Abstract
Immunization with DNA followed by modified vaccinia virus Ankara strain, both expressing the antigen 85A, induced both CD4+- and CD8+-T-cell responses in BALB/c mice. Following challenge with Mycobacterium tuberculosis, this prime-boost regimen produced protection equivalent to that conferred by Mycobacterium bovis BCG. Following immunization with dendritic cells pulsed with an antigen 85A CD4+- or CD8+-restricted epitope, alone or in combination, copresentation of both epitopes on the same dendritic cell was required for protection, demonstrating that induced CD8+ T cells can play a protective role against tuberculosis.
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) and are central to the development of cellular immunity. Immature DCs undergo activation and maturation upon contact with pathogens. Mature DCs migrate to secondary lymphoid organs and prime T cells. DCs can present exogenous antigen directly to naïve T cells in situ (11), and this function is likely to be important in the induction of cellular immunity against Mycobacterium tuberculosis (8). CD4+ T cells are essential for immunity against M. tuberculosis (20). Classical class I-restricted CD8+ T cells may also be important (20), but their functional significance in vivo remains unclear. A promising candidate antigen for a new tuberculosis (TB) vaccine is antigen 85A, which is protective in mice and guinea pigs (10, 9). Antigen 85A contains several CD4+-T-cell epitopes and at least one CD8+-T-cell epitope in BALB/c mice (4), and CD4+- and CD8+-T-cell responses have been identified in humans (12, 18). Heterologous prime-boost immunization strategies can induce high levels of CD8+ and CD4+ T cells in patients with malaria, human immunodeficiency virus, and TB (7, 14, 15). We used DNA and recombinant modified vaccinia virus Ankara strain (MVA) vaccines, each encoding antigen 85A, to identify two immunodominant epitopes within antigen 85A, a CD4+-restricted epitope and a CD8+-restricted epitope. DCs pulsed with both of these epitopes but not a mixture of DCs pulsed with each epitope separately conferred protection against challenge equivalent to the protection conferred by the prime-boost immunization regimen and by Mycobacterium bovis BCG.
M. tuberculosis H37Rv stocks were prepared as previously described (14). The antigen 85A gene and the tissue plasminogen activator leader sequence were ligated together as a single coding sequence in plasmid vector pSG2 and vaccinia virus shuttle vector pSC11. BHK cells were infected with nonrecombinant MVA at a multiplicity of infection of 0.05 and then transfected with the recombinant shuttle vector. Recombinant virus was selected for by plaque purification. Autologous bone marrow cells were cultured in RPMI 1640 containing 10% fetal calf serum (Labtech International, Uckfield, United Kingdom) and 1 ng of recombinant granulocyte-macrophage colony-stimulating factor (Peprotech, Rocky Hill, N.J.) per ml for 7 days. The medium and recombinant granulocyte-macrophage colony-stimulating factor (0.5 ng/ml) were replenished on days 3 and 6. DCs were pulsed with peptide (2 μg/ml) overnight, washed twice, and counted before injection. Incubation of DCs with peptide did not induce DC maturation, as indicated by expression of surface molecules, such as major histocompatibility complex classes I and II, CD86, and CD40 (determined by fluorescence-activated cell sorter analysis) (data not shown). Female BALB/c mice (age, 4 to 6 weeks; Harlan Orlac, Shaws Farm, Blackthorn, United Kingdom) were injected intramuscularly with plasmid DNA (50 μg/immunization). Recombinant MVA (106 PFU) and DCs (106 cells) were injected intravenously. BCG Glaxo (4 × 105 CFU) was injected intradermally at the time of the first immunization. Immunizations were given at 2-week intervals, and immunogenicity was evaluated 2 weeks after the last immunization. Splenocytes were prepared as previously described (14). The number of gamma interferon-secreting specific T cells was determined by using an ex vivo Elispot assay and 20-mer peptides (overlapping by 10 amino acids) spanning the length of antigen 85A (Research Genetics, Huntsville, Ala.) (14). The nonamer H-2Kd-restricted epitope within peptide p11 (WYDQSGLSV) was used for some DC experiments. The numbers of spot-forming cells (SFC) per 106 splenocytes were determined. Cell depletion was performed on cells restimulated in vitro for 7 days as previously described (14). In the challenge experiments, mice were infected with 106 or 5 × 106 CFU of M. tuberculosis by intraperitoneal injection 2 weeks after the final immunization. Organs were homogenized 8 weeks later with a mini-bead beater (Biospec Products, Bartlesville, Okla.), and dilutions were plated onto Middlebrook plates. The plates were incubated for 21 days at 37°C, and the numbers of colony counts per organ were calculated. Data were expressed as the log10 mean and standard error for each experimental group. Student's t test was used to determine statistical significance between groups. The P values presented below are one-tailed values determined by comparing immunized groups with nonimmunized controls.
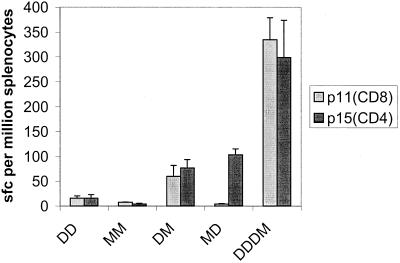
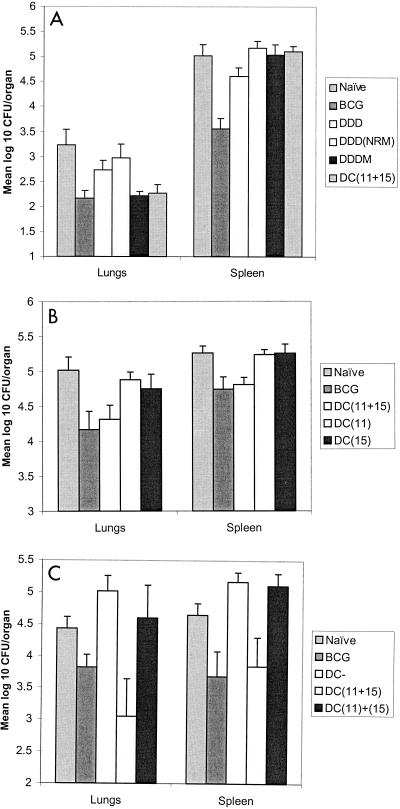
BALB/c mice were immunized with DNA (D regimen), MVA (M regimen), or a combination of the two (DM or MD regimen). Using an ex vivo Elispot assay, we identified responses to the following four peptides from the splenocytes of immunized mice: p11, p15, p24, and p27 (Table 1). The strongest responses were the responses to peptides p11 and p15. Magnetic bead depletion studies performed with restimulated splenocytes from mice immunized with DNA and then MVA showed that the response to p11 was eliminated by CD8+-T-cell depletion. The responses to p15, p24, and p27 were eliminated by CD4+-T-cell depletion. In this study we focused on the dominant epitopes contained in p11 and p15. Figure 1 shows that the responses generated by single or repeated immunizations with either construct were weak. Heterologous boosting of DNA with MVA resulted in higher frequencies of both the CD4+(p15) and CD8+(p11) T cells. Heterologous boosting of MVA with DNA increased only the frequency of CD4+ T cells. The most immunogenic regimen was three doses of DNA followed by a single boost with MVA (DDDM regimen) (Fig. 1). In challenge studies, the DDDM regimen conferred protection in the lungs equivalent to the protection conferred by BCG (Fig. 2A). Immunization with DNA alone (DDD regimen) or immunization with DNA boosted with nonrecombinant MVA (DDDNRM regimen) conferred no protection. In order to determine whether the protective immunity induced by the DDDM immunization regiment was attributable to T cells specific to the 85A CD4+ and CD8+ epitopes identified in the Elispot assay, peptide-pulsed DCs were used. Mice were immunized intravenously with DCs pulsed with peptides p15 (CD4+) and p11 (CD8+). When challenged, mice immunized with DCs pulsed with both epitopes showed levels of protection in the lungs comparable to the levels of protection obtained with both BCG and the heterologous DDDM regimen (Fig. 2A). Mice immunized with DCs pulsed with either the CD4+- or the CD8+-T-cell epitope alone were not protected against challenge (Fig. 2B). Mice immunized with DCs pulsed with both epitopes simultaneously again were protected against challenge. We then investigated the interaction between the CD4+- and CD8+-T-cell responses by immunizing mice with either a mixture of DCs, 5 × 105 cells of which had been pulsed with the CD4+ epitope and 5 × 105 cells of which had been pulsed with the CD8+ epitope, or with DCs pulsed with both epitopes simultaneously. Three mice from each group were analyzed for immunogenicity. The mean Elispot responses per 106 splenocytes were 57 SFC (p11) and 104 SFC (p15) in the mice immunized with the mixture of DCs, compared with 129 SFC (p11) and 116 SFC (p15) in the mice immunized with DCs pulsed with both peptides simultaneously; the latter immunization regimen also induced cytotoxic T lymphocytes (CTL) to p11 (data not shown). Mice immunized with the DC mixture were not protected against challenge, despite having specific CD4+ and CD8+ T cells against antigen 85A. Mice immunized with DCs pulsed with both epitopes together showed significant protection (Fig. 2C).
TABLE 1.
Prime-boost immunization regimens with DNA and MVA constructs induced responses to four of the overlapping peptides from antigen 85Aa
| Peptide | Sequence | Undepleted response (no. of SFC) | CD4+ depletion response (no. of SFC) | CD8+ depletion response (no. of SFC) |
|---|---|---|---|---|
| p11b | EWYDQSGLSVVMPVGGQSSF | 250 | 200 | 10 |
| p15c | TFLTSELPGWLQANRHVKPT | 300 | 0 | 250 |
| p24c | QRNDPLLNVGKLIANNTRVW | 30 | 0 | 20 |
| p27c | LGGNNLPAKFLEGFVRTSNI | 30 | 0 | 25 |
Depletion studies were performed with splenocytes obtained from mice immunized with DNA and MVA and restimulated in vitro with peptide for 7 days.
The response to peptide p11 was eliminated by CD8+-T-cell depletion.
The responses to peptides p15, p24, and p27 were eliminated by CD4+-T-cell depletion.
FIG. 1.
Comparative immunogenicity results obtained by ex vivo gamma interferon Elispot assay for homologous and heterologous prime-boost immunization regimens. The bars indicate mean numbers of SFC per 106 splenocytes; the error bars indicate standard errors.
FIG. 2.
Mean log10 number of CFU per organ. The error bars indicate standard errors. In all experiments, BCG immunization conferred significant protection against challenge in the lungs and spleens. There were 10 to 15 mice per group in most experiments; in the experiments whose results are shown in panel B there were only 6 mice in the control group. Mice were challenged with 106 CFU (A) or 5 × 106 CFU (B and C). (C) DCs with no peptide (DC−) and Prime-boost immunization with the DDDM regimen conferred significant protection (P = 0.005) in the lungs, as did immunization with DCs pulsed with both the CD4+-T-cell (p15) and CD8+-T-cell (p11) epitopes from antigen 85A (P = 0.008). (B). DCs pulsed with either the CD4+ epitope alone or the CD8+ epitope alone did not confer protection, whereas DCs pulsed with both epitopes conferred significant protection in the lungs (P = 0.016) and spleens (P < 0.001). (C) DCs with no peptide (DC−) and DCs pulsed with the CD4+ epitope and the CD8+ epitope separately and then mixed prior to immunization [DC(11)+(15)] did not confer protection against challenge. DCs pulsed with both epitopes conferred significant protection in the lungs (P = 0.022).
We found that induction of classical class I-restricted CD8+ T cells by vaccination can confer protective immunity against M. tuberculosis. Direct evidence that these cells play a role has not been obtained previously. β2M knockout mice, which lack functional CD8+ T cells, are more susceptible to challenge with M. tuberculosis (6), but this does not eliminate the possibility that nonclassically restricted T cells, which are well described in TB patients (13, 19), play a role. The protective effect of DNA vaccines has been attributed, at least in part, to the CD8+ T cells (10, 21). However, recently, a DNA vaccine encoding antigen 85A has been shown to induce protective immunity in β2M knockout mice, suggesting that the protective effect of this DNA vaccine is mediated through CD4+ T cells (5). The adoptive transfer of CD8+-T-cell clones induced by DNA vaccination conferred protection, but these cells were expanded in vitro before transfer (2). The results do not indicate whether CD8+ T cells can protect when they are present at the more physiological numbers found in vivo. We found that protection induced by DC-peptide immunization is dependent on a classically class I-restricted CTL epitope, suggesting that H-2Kd-restricted (4) T cells specific for this epitope mediate protective immunity. The presence of a CD4+-T-cell epitope on the same APC was also required. Our finding is consistent with a model of CTL activation in which the CD4+ T cell activates the APC, which in turn activates the CTL (1). The CD40 ligand (CD40L) on the surface of activated CD4+ T cells interacts with CD40 on the surface of the APC, which facilitates CTL priming (16). The mechanism by which this CD40-CD40L interaction enables CTL priming is unclear. In DCs, ligation of CD40 by CD40L induces high levels of interleukin-12. In addition, CD40L is extremely potent at upregulating the expression of costimulatory molecules on DCs (3). CTL priming in vivo is dependant on the maturation of DCs, and DC maturation can be triggered by CD4+-T-helper cells, agonistic CD40 antibody, or lipopolysaccharide (17). In this study immature DCs were used in order to allow the CD40-CD40L interaction to induce DC maturation in vivo. The lack of protection in the mice immunized with the DDD or DDD(NRM) regimen effectively rules out the possibility that these vaccines administered 2 weeks before challenge have a nonspecific protective effect. In addition, the lack of protection in the DC groups pulsed with either epitope alone (Fig. 2B), the mixed DC group, or the DC with no peptide group (Fig. 2C) rules out the possibility that DC immunization 2 weeks prior to challenge has a nonspecific protective effect. Although the results obtained with these control groups ruled out the possibility that there was a nonspecific protective effect, in this study we did not investigate the durability of the protection observed. It would be interesting in future studies to increase the time interval between DC immunization and challenge and assess the sustainability of the protection conferred in this model. The aerosol challenge model that is now more widely available is considered a more relevant model of human TB, and it would be interesting to see whether the differential protective effect in the lungs is maintained with an aerosol challenge. It would also be interesting to investigate the importance of mucosal immunity by comparing systemic and aerosol delivery of DCs. We found that induction of classical class I-restricted CD8+ T cells by immunization conferred protection against challenge and implicated these cells in protective immunity against TB. An optimal TB vaccine should aim to induce classically restricted CD8+ T cells as well as CD4+ T cells. Further use of the DC model should help in the identification of protective antigens and epitopes and in the optimization of vaccine design.
Acknowledgments
We thank Jiang-Ting Hu, Magdalena Plebanski, and Ernie Gould for assistance and advice.
H.M. was supported by an MRC clinical training fellowship. A.V.S.H. is a Wellcome Trust Principal Research Fellow.
REFERENCES
- 1.Bennett, S. R. M., F. R. Carbone, F. Karamalis, J. F. A. P. Miller, and W. R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonato, V. L. D., V. M. F. Lima, R. E. Tascon, D. B. Lowrie, and C. L. Silva. 1998. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect. Immun. 66:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T. P. Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Souza, S., O. Denis, T. Scorza, F. Nzabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding antigen 85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 6.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 2017. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 9.Horwitz, M. A., B. W. E. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 11.Inaba, K., J. P. Metlay, M. T. Crowley, and R. M. Steinman. 1990. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Launois, P., R. DeLeys, M. N. Niang, A. Drowart, M. Andrien, P. Dierckx, J. L. Cartel, J. L. Sarthou, J. P. Van Vooren, and K. Huygen. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewinsohn, D. M., M. R. Alderson, A. L. Briden, S. R. Riddell, S. G. Reed, and K. H. Grabstein. 1998. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen presenting cells. J. Exp. Med. 187:1633-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McShane, H., R. Brookes, S. C. Gilbert, and A. V. S. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. S. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 16.Schoenberger, S. P., R. E. M. Toes, E. I. H. van der Voort, R. Offringa, and C. J. M. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 17.Schuurhuis, D. H., S. Laban, R. E. M. Toes, P. Ricciardi-Castagnoli, M. J. Kleijmeer, E. I. H. van der Voort, D. Rea, R. Offringa, H. J. Geuze, C. J. M. Melief, and F. Ossendorp. 2000. Immature dendritic cells acquire CD8+ cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J. Exp. Med. 192:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, S. M., A. Malin, P. T. Lukey, S. E. Atkinson, J. Content, K. Huygen, and H. M. Dockrell. 1999. Characterization of human Mycobacterium bovis Bacille Calmette-Guérin-reactive CD8+ T cells. Infect. Immun. 67:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 20.Stenger, S., and R. L. Modlin. 1999. T cell mediated immunity to Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2:89-93. [DOI] [PubMed] [Google Scholar]
- 21.Tascon, R. E., M. J. Colston, S. Ragno, E. Stavropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]