table 1), and this effect was slightly enhanced by addition of IL-4 to the cultures. To exclude an influence of endogenous IL-4 produced by dendritic epidermal T cells in the cultures of freshly prepared LC (11), we also tested LC cultures that had been pretreated with anti-IL-4 MAb (Table 1). The results demonstrated that IL-4 strongly reduces the LPS-induced production of IL-12 p40 by pretreated LC. This reduction was most pronounced after infection of LC with L. major. Notably, IL-12 p40 production by LC from C57BL/6 mice was not influenced by IL-4 (data not shown).
TABLE 1.
Production of IL-12 p40 by BALB/c LC after treatment with IL-4 and/or LPSa
| Cells | Infection with L. major | Amt of IL-12 p40 (ng/ml)b in cells cultured in:
|
|||
|---|---|---|---|---|---|
| Medium | Medium + LPS | Medium + IL-4 | Medium + LPS and IL-4 | ||
| LC | − | 0.8 | 1.2 | 1.3 | 0.8 |
| + | 0.7 | 0.7 | 0.6 | 0.4 | |
| LC pretreated with anti-IL-4 MAb | − | 0.3 | 1.3 | 0.7 | 0.5 |
| + | 0.4 | 1.2 | 0.5 | 0.2 | |
LC or LC pretreated with anti-IL-4 MAb, uninfected or infected with L. major, were cultured in the absence or presence of LPS (100 ng/ml) and/or IL-4 (5 ng/ml). IL-12 p40 concentrations in culture supernatants were determined after 6 days by ELISA. Shown are the results of one representative experiment of four experiments (LC) or three experiments (LC plus anti-IL-4 MAb) with similar results.
Standard deviation amounts were below 0.15 ng/ml.
Concluding remarks.
It has been shown that the development of a Th2 response in L. major-susceptible BALB/c mice is caused by the lack of IL-12 (15). In the absence of IL-12, normally resistant C57BL/6 mice also fail to develop a Th1 phenotype and are unable to control the infection (6, 12). Since DC, but not macrophages, produce IL-12 in the early phase of Leishmania infection (5), these cells are critical for the development of a protective Th1 response. IL-4, on the other hand, supports the differentiation of Th2 cells (9). In BALB/c mice, but not in C57BL/6 mice, an early peak of IL-4 expression in the draining lymph node can be observed within 1 day of L. major infection (10). The data reported here suggest that the increased sensitivity of infected LC from BALB/c mice to IL-4, which is caused by receptor upregulation, and the resulting decrease in IL-12 expression by activated LC contribute to the induction of Th2 development in susceptible mice. Interestingly, the level of IL-12 production of LC from C57BL/6 mice was not altered by IL-4, although these cells express a considerable level of IL-4R, which, however, is independent of infection. This finding suggests that the IL-12 response of LC from C57BL/6 mice is not regulated by the level of IL-4R expression and may provide an explanation for the previous observation that resistant mice, despite the presence of an IL-4 response that is comparable to that in susceptible mice in the early phase of infection, develop a dominant Th1 response and heal (19).
The present study revealed that IL-4R expression was increased on infected LC from susceptible BALB/c mice but not on those from resistant C57BL/6 mice. Enhanced IL-4R levels correlated with suppression of LPS-induced IL-12 expression by DC after treatment with IL-4. These data provide the first evidence that infection of DC results in a mouse strain-dependent modulation of cytokine receptor expression, a mechanism that may contribute to the development of Th2 cells and to susceptibility to L. major in BALB/c mice via its effect on the ability of DC to express IL-12.
Acknowledgments
This work was supported by the German Bundesministerium für Bildung und Forschung (BMBF).
REFERENCES
- 1.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 2.Blank, C., H. Fuchs, K. Rappersberger, M. Röllinghoff, and H. Moll. 1993. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 167:418-425. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C., H. Moll, W. Solbach, and M. Röllinghoff. 1990. Tumor necrosis factor α in combination with interferon γ but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur. J. Immunol. 20:1131-1135. [DOI] [PubMed] [Google Scholar]
- 4.Flohé, S. B., C. Bauer, S. Flohé, and H. Moll. 1998. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur. J. Immunol. 28:3800-3811. [DOI] [PubMed] [Google Scholar]
- 5.Gorak, P. M. A., C. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687-695. [DOI] [PubMed] [Google Scholar]
- 6.Hondowicz, B. D., T. M. Scharton-Kersten, D. E. Jones, and P. Scott. 1997. Leishmania major-infected C3H mice treated with anti-IL-12 mAb develop but do not maintain a Th2 response. J. Immunol. 159:5024-5031. [PubMed] [Google Scholar]
- 7.Kämpgen, E., F. Koch, C. Heufler, A. Eggert, L. L. Gill, S. Gillis, S. K. Dower, N. Romani, and G. Schuler. 1994. Understanding the dendritic cell lineage through a study of cytokine receptors. J. Exp. Med. 179:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konecny, P., A. J. Stagg, H. Jebbari, N. English, R. N. Davidson, and S. C. Knight. 1999. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur. J. Immunol. 29:1803-1811. [DOI] [PubMed] [Google Scholar]
- 9.Kopf, M., F. Brombacher, G. Köhler, G. Kienzle, K.-H. Widmann, K. Lefrang, C. Humborg, B. Ledermann, and W. Solbach. 1996. IL-4-deficient BALB/c mice resist infection with Leishmania major. J. Exp. Med. 184:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by Vβ4Vα8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 11.Matsue, H., P. D. Cruz, Jr., P. R. Bergstresser, and A. Takashima. 1993. Profiles of cytokine mRNA expressed by dendritic epidermal T cells in mice. J. Investig. Dermatol. 101:537-542. [DOI] [PubMed] [Google Scholar]
- 12.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 13.Moll, H. 1993. Epidermal Langerhans cells are critical for immunoregulation of cutaneous leishmaniasis. Immunol. Today 14:383-387. [DOI] [PubMed] [Google Scholar]
- 14.Moll, H., H. Fuchs, C. Blank, and M. Röllinghoff. 1993. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 23:1595-1601. [DOI] [PubMed] [Google Scholar]
- 15.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 16.Reiner, S. L., S. Zeng, S. E. Wang, L. Stowing, and R. M. Locksley. 1994. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 179:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scharton-Kersten, T., L. C. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320-5330. [PubMed] [Google Scholar]
- 18.Schuler, G., and F. Koch. 1990. Enrichment of epidermal Langerhans cells, p. 139-157. In G. Schuler (ed.), Epidermal Langerhans cells. CRC Press, Boca Raton, Fla.
- 19.Scott, P., A. Eaton, W. C. Gause, X. di Zhou, and B. Hondowicz. 1996. Early IL-4 production does not predict susceptibility to Leishmania major. Exp. Parasitol. 84:178-187. [DOI] [PubMed] [Google Scholar]
- 20.Von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, B., S. Kondo, G. M. Shivji, H. Fujisawa, T. W. Mak, and D. N. Sauder. 1996. Tumour necrosis factor receptor II (p75) signalling is required for the migration of Langerhans cells. Immunology 88:284-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinheber, N., M. Wolfram, D. Harbecke, and T. Aebischer. 1998. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur. J. Immunol. 28:2467-2477. [DOI] [PubMed] [Google Scholar]