Abstract
In order to study the immune response elicited by asymptomatic carriage of Neisseria meningitidis, samples of serum, peripheral blood mononuclear cells (PBMCs), and saliva were collected from a cohort of more than 200 undergraduate students in Nottingham, United Kingdom, who were subject to high rates of acquisition and carriage of meningococci. Serum immunoglobulin G levels were elevated following increases in the rate of carriage, and these responses were specific for the colonizing strains. In order to investigate T-cell responses, PBMCs from 15 individuals were stimulated with a whole-cell lysate of the H44/76 meningococcal strain (B:15:P1.7,16), stained to detect cell surface markers and intracellular cytokines, and examined by flow cytometry. The cells were analyzed for expression of CD69 (to indicate activation), gamma interferon (IFN-γ) (a representative T-helper 1 subset [Th1]-associated cytokine), and interleukin-5 (IL-5) (a Th2-associated cytokine). Following a brief meningococcal stimulation, the numbers of CD69+ IFN-γ+ CD56/16+ NK cells were much higher than cytokine-positive CD4+ events. Both IFN-γ+ and IL-5+ events were detected among the CD69+ CD4+ population, leading to the conclusion that an unbiased T-helper subset response was elicited by meningococcal carriage.
Neisseria meningitidis colonizes the human nasopharynx, from where invasion of underlying tissues may result in a number of severe, possibly fatal, clinical syndromes. It is the most common cause of pyogenic meningitis, and causes outbreaks of invasive disease. A number of such outbreaks have recently been reported at universities in the United Kingdom (12), where the rates of asymptomatic carriage are known to be particularly high (30). The worldwide incidence of meningococcal infections is increasing, particularly those due to serogroup B, for which there is no available vaccine.
Immunity to invasive meningococcal disease appears to be dependent upon serum immunoglobulin G (IgG), which, together with complement, elicits bactericidal activity (20). Those who recover from an invasive infection usually remain protected from disease for life (25). There appears to be an inverse relationship between serum IgG levels and incidence of invasive disease, highlighting the importance of IgG in protection. A cellular response is extremely important in generating and maintaining this protective immunity. T-helper cells are required for the generation and maturation of humoral responses against T-cell-dependent protein antigens, providing immunological memory. The cellular response and cytokine profiles elicited by invasive disease have been studied, although not extensively. Kornelisse et al. (24) measured cytokines in the serum and cerebrospinal fluid of children with meningitis and found elevated levels of interleukin-12 (IL-12) and gamma interferon (IFN-γ) but not IL-6, IL-8, or IL-10. A study of cytokine secretion by peripheral blood mononuclear cells (PBMCs) from convalescent patients (33) revealed that cells from older children produced a much higher IL-10/IFN-γ ratio, and therefore T-helper 2 subset (Th2) response, than those of younger children, who are more vulnerable because of their immature immune status. Those authors concluded that a vaccine, designed to stimulate immune responses mimicking those following invasive disease, should stimulate IL-10 production. The cellular source of cytokines in these studies was not identified, and therefore the T-helper subset response per se to meningococcal infection remains unknown.
Little is known about immune responses elicited by meningococcal carriage, and studies have thus far been concerned only with serum antibody. Carriage rates rise rapidly in childhood and peak at around 16 to 20 years before falling steadily with increasing age (25). In contrast, serum IgG antibody levels remain low until adolescence and then steadily increase. It has been known for many years that carriage elicits a bactericidal antibody response that is specific for the strain carried but also cross-reactive with heterologous strains (13, 22, 34). This response may provide high levels of protective antibody for several months after the carried strain has been lost. The members of the neisserial genus share several cross-reactive antigens, and it is thought that natural immunity against N. meningitidis may be obtained through colonization by commensal species such as Neisseria lactamica (45). This mechanism is thought to be especially important in childhood, where carriage of N. lactamica fluctuates in the first 5 years of life (13). Carriage elicits a response against a variety of meningococcal antigens, including class 1 (22), class 2 (21), and class 5 (37) outer membrane proteins and lipopolysaccharide (LPS) (21). A detailed study of carriage and humoral immunity among military recruits demonstrated that the response was dominated by antibodies with specificity for the class 1 outer membrane protein (22). This important molecule is a major component of the outer membrane and forms the basis for serotype and subtype classification of meningococci, since it exhibits antigenic diversity between strains.
Although colonization with a variety of meningococcal strains throughout life appears to be beneficial in inducing immune responses, colonization is not protective against subsequent recolonization with the homologous strain or a heterologous strain and is not always protective against invasive disease (4). This may be in contrast with the immune response elicited by invasive disease, which is said to be cross-protective against further episodes of disease (13). An analysis of immune responses elicited by carriage may therefore provide some clues concerning resistance and susceptibility to infection.
The aim of this cohort study was to investigate humoral and cellular immune responses elicited by meningococcal colonization among new university students, who are known to be subject to increased rates of carriage and acquisition of new strains (4, 30). Salivary antibody levels were measured in addition to those in serum, so that mucosal as well as systemic antibody responses could be detected. In order to investigate carriage-induced immune responses more fully, and considering the lack of published data on antimeningococcal T-cell responses, several parameters of cellular immunity were studied, with a focus on T-helper cell subsets.
MATERIALS AND METHODS
Subjects.
A cohort of 228 first-year undergraduates were recruited at the start of the academic year, on the day of arrival at their hall of residence (dormitory), and a posterior pharyngeal swab was taken. An additional 46 first-year medical students were recruited 2 days later. Seventy-eight of the residence hall students and 46 medical students also volunteered to donate 50 ml of venous blood. One week later, 190 of the original 228 students were reswabbed.
At weeks 7 to 8 of the study, 48 students from the residence hall cohort and 30 medical students were swabbed again. Further samples of blood and also of saliva were collected. At weeks 20 to 21, 18 students from the residence hall and 17 medical students volunteered for the final swabs and blood and saliva collections. A total of 43 students provided samples at all three time points.
Cultures.
Pharyngeal swabs were directly plated onto GC agar containing vancomycin, colistin, nystatin, and trimethoprim (selective supplement SR91) (Oxoid Ltd., Basingstoke, United Kingdom) at the point of sampling. Plates were incubated at 37°C with 5% CO2 for 24 to 48 h. Isolates that produced colonies morphologically similar to N. meningitidis were subcultured and Gram stained. Gram-negative diplococci were tested further using a Gonochek kit (EY Laboratories Inc., San Mateo, Calif.), and those thought to be N. meningitidis were serologically characterized at the Public Health Laboratory Service Meningococcal Reference Laboratory.
Clinical materials.
Fifty-milliliter blood samples were collected in 10-ml Vacutainer tubes containing EDTA anticoagulant. Samples of serum were collected, frozen, and stored at −20°C. PBMCs were purified by density gradient centrifugation using Histopaque1077 (Sigma-Aldrich Company Ltd., Poole, United Kingdom). The washed PBMCs were split into three aliquots and frozen with 10% dimethyl sulfoxide in fetal calf serum (FCS) (Sigma) at −80°C overnight before being stored in liquid nitrogen vapor.
Saliva samples were collected into tubes and placed immediately on ice. The samples were stabilized with a cocktail of protease inhibitors with broad-range activity (Sigma) before being frozen and stored at −80°C.
Meningococcal antigen preparation.
Using a method based upon that of Jones et al. (22), overnight cultures of individual meningococcal isolates and the H44/76 strain in Muller-Hinton broth (Oxoid Ltd.) were centrifuged at 1,600 × g for 15 min. The bacteria were harvested, washed, and then resuspended in sterile phosphate-buffered saline (PBS) before heat inactivation at 56°C for 30 min. Optical densities (ODs) of the suspensions at a wavelength of 600 nm were recorded. An OD of 0.30 was determined to be the optimum coating concentration for enzyme-linked immunosorbent assays (ELISAs).
Serum and salivary antibody ELISA.
The ELISA protocol was based upon that described by Guttormsen et al. (15). Briefly, 96-well ELISA plates (NUNC Maxisorp; Life Technologies, Paisley, Scotland) were coated overnight at 4°C with (per well) 50 μl of meningococcal lysate diluted to give an OD of 0.30 in 0.05 M carbonate-bicarbonate buffer (pH 9.6). In order that a standard curve could be included, a portion of each plate was also coated with an optimal concentration of monoclonal anti-human IgG, IgA, or IgM Fab-specific antibody (Sigma). Plates were washed with PBS containing 0.05% Tween 20 (PBS-Tween) (Sigma) before blocking for 1 h at room temperature with 100 μl of 3% bovine serum albumin (BSA) (Sigma) in PBS-Tween per well. Sera and saliva samples were diluted in PBS-Tween before 50 μl/well was applied to the washed plates in duplicates. Dilutions from a standard curve of human IgG, IgA, or IgM (Sigma) were placed in the wells coated with anti-immunoglobulin antibody and were present on every plate. Plates were incubated at room temperature for 90 min before washing and addition of (per well) 50 μl of alkaline phosphatase-conjugated anti-human IgG, IgA, or IgM diluted (to the working concentration suggested by the manufacturer) in PBS-Tween. After a further 90 min of incubation and rigorous washing, 100 μl of the substrate solution was added per well. This solution was composed of Sigma 104 phosphatase tablets dissolved in diethanolamine buffer (pH 9.8) (Sigma). The plates were incubated for 30 min for IgG and IgM assays and for 90 min for IgA assays. ODs were read at 405 nm using an Emax Precision Microplate Reader (Molecular Devices), and standard curves were plotted. The limit of sensitivity of each assay plate was calculated as the mean OD plus three times the standard deviation for six control wells receiving no primary serum. The relative concentration of specific antibody in each sample was calculated by direct reference to the standard curve. The relative concentrations of antigen-specific and total IgA and IgG in saliva were measured.
Bactericidal assays.
The method used for bactericidal assays was based upon that of Borrow et al. (6). Briefly, Muller-Hinton broth was inoculated with 5 to 10 colonies from a fresh culture of N. meningitidis and incubated for 2 h at 37°C with shaking. Cells were removed, washed with sterile 0.5% BSA in PBS (Sigma), and diluted to 800 CFU per 10 μl using an OD equivalence of 0.1 = 2 × 108 CFU/ml at a wavelength of 650 nm. Twenty microliters of diluted heat-inactivated test serum was mixed with 10 μl of baby rabbit complement and 10 μl of bacterial suspension. After 1 h of incubation at 37°C, the contents of each reaction well were serially diluted, 10-μl aliquots were placed onto blood agar, and the plates were incubated overnight at 37°C with 5% CO2 before the colonies were counted. The number of CFU in each reaction well was then calculated, and the percent reduction in CFU was determined by comparison with control wells which received no serum. A second set of control wells which contained serum but no complement was included in each test. The bactericidal antibody titer was defined as the dilution of serum resulting in a 50% reduction in CFU.
Cellular proliferation assays.
As described previously (1, 23), frozen aliquots of PBMCs were resuscitated, washed, and resuspended to 106 per ml in complete medium (RPMI 1640 supplemented with 10% human AB serum, 10 mM HEPES, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml) (Sigma). Two-hundred-microliter aliquots of cells were placed into the wells of a sterile 96-well flat-bottomed plate in quadruplicate together with 20 μl of antigen, mitogen, or medium. A range of final concentrations of the H44/76 meningococcal lysate from 1 to 50 μg of protein/ml was used in the assays together with tuberculin purified protein derivative (Statens Seruminstitut, Copenhagen, Denmark) at 20 μg/ml as a positive control antigen and phytohemagglutinin (Sigma) at 10 μg/ml as a mitogen control.
The cells were incubated at 37°C in 5% CO2 for 7 days. For the final 18 h of culture, 1 μCi of [3H]thymidine (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) was added to each well. The cells were harvested, and proliferation was detected by measurement of cellular [3H]thymidine incorporation. Stimulation indices were calculated as the ratio of mean counts per minute from stimulated and unstimulated cells. Pilot experiments determined that fresh and frozen PBMCs gave reproducible results in these assays.
Assay of cytokine responses by intracellular staining and flow cytometry.
According to a method based on that of Maino and Picker (28), 5 × 105 PBMCs in 1 ml of complete medium were aliquoted into sterile 12- by 75-mm culture test tubes (Elkay Laboratory Products Ltd., Basingstoke, United Kingdom). Anti-CD28 antibody (Beckman Coulter, High Wycombe, United Kingdom) was added to each tube to give a concentration of 1 μg/ml. The lysate from meningococcal strain H44/76 was added to give a final concentration of 20 μg/ml. As a positive control, phorbol myristate acetate (PMA) and ionomycin were added to give final concentrations of 20 ng/ml and 1 μM, respectively. Negative control tubes received anti-CD28 but no antigens or mitogens. Anti-CD28 antibody is commonly used to improve the sensitivity of ex vivo assays of T-cell cytokine expression, but there is no evidence from other studies that it causes skewing of the response profile (38).
The cultures were incubated for 2 h at 37°C in 5% CO2 before addition of brefeldin A (Sigma) at 10 μg/ml and then replaced in the incubator for a further 8 h (which was determined by experimentation to be the optimal incubation time for measurement of intracellular cytokines following antigenic stimulation). The positive control PMA-ionomycin tubes were incubated for only 4 h after addition of brefeldin A.
The cells were pelleted at 200 × g for 5 min before incubation on ice for 30 min during staining with anti-CD4-phycoerythrin-Texas red (anti-CD4-ECD), anti-CD8-ECD, anti-CD16-phycoerythrin (anti-CD16-PE), and anti-CD56-PE antibody conjugates (Beckman Coulter). The cells were washed three times with 1 ml of PBA buffer (PBS containing 0.1% BSA and 0.1% sodium azide) supplemented with 2% FCS (Sigma) before being fixed in 1 ml of 0.5% formaldehyde in borate-buffered saline at 4°C overnight. The cells were then washed with 1 ml of PBA, permeabilized with 1 ml of PBA containing 0.1% saponin (Sigma), and washed with 1 ml of PBA-0.1% saponin-10% FCS before being stained with anti-CD69-PC5, anti-IFN-γ-fluorescein isothiocyanate (Beckman Coulter), and anti-IL-5-PE (Pharmingen, San Diego, Calif.) antibody conjugates at 4°C for at least 2 h. The cells were washed three times with PBA-0.1% saponin before being fixed in 0.5% formaldehyde.
The data on fluorescently labeled cells were acquired using a Coulter EPICS XL-MCL flow cytometer and analyzed using WinMDI version 2.8 (http://facs.scripps.edu/).
Assay of TGF-β1 in culture supernatants.
PBMCs were cultured at 106/ml in AIM V serum-free medium (GIBCO, Life Technologies, Paisley, Scotland) in 96-well plates for 72 h in the presence of optimum stimulatory concentrations of a whole-cell lysate from the H44/76 meningococcal strain, as previously determined by the proliferation assay. The sterile culture supernatants were collected by centrifugation and frozen at −80°C. A DuoSet ELISA kit (R&D Systems Europe, Ltd., Abingdon, United Kingdom) was used to quantify transforming growth factor β1 (TGF-β1) according to the manufacturer's instructions.
Statistics.
Statistical tests of paired sets of data were carried out using the Wilcoxon signed rank test. For unpaired data, the Mann-Whitney U test was used. The Spearman rank correlation coefficient was calculated to detect significant correlations in the data. In all cases, a difference or correlation was considered to be significant at a P value of ≤0.05.
RESULTS
Meningococcal carriage.
As shown in Table 1, the rate of carriage of N. meningitidis on the first day of the academic year (day 0 of the study) of the residence hall cohort of 228 students was 4.4%. Seven days later (at week 1) an increase in carriage to a rate of 20.5% was found. Carriage rates remained elevated at week 8 of the study (14.6%) and also at week 20 (45.0%), although there were fewer volunteers for these time points. Carriage rates among the cohort of medical students followed a similar trend, with the rate during the middle of the first week being elevated already at 19.6%.
TABLE 1.
Meningococcal carriage rates among students from the beginning of the academic yeara
| Cohort | Wk of term | Carriage rate (%)b |
|---|---|---|
| Residence hall students | 0 (day 0) | 10/228 (4.4) |
| 1 (day 7) | 39/190 (20.5) | |
| 8 | 7/48 (14.6) | |
| 20 | 18/40 (45.0) | |
| Medical students | 0 (day 4) | 9/46 (19.6) |
| 7 | 9/28 (32.1) | |
| 21 | 8/17 (47.1) |
Pharyngeal swabs collected from two cohorts of students at weeks 0, 1, 7 to 8, and 20 to 21 were plated directly onto selective agar plates and incubated immediately.
Swabs yielding confirmed cultures of N. meningitidis.
Thirty-seven of the 77 isolates recovered during the study were nonserogroupable acapsulate strains (48.1%). Nineteen isolates were of group B (24.7%), the most prevalent serogroup. Seven strains (10.3%) were of group Y, two (9.1%) were of group W135, one was of group 29E, and one strain belonged to serogroup C.
Serum antimeningococcal antibody responses.
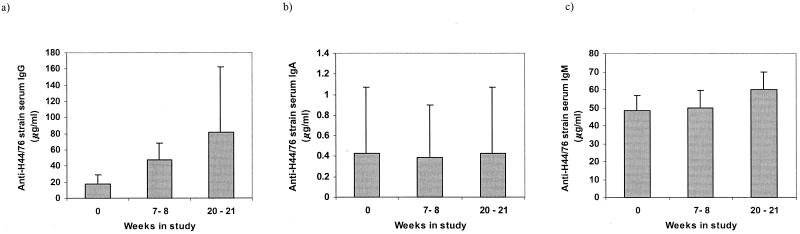
The sera from 43 students who provided samples at all of the time points of the study were tested by ELISA for levels of antibody with specificity for the H44/76 strain (B:15:P1.7,16), belonging to the ET-5 complex of N. meningitidis. As the rate of meningococcal carriage increased among the group, the levels of meningococcus-specific serum IgG also increased (Fig 1a). There was a significant (P < 0.001) twofold median increase in serum IgG between week 0 and weeks 7 to 8 and a further significant increase (P < 0.001) in median IgG concentrations from 47.6 μg/ml at weeks 7 to 8 to 81.25 μg/ml at weeks 20 to 21. All except 3 of these 43 students had an increase in their levels of antimeningococcal serum IgG of at least 30 μg/ml. In contrast with the IgG data, however, no significant differences in serum IgA or IgM levels were detected over the course of the study (Fig. 1b and c). Serum IgA levels remained at median levels of approximately 0.4 μg/ml for the duration of the 21 weeks. The concentration of H44/76 strain-specific IgM in serum samples increased from a median of 48.5 μg/ml at week 0 to 50.0 μg/ml at weeks 7 to 8 and 60.0 μg/ml at weeks 20 to 21. These differences, however, were not significant.
FIG. 1.
Relative concentrations of serum antibodies with specificity for the H44/76 meningococcal strain. Serum samples collected from 43 individuals at three time points during the course of the study were tested by indirect ELISA for meningococcus-specific IgG (a), IgA (b), and IgM (c) antibodies. The shaded bars represent the geometric mean concentrations; error bars depict the 95% CIs.
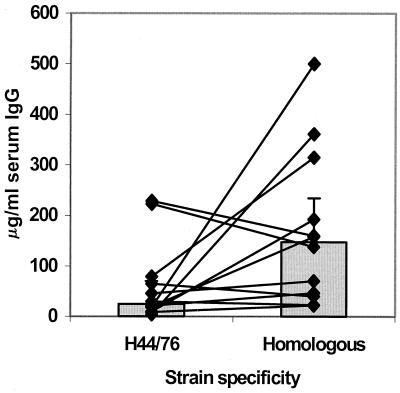
The levels of serum IgG specific for carriers' own homologous isolates were significantly higher (P < 0.05) than those responses detected against the heterologous H44/76 strain. At weeks 7 to 8, the responses of a group of 12 students to their homologous strains were found to be on average 2.74-fold higher (median, 147.6 μg/ml), than those detected against the H44/76 strain (median, 25.10 μg/ml) (Fig. 2).
FIG. 2.
Strain specificity of serum antibody responses among a group of 12 confirmed meningococcal carriers. The points and lines represent, for each individual, concentrations of serum IgG with specificity for homologous isolates recovered from pharyngeal swabs compared with those of anti-strain H44/76 IgG. The shaded bars represent the geometric mean concentrations; error bars depict the 95% CIs.
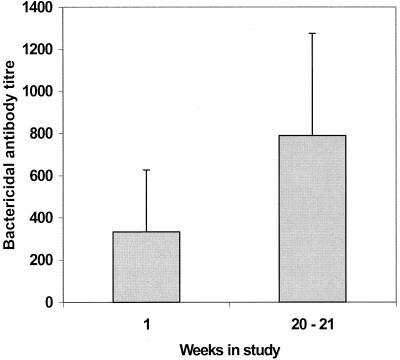
In addition to the increase in concentration of H44/76 strain-specific IgG, a significant increase in the bactericidal activity of sera was detected over the 20 weeks of the study. The bactericidal activity of a range of dilutions of sera was tested against the H44/76 strain (Fig. 3). At week 0, the mean bactericidal titer was 332.0. By weeks 20 to 21, however, this had increased significantly (P = 0.02) to 789.6, suggesting an increase in the functional activity of the immune response.
FIG. 3.
Bactericidal activity of serum samples collected from 19 students at week 1 and weeks 20 to 21. Sets of serially diluted serum samples were incubated with 900 CFU of the H44/76 meningococcal strain per reaction well in the presence of human complement. Bactericidal antibody titers were calculated as the serum dilution that yielded a 50% reduction in CFU per well. Shaded bars show the geometric means; error bars represent the 95% CIs.
Antimeningococcal antibody levels in saliva.
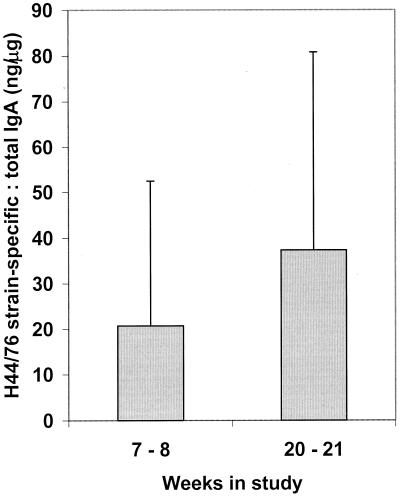
Saliva samples collected from 37 students at weeks 7 to 8 and weeks 20 to 21 were tested by indirect ELISA to quantify IgA and IgG antibodies with specificity for the H44/76 strain. An increase in the relative concentration of specific IgA was detected. The median concentration in samples from weeks 7 to 8 was 600 ng/ml, while that in samples from weeks 20 to 21 was 745 ng/ml. There was no change in the median concentration of total IgA from weeks 7 to 8 (24.5 μg/ml) to weeks 20 to 21 (23.75 μg/ml); however, an increase in the proportion of meningococcus-specific IgA (from 22.9 to 37.3 ng of specific IgA:μg total IgA; P = 0.073) was calculated (Fig. 4).
FIG. 4.
Salivary IgA antibody responses elicited by meningococcal carriage. Saliva samples were collected from 37 students at two time points during the study and stabilized against proteolytic damage. The samples were tested by indirect ELISA for total and H44/76 strain-specific IgA. Shaded bars represent geometric mean ratios of specific to total immunoglobulin; error bars show 95% CIs.
No significant differences in the relative concentrations of IgG were detected in the saliva samples during the study. An increase in the median ratio of specific to total IgG was noted, from 8.18 ng/μg to 18.52 ng/μg (geometric means, 8.79 and 18.37 ng/μg, respectively), but the variation among the group was extremely wide (95% confidence interval [CI], 44.69 and 53.35 ng/μg).
Antigen-specific proliferative responses of PBMCs.
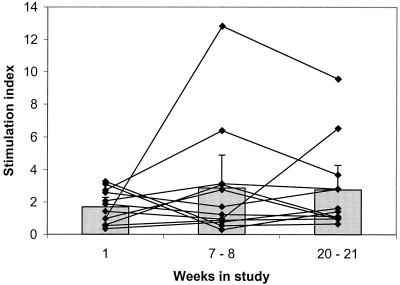
PBMCs from 15 students who had provided blood samples at all of the time points of the study were tested for proliferative responses to a range of concentrations from 1 to 50 μg of protein per ml from a whole-cell lysate of the H44/76 meningococcal strain. Eight of these volunteers were confirmed as carriers by pharyngeal swabbing, but all had significantly elevated serum IgG responses over the course of the study, indicating that carriage had probably occurred. A stimulation index (SI) value of greater than 2.0 was taken to indicate that proliferation had occurred (35).
Extremely high SI values were obtained following mitogenic phytohemagglutinin stimulation of all of the samples of PBMCs, indicating highly proliferative responses. The median SIs of cells taken at weeks 1, 7 to 8, and 20 to 21 were 768.8, 417.4, and 208.6, respectively. There were no significant differences with time. The median SI values resulting from stimulating PBMCs from the three time points with PPD were 2.76, 3.31, and 2.40, indicating proliferative responses.
Following stimulation with meningococcal whole-cell lysate, however, the peak in the proliferative response to an optimal stimulatory concentration varied from individual to individual (Fig. 5). No significant trends could be detected among the group, and sets of cells from some of the individuals responded with higher SI values than others. Indeed, PBMCs from three of the individuals did not appear to respond to stimulation with the H44/76 lysate, since their SI values did not exceed 2.0 at any of the three time points.
FIG. 5.
Proliferative responses of PBMCs to optimum stimulatory concentrations of a heat-killed whole cell lysate of the H44/76 meningococcal strain. PBMCs were incubated with 1 to 50 μg of the H44/76 strain lysate per ml for 7 days, and [3H]thymidine was included for the final 18 h. SIs were calculated as the ratio of mean counts per minute from antigen-stimulated cells to mean counts per minute from unstimulated cells. The points and lines show the SI values obtained from the PBMCs collected from 15 students at three time points during the study. Shaded bars represent the geometric mean SIs for the group; error bars depict the 95% CIs.
Characterization of cellular immune responses by using flow cytometry.
The 15 sets of PBMCs tested in proliferation assays (see above) were incubated with a lysate of the H44/76 meningococcal strain in the presence of anti-CD28 monoclonal antibody for an optimal 10 h. Brefeldin A was added after the first 2 h in order to block cytokine secretion. The cells were stained for surface markers and then fixed and permeabilized in order to detect the intracellular cytokines IFN-γ and IL-5 and also CD69.
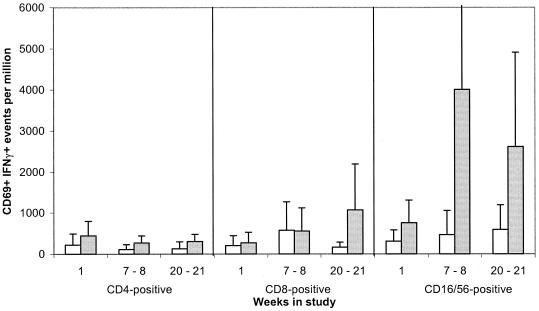
In comparison with unstimulated controls, a significant increase in the percentage of CD69-positive events was detected in cultures stimulated with PMA and ionomycin (>threefold; P = 0.000 at all time points) and also in those stimulated with the meningococcal lysate (twofold; P < 0.005 at all time points). Following meningococcal stimulation, the majority of IFN-γ+ CD69+ events proved to be CD16 CD56+ NK cells (Fig. 6). Indeed, a greater-than-fourfold-higher median frequency of CD69+ IFN-γ+ NK cells compared with CD4+ events was detected in cultures from all three of the time points (week 1, P = 0.06; weeks 7 to 8, P = 0.001; weeks 20 to 21, P = 0.013).
FIG. 6.
Frequencies of activated IFN-γ-producing CD4, CD8, and NK cells following incubation of PBMCs with a meningococcal whole-cell lysate. PBMCs collected at three time points from 15 students were incubated for 10 h in the presence of a heat-killed lysate (20 μg/ml) from the H44/76 strain (░⃞) or medium only (□). The cells were stained for CD4, CD8, and CD16/56 prior to fixation, permeabilization, intracellular staining for CD69 and IFN-γ, and flow cytometry. Error bars depict 95% CIs.
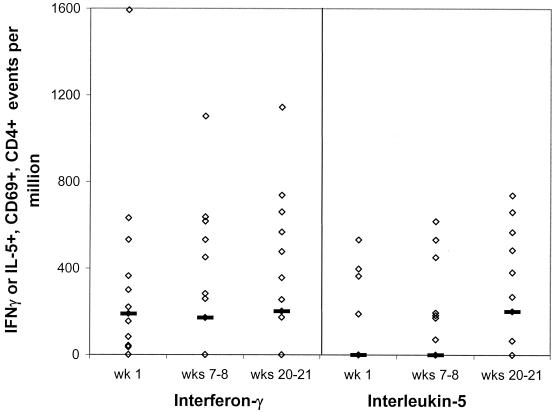
The median frequency of CD69+ CD4+ IFN-γ+ events following PMA stimulation was greater than 1.2 × 104 per million cells at each time point, whereas that of CD69+ CD4+ IL-5+ events was greater than 3,100, demonstrating that the cells produced a cytokine response to mitogenic stimulation. No significant trends in either CD4+ IFN-γ+ or CD4+ IL-5+ events were detected among the CD69+ population following meningococcal stimulation of PBMCs throughout the time course (Fig. 7). Additionally, there was no clear bias in the numbers of events positive for IFN-γ or IL-5. The ratios of mean IFN-γ+ to IL-5+ events among CD69+ CD4+ cells in cultures from weeks 1, 7 to 8, and 20 to 21 were 1.02, 1.83, and 0.81, respectively, and a positive correlation of IFN-γ and IL-5 events was detected at all of the time points (rs = 0.740, P < 0.005; rs = 0.898, P < 0.005; and rs = 0.946, P < 0.005).
FIG. 7.
Frequencies of activated CD4 cells staining positive for IFN-γ or IL-5 following incubation of PBMCs with a meningococcal whole-cell lysate. The CD4 cytokine responses of PBMCs from 15 students were detected by intracellular staining and flow cytometry following 10 h of incubation with a heat-killed whole-cell lysate of the H44/76 strain. Points represent the individual frequencies of cytokine-positive CD69+ CD4+ events. Lines depict the median frequency for the group. Stimulating the PBMCs with PMA and ionomycin resulted in >11,000 IFN-γ+ and >3,100 IL-5+ CD69+ CD4+ events per 106 cells at each of the time points. Unstimulated PBMCs from weeks 1, 7 to 8, and 20 to 21 contained median frequencies of 95, 92, and 81 IFN-γ+ CD4+ CD69+ events and 43, 0, and 56 IL-5+ CD4+ CD69+ events per 106 cells, respectively.
A representative number of culture supernatants (from six samples of PBMCs) were assayed for the presence of TGF-β1. Median TGF-β1 concentrations in meningococcus-stimulated PBMCs from weeks 1, 7 to 8, and 20 to 21 were 56.4, 69.8, and 61.7 pg/ml, respectively. A significant negative correlation (P = 0.05) between TGF-β1 concentrations and cellular proliferation was detected (weeks 7 to 8, rs = −0.80; weeks 20 to 21, rs = −0.90).
DISCUSSION
Interest in immunity to meningococcal disease has grown over the last 10 years (19). It is now recognized that rates of meningococcal carriage and invasive disease are higher among university undergraduates (30) than among others of the same age, and outbreaks of meningococcal disease have been reported at United Kingdom universities (12). Several groups have investigated meningococcal carriage, but studies of the immune responses elicited have been limited to measurement of serum antibodies (22, 25, 34). The present study was unique since it aimed to address both mucosal and systemic antibody responses and, more importantly, to investigate the cellular immune response elicited by carriage.
The study population of undergraduate students indeed proved to be subject to increasing rates of carriage, similar to those found in a previous and much larger study (30). On the day of arrival at the university, fewer than 10% of the pharyngeal swabs proved to be positive for N. meningitidis, but a dramatic increase to greater than 20% was detected within 7 days. The elevated rate of carriage continued throughout the 21 weeks of the study and confirmed earlier findings, which also showed that the majority of recovered isolates were nonserogroupable (4, 30). Despite the fact that only three highly experienced personnel collected the pharyngeal swabs, the rates of recovery of meningococcal isolates may be underestimates. It has been demonstrated that meningococci reside not only on the mucosal surface but also beneath it and therefore would be inaccessible to conventional swabs (39). The data presented here do not attempt to define individuals as carriers and noncarriers, since we collected swabs on only a few occasions over the 21-week period and had no way to continuously determine carriage status, especially if carriage was of a short duration between swabbing points. The collection of pharyngeal swabs demonstrated that our selected students were representative of the dynamic patterns of transmission of meningococci in university halls of residence.
It has been known for many years that immunity to meningococcal disease is mediated by serum antibodies with bactericidal activity (17) and that asymptomatic carriage stimulates such responses (13, 34). Over the period of the present study, as the subjects were exposed to high rates of carriage, it was possible to reproduce these early findings, detecting a trend of increasing concentrations and bactericidal activity of serum IgG. The data showed a greater specificity of serological responses for the homologous colonizing strains, as found previously by Jones et al. (22), who demonstrated that the class 1 protein (PorA) of homologous strains elicited this enhancement in antibody specificity. Measurement of strain specificity in such antibody responses, however, may be complicated by “original antigenic sin.” This phenomenon has been reported to occur when repeated infections with serologically different bacteria elicit the recall of antibodies with specificity for previously encountered similar strains (5).
An important finding of the present study was that carriage also elicits a mucosal immune response, as detected by the presence of increasing concentrations of specific (compared with total) IgA in saliva. This increase was not significant (P = 0.07); however, during a similar study of immunity and meningococcal carriage during the 2000 to 2001 academic year, an increase of more than twofold in IgA specific for the H44/76 meningococcal strain (P < 0.05) was detected in saliva samples from week 1 to week 8 of the term (data not shown). During these experiments the mucosal origin of the IgA was not confirmed with assays for secretory component, but the absence of any change in serum IgA levels over the study period strongly indicates that the IgA detected in saliva was not derived from serum. Human salivary IgA responses with specificity for bacteria, such as oral commensal streptococci, have been described by many investigators (2, 9, 11). Studies of antimeningococcal mucosal antibodies, however, have been concerned only with assessment of vaccine-induced immunity, where it was reported that parenteral vaccination with A/C polysaccharide (31) or conjugate vaccines (7, 50) elicits high serum IgG titers but also salivary IgA antibodies. Mucosal antibody responses appear to be important, since individuals who are nonsecretors of ABO blood group antigens are more prone to meningococcal colonization and this is said to be due to a deficiency of immunoglobulins in secretions (51). The role of this mucosal response in protection has yet to be determined, but it is likely to be involved in limiting colonization of the nasopharynx and perhaps in an inhibition of invasion of submucosal tissues. Carriage of meningococci could therefore elicit a twofold mechanism of humoral immunity. IgA at the mucosal surface could provide a barrier at the portal of entry to the body (41), while serum IgG efficiently opsonizes invading organisms, targeting them for phagocytosis and complement-mediated lysis (32).
The in vitro proliferative responses of PBMCs to the meningococcal lysate were surprisingly low; indeed, cells from three of the students failed to yield an SI of greater than 2.0 at any of the time points. Pollard et al. (33) described that high levels of IL-10 were secreted by PBMCs, collected from convalescent patients and healthy adults, in response to stimulation with meningococcal outer membrane vesicles. IL-10 produced by monocytes during the incubation of PBMCs with LPS or Escherichia coli cells has been reported to result in the inhibition of cellular proliferation (18). It seems likely that such a mechanism could have inhibited the meningococcus-specific proliferation assays. Although the secretion of proinflammatory cytokines by monocytes in responses to meningococci has been described (46), there are presently no data on suppressive factors such as IL-10. It is intended that IL-10 assays will be incorporated into future experiments.
The measurement of cellular immune responses to complex antigenic structures such as bacteria remains difficult. When PBMCs are stimulated in vitro with bacterial antigens, both innate and adaptive immune mechanisms may be activated. It may not be appropriate to separate these pathways in culture, since in vivo an immune response encompasses a network of effector cells, and adaptive immune responses are influenced by the local cytokine environment, which may be provided by innate mechanisms (36).
Our experiments were designed to measure the T-cell response to meningococci and employed detection of CD69 expression, an early activation marker (44), as a tool for differential detection of memory cells and cells of the innate response activated by meningococcal stimulation. CD16/56+ NK cells were the predominant CD69+ IFN-γ-producing cells following stimulation of PBMCs with a meningococcal whole-cell lysate. Lower frequencies of CD8+ and CD4+ IFN-γ+ events were also detected. These data were consistent with the findings of Haller et al. (16), who demonstrated elevated CD69 expression and IFN-γ secretion by NK cells in cultures of human PBMCs with E. coli or purified LPS, although NK cells may produce may other cytokines. Lertmemongkolchai et al. (26) recently showed that murine NK cells produced IFN-γ within 5 h of incubation with the gram-negative bacterium Burkholderia pseudomallei. The activation of Toll-like receptors by bacterial components is known to elicit the secretion of proinflammatory cytokines such as tumor necrosis factor alpha, IL-12, and IL-18 from monocytes and macrophages in culture (8). Lertmemongkolchai et al. (26) postulated that IL-12 and IL-18, which are known to be capable of inducing IFN-γ production independently of T-cell receptor signaling (49), could quickly elicit IFN-γ from NK cells, which constitutively express IL-12 and IL-18 receptors. They also demonstrated the bystander activation of memory CD8+ T cells, which express increased levels of IL-18 receptor compared with CD4+ Th1 cells. Th2 cells, however, cannot be induced to express this receptor (3). The detected CD69+ CD4 cell response of PBMCs stimulated with meningococci was considered to represent the memory response, although it is possible that the cytokine profiles were influenced by innate responses in vitro.
Cellular immunity to meningococcal infection has been poorly studied, and previous workers have described Th2 subset responses by comparison of IL-10 and IFN-γ concentrations in antigen-stimulated PBMC culture supernatants (33). Clearly, this method could not determine the cellular source of cytokines, especially since factors such as IL-10 are now known to be produced by a variety of human cell types, including Th0 cells, Th1 cells, Th2 cells, CD8-positive cells, B-cells, monocytes, and macrophages (29). In the present study, therefore, the powerful technique of intracellular cytokine staining for flow cytometry (28) was used to measure cytokine expression among circulating memory CD4 clones, which may have been polarized Th0, Th1, or Th2 cells (42). IFN-γ and IL-5 were chosen as representative Th1- and Th2-associated factors, respectively. IL-4 is usually described as the most important Th2-defining cytokine, but in our hands IL-4-positive cells yielded low-intensity fluorescence, making fluorescence-activated cell sorter analysis problematic.
The assays of PBMCs from meningococcal carriers revealed no bias in the frequencies of activated IFN-γ- or IL-5-positive CD4 events after 10 h of culture, and indeed a positive correlation of these responses was detected. Such cytokine-positive events were a mixture of single-positive IFN-γ+ and IL-5+ cells and double-positive IFN-γ+ IL-5+ cells. This indicated a mixed Th1, Th2, and Th0 response, which is elicited under circumstances where there is no clear Th1-Th2 polarizing signal (36). The data differ from those of Simpson et al. (40), who examined a different feature of the recall cytokine response but used similar methodology. After 7 days of culture, human T-cell responses to porins from Neisseria gonorrhoeae were dominated by Th2 and T-cytotoxic 2 (Tc2) subsets. The incubation time for these assays was much longer (at 7 days), and since the expression of certain cytokines is known to occur in a time-dependent manner (38), this may account for the differences.
The majority of the sets of meningococcus-stimulated PBMC cultures exhibited increasing T-cell responses of an unbiased Th phenotype over the course of the study. A proportion of samples, however, responded with a marked reduction in IFN-γ- and IL-5-positive CD4 events from week 7, despite maintaining levels of CD69+ CD4 cells. Lowered cellular proliferation was also detected among these samples, which correlated with an increase in TGF-β in culture supernatants. It is known that meningococci induce proinflammatory cytokines when in contact with epithelium, endothelium, and leukocytes (27, 43, 48); therefore, the asymptomatic nature of carriage becomes a paradox. A possible parallel may be drawn with mucosal tolerance to commensal bacteria of the intestine, which are also capable of eliciting proinflammatory cytokines in vitro (41). Responses to such commensals are mediated by Th3 (or Tr1) cells, which are poorly proliferative, primarily secrete the suppressive cytokines TGF-β and IL-10, but can secrete low levels of Th1 and Th2 cytokines (14, 47) and may be detected in the peripheral circulation as well as in mucosa-associated lymphoid tissues (10). It is possible, therefore, that the poorly proliferative cellular responses of some individuals were of the Th3 subset or that elevated levels of non-CD4 cell-derived suppressive cytokines such as TGF-β and IL-10 inhibited proliferation and cytokine production. Further experiments will be required to address this question, and future studies will include analyses of the response to purified meningococcal antigens.
When analyzing the immune responses of human subjects, considerable variation between the genetically diverse individuals gives rise to difficulties in determining significant trends. Despite these problems, the results of the study have shown for the first time that meningococcal carriage induces both systemic and mucosal antibody responses. Using intracellular staining and flow cytometry, it was possible to study the expression of cytokines among activated CD4 cells to determine that an unbiased Th subset response was elicited. A far higher frequency of IFN-γ-producing activated NK cells than of CD4 cells was detected in meningococcus-stimulated PBMC cultures. These results emphasized that merely quantifying IFN-γ and IL-5 in culture supernatants could have given misleading indications of the T-helper subset response and demonstrate the importance of determining the cellular origin of such factors.
Acknowledgments
K.R. was supported by the Meningitis Research Foundation. Partial funding for this study was obtained from the League of Friends at Jersey General Hospital.
We express our gratitude to the students who provided the biological samples tested in this study, to K. Ait-Tahar for his time and assistance in processing the blood samples, and to A. Galvin for much help and advice with flow cytometry.
REFERENCES
- 1.Abdel-Hadi, H., K. G. Wooldridge, K. Robinson, and D. A. A. Ala'Aldeen. 2001. Identification and characterisation of App: an immunogenic autotransporter protein of Neisseria meningitidis. Mol. Microbiol. 41:611-623. [DOI] [PubMed]
- 2.Ahl, T., and J. Reinholdt. 1991. Subclass distribution of salivary secretory immunoglobulin A antibodies to oral streptococci. Infect. Immun. 59:3619-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira, S. 2000. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 12:59-63. [DOI] [PubMed] [Google Scholar]
- 4.Ala'Aldeen, D. A. A., K. R. Neal, K. Ait-Tahar, J. S. Nguyen-Van-Tam, A. English, T. J. Falla, P. M. Hawkey, and R. C. B. Slack. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J. Clin. Microbiol. 38:2311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, J. D., R. W. Peeling, and R. C. Brunham. 1999. Analysis of the original antigenic sin antibody responses to the major outer membrane protein of Chlamydia trachomatis. J. Infect. Dis. 179:180-186. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, R., A. J. Fox, K. Cartwright, N. T. Begg, and D. M. Jones. 1999. Salivary antibodies following parenteral immunisation of infants with a meningococcal serogroup A and C conjugated vaccine. Epidemiol. Infect. 123:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brightbill, H. D., and R. L. Modlin. 2000. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology 101:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, T. A., and J. Mestecky. 1985. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect. Immun. 49:459-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, W., W. Jin, M. Cook, H. L. Weiner, and S. M. Wahl. 1998. Oral delivery of group A streptococcal cell walls augments circulating TGFβ and suppresses streptococcal arthritis. J. Immunol. 161:6297-6304. [PubMed] [Google Scholar]
- 11.Cole, M. F., S. Bryan, M. K. Evans, C. L. Pearce, M. J. Sheridan, P. A. Sura, R. L. Wientzen, and G. H. Bowden. 1999. Humoral immunity to commensal oral bacteria in human infants: salivary secretory immunoglobulin A antibodies reactive with Streptococcus mitis biovar 1, Streptococcus oralis, Streptococcus mutans, and Enterococcus faecalis during the first two years of life. Infect. Immun. 67:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore, A., G. Jones, M. Barker, N. Soltanpoor, and J. M. Stuart. 1999. Meningococcal disease at the University of Southampton: outbreak investigation. Epidemiol. Infect. 123:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groux, H., and F. Powrie. 1999. Regulatory T cells and inflammatory bowel disease. Immunol. Today 20:442-446. [DOI] [PubMed] [Google Scholar]
- 15.Guttormsen, H. K., L. M. Wetzler, and C. O. Solberg. 1994. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect. Immun. 62:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller, D., S. Blum, C. Bode, W. P. Hammes, and E. J. Schiffrin. 2000. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect. Immun. 68:752-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heist, G. D., S. Solis-Cohen, and M. Solis-Cohen. 1922. A study of the virulence of meningococci for man and of human susceptibility to meningococcal infection. J. Immunol. 7:1-33.
- 18.Hessle, C., L. A. Hanson, and A. E. Wold. 2000. Interleukin-10 production by the innate immune system masks in vitro evidence of acquired T-cell immunity to E. coli. Scand. J. Immunol. 52:13-20. [DOI] [PubMed] [Google Scholar]
- 19.Hubert, B., and D. A. Caugant. 1997. Recent changes in meningococcal disease in Europe. Eur. Surveillance 2:69-71. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis, G. A. 1995. Recognition and control of neisserial infection by antibody and complement. Trends Microbiol. 3:198-201. [DOI] [PubMed] [Google Scholar]
- 21.Jones, D. M., and J. Eldridge. 1978. Development of antibodies to meningococcal protein and lipopolysaccharide serotype antigens in healthy carriers. J. Med. Microbiol. 12:107-111. [DOI] [PubMed] [Google Scholar]
- 22.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. O. Miller, K. A. V. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 23.Kizil, G., I. Todd, M. Atta, S. P. Borriello, K. Ait-Tahar, and D. A. A. Ala'Aldeen. 1999. Identification and characterization of TspA, a major CD4+-T-cell and B-cell-stimulating Neisseria-specific antigen. Infect. Immun. 67:3533-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kornelisse, R. F., C. E. Hack, H. F. J. Savelkoup, T.C. van der Pouw Kraan, W. Hop, G. van Mierlo, M. H. Suur, H. J. Neijens, and R. de Groot. 1997. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect. Immun. 65:877-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristiansen, B.-E., K. W. Lind, K. Mevold, B. Sorensen, L. O. Froholm, K. Bryn, T. Tjade, and K. Bovre. 1998. Meningococcal phenotypic and genotypic characteristics and human antibody levels. J. Clin. Microbiol. 26:1988-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFNγ in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzen, D. R., F. Dux, U. Wolk, A. Tsirpouchtsidis, G. Haas, and T. F. Meyer. 1999. Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines. J. Exp. Med. 190:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maino, V. C., and L. J. Picker. 1998. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34:207-215. [DOI] [PubMed] [Google Scholar]
- 29.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 30.Neal, K. R., J. S. Nguyen-Van-Tam, N. Jeffrey, R. C. B. Slack, R. J. Madeley, K. Ait-Tahar, K. Job, M. C. J. Wale, and D. A. A. Ala'Aldeen. 2000. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. Br. Med. J. 320:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurkka, A., J. MacLennan, V. Jäntti, S. Obaro, B. Greenwood, and H. Käythy. 2001. Salivary antibody response to vaccination with meningococcal A/C polysaccharide vaccine in previously unvaccinated Gambian children. Vaccine 19:547-556. [DOI] [PubMed] [Google Scholar]
- 32.Pollard, A. J., and C. Frasch. 2001. Development of natural immunity to Neisseria meningitidis. Vaccine 19:1327-1346. [DOI] [PubMed] [Google Scholar]
- 33.Pollard, A. J., R. Galassini, E. M. Rouppe van der Voort, M. Hibberd, R. Booy, P. Langford, S. Nadel, C. Ison, J. S. Kroll, J. Poolman, and M. Levin. 1999. Cellular immune responses to Neisseria meningitidis in children. Infect. Immun. 67:2452-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reller, L. B., R. R. MacGregor, and H. N. Beaty. 1973. Bactericidal antibody after colonization with Neisseria meningitidis. J. Infect. Dis. 127:56-62. [DOI] [PubMed] [Google Scholar]
- 35.Robinson, K., T. Bellaby, and D. Wakelin. 1994. Vaccination against the nematode Trichinella spiralis in high- and low-responder mice. Effects of different adjuvants upon protective immunity and immune responsiveness. Immunology 82:261-267. [PMC free article] [PubMed] [Google Scholar]
- 36.Romagnani, S. 2000. Cytokines and the Th1/Th2 paradigm, p. 71-102. In F. Balkwill (ed.), The cytokine network. Oxford University Press, Oxford, United Kingdom.
- 37.Rosenqvist, E., E. A. Hoiby, E. Wedege, B. Kusecek, and M. Achtman. 1993. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J. Infect. Dis. 167:1065-1073. [DOI] [PubMed] [Google Scholar]
- 38.Rostaing, L., J. Tkaczuk, M. Durand, C. Peres, D. Durand, C. de Préval, E. Ohayon, and M. Abbal. 1999. Kinetics of intracytoplasmic Th1 and Th2 cytokine production assessed by flow cytometry following in vitro activation of peripheral blood mononuclear cells. Cytometry 35:318-328. [DOI] [PubMed] [Google Scholar]
- 39.Sim, R. J., M. M. Harrison, E. R. Moxon, and C. M. Tang. 2000. Underestimation of meningococci in tonsillar tissue by nasopharyngeal swabbing. Lancet 356:1653-1654. [DOI] [PubMed] [Google Scholar]
- 40.Simpson, S. D., Y. Ho, P. A. Rice, and L. M. Wetzler. 1999. T lymphocyte response to Neisseria gonorrhoea porin in individuals with mucosal gonococcal infections. J. Infect. Dis. 180:762-773. [DOI] [PubMed] [Google Scholar]
- 41.Svanborg, C. 1994. Bacterial adherence and mucosal immunity, p. 71-78. In P. L. Ogra, W. Strober, J. Mestecky, J. R. McGhee, M. E. Lamm, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press Inc., San Diego, Calif.
- 42.Swain, S. L. 1994. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity 1:543-552. [DOI] [PubMed] [Google Scholar]
- 43.Taha, M-K. 1999. Neisseria meningitidis induces the expression of the TNFα gene in endothelial cells. Cytokine 12:21-25. [DOI] [PubMed] [Google Scholar]
- 44.Testi, R., D. D'Ambrosio, R. De Maria, and A. Santoni. 1994. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol. Today 15:479-483. [DOI] [PubMed] [Google Scholar]
- 45.Troncoso, G., S. Sanchez, M. Moreda, M. T. Criado, and C. M. Ferreiros. 2000. Antigenic cross-reactivity between outer membrane proteins of Neisseria meningitidis and commensal Neisseria species. FEMS Immunol. Med. Microbiol. 27:103-109. [DOI] [PubMed] [Google Scholar]
- 46.Uronen, H., A. J. Williams, G. Dixon, S. R. Andersen, P. van der Ley, and M. van Deuren. 2000. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 122:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiner, H. L. 1997. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol. Today 18:335-343. [DOI] [PubMed] [Google Scholar]
- 48.Wells, D. B., P. J. Tighe, K. G. Wooldridge, K. Robinson, and D. A. A. Ala'Aldeen. 2001. Differential gene expression during meningeal-meningococcal interaction: evidence for self-defense and early release of cytokines and chemokines. Infect. Immun. 69:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, J., H. Zhu, T. L. Murphy, W. Ouyang, and K. M. Murphy. 2001. IL-18-stimulated GADD45β required in cytokine-induced, but not TCR-induced, IFNγ production. Nat. Immunol. 2:157-164. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, Q., S. Choo, J. Everard, R. Jennings, and A. Finn. 2000. Mucosal immune responses to meningococcal group C conjugate and group A and C polysaccharide vaccines in adolescents. Infect. Immun. 68:2692-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zorgani, A. A., J. Stewart, C. C. Blackwell, R. A., Elton, and D. M. Weir. 1994. Inhibitory effect of saliva from secretors and non-secretors on binding of meningococci to epithelial cells. FEMS Immunol. Med. Microbiol. 9:135-142. [DOI] [PubMed] [Google Scholar]