Abstract
The effect of Mycobacterium bovis BCG vaccination on interleukin-1β (IL-1β) or regulated-upon-activation, normally T-cell-expressed and -secreted chemokine (RANTES) mRNA expression in guinea pig spleen cells stimulated with concanavalin A, lipopolysaccharide (LPS), phorbol myristate acetate (PMA) plus ionomycin, or purified protein derivative (PPD) was studied in vitro. Similarly, peritoneal exudate cell-derived macrophages from naïve and BCG-vaccinated guinea pigs were infected with M. bovis BCG, Mycobacterium avium, the attenuated Mycobacterium tuberculosis H37Ra strain, or virulent strains H37Rv and Erdman of M. tuberculosis. Total RNA was subjected to Northern blot analysis using probes generated from guinea pig IL-1β or RANTES cDNA. Although IL-1β and RANTES mRNA could be detected in the spleen cells from naïve animals stimulated with LPS or PMA plus ionomycin, the levels were significantly enhanced after BCG vaccination. mRNA expression was also elevated in macrophages infected with live mycobacteria after BCG vaccination. However, macrophages infected with the virulent H37Rv strain of M. tuberculosis showed 75 to 90% reductions in IL-1β expression and 25 to 60% reductions in RANTES mRNA expression compared with macrophages infected with the attenuated H37Ra strain. The IL-1β mRNA levels peaked as soon as 1 h after PPD stimulation and 4 h after M. tuberculosis H37Rv infection of macrophages. In contrast, RANTES mRNA expression was delayed until 48 h after infection. These results indicate that molecular mediators produced in response to various stimuli associated with protective immunity against mycobacteria are upregulated after BCG vaccination; however, a significantly weaker response was observed with virulent M. tuberculosis. These initial studies indicate that BCG vaccination has a positive effect on IL-1β and RANTES mRNA expression by host cells in a highly relevant animal tuberculosis model.
Tuberculosis remains a major public health problem in many parts of the world. It is estimated that one-third of the world's population is persistently infected with Mycobacterium tuberculosis (20). The emergence of drug-resistant bacteria and the increased prevalence of mycobacterial infections in AIDS patients have only magnified this problem (47). The only vaccine currently available for tuberculosis is Mycobacterium bovis BCG. Although BCG vaccine is widely used throughout the developing world, the efficacy of this vaccine is affected by unknown environmental and host factors in certain high-risk populations (9). It is known that resistance to tuberculosis requires the development of an effective cell-mediated immune response involving cooperation between the antigen-specific T cells and macrophages (26). Several cytokines and chemokines have been identified as critical factors in the interaction between sensitized lymphocytes and infected macrophages during the initial stages of infection in a previously vaccinated host (1). Among the many cytokines, gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) appear to be important in antimycobacterial defense, while other cytokines, such as interleukin-10 (IL-10) or transforming growth factor β (TGF-β), have been shown to downregulate immune responses to mycobacteria (12).
IL-1β has been shown to play an important role in host resistance to mycobacteria (48). Both M. tuberculosis and its cell wall components are known to induce IL-1β expression in human monocytes and macrophages (36). In addition, increased gene expression of IL-1β was observed in bronchoalveolar lavage (BAL) cells from tuberculosis patients when they were compared with cells from healthy individuals (25). The levels of certain proinflammatory cytokines, including IL-1β, produced after mycobacterial stimulation have been reported to depend on the virulence of the organism (25). In vitro studies have indicated that virulent organisms induce lower levels of IL-1β, thereby escaping host defenses by limiting the production of proinflammatory cytokines that contribute to the development of resistance (33).
Regulated-upon-activation, normally T-cell-expressed and -secreted chemokine (RANTES), a CC chemokine, is produced by a variety of cell types, including T cells and macrophages. RANTES induces migration of T cells with the memory phenotype, as well as monocytes and macrophages, the two principal cell types seen in granulomas (3). Expression of RANTES has been detected predominantly in the macrophages and endothelial cells in the granulomatous lesions of both sarcoidosis and tuberculosis patients (13). In vitro stimulation of human umbilical venous endothelial cells with IFN-γ and TNF-α, two cytokines that are found at high levels in granulomas, induced the production of RANTES protein (27). Granulomas elicited by purified protein derivative (PPD)-coated beads in IFN-γ knockout mice had decreased levels of TNF-α and RANTES mRNA, suggesting that reduced expression of RANTES is associated with a loss of protective cytokines (6). Recent findings indicated that RANTES mRNA was expressed in the spleen and lymph node cells of nonimmunized and BCG-vaccinated guinea pigs (21).
The mechanism regulating the immune responses against mycobacterial infections in mice has been extensively studied (10, 15). However, a large inoculum has usually been employed to establish the infection in the mouse model. Moreover, there are differences in disease pathogenesis between mice and humans. For several years, we have been using a well-established guinea pig model of low-dose pulmonary tuberculosis (31). We have characterized the course of the progressive disease caused by the virulent strain M. tuberculosis H37Rv and have begun to elucidate the mechanisms by which BCG vaccination protects guinea pigs. We have also studied the self-resolving disease induced by infection with the attenuated H37Ra strain (28). Recently, the availability of guinea pig chemokine and cytokine cDNA clones (3, 46) allowed us to undertake novel studies with this model to examine the immunobiology of vaccine-induced resistance.
In the present study, we determined whether BCG vaccination influenced the expression of IL-1β and RANTES mRNA in cultured guinea pig cells stimulated in vitro with various agonists or live mycobacteria with probes derived from guinea pig cDNA clones. Our results indicate that expression of IL-1β and RANTES mRNA increases after BCG vaccination. Furthermore, infection of macrophages with the virulent strain of M. tuberculosis, but not infection of macrophages with an attenuated strain, resulted in reduced expression of these molecular mediators of a protective immune response against mycobacteria.
MATERIALS AND METHODS
Animals.
Outbred Hartley strain guinea pigs (Charles River Breeding Laboratories, Inc., Wilmington, Mass.) weighing 200 to 300 g were used in this study. The animals were housed individually in polycarbonate cages in a temperature- and humidity-controlled environment with a cycle consisting of 12 h of darkness and 12 h of light. Animals were given commercial chow (Ralston Purina, St. Louis, Mo.) and tap water ad libitum. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee.
BCG vaccination.
Guinea pigs were vaccinated intradermally with 0.1 ml (103 viable units) of M. bovis BCG (Danish 1331 strain; Statens Seruminstitut, Copenhagen, Denmark) in the left and right inguinal regions. The lyophilized vaccine was reconstituted with physiological saline just prior to use.
Preparation of spleen cells.
At 4 to 6 weeks postvaccination, guinea pigs were anesthetized by intramuscular injection of ketamine hydrochloride (30 mg/kg of body weight; Ketaset; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) and xylazine (2.5 mg/kg; Rompun; Fort Dodge Laboratories, Inc.). The guinea pigs were then euthanized by cardiac injection of 100 mg of sodium barbital (Sleepaway; Fort Dodge Laboratories, Inc.) per kg. After peritoneal exudate cells (PEC) were collected as described below, the spleen was removed aseptically from each animal and homogenized in RPMI 1640 (Irvine Scientific, Santa Ana, Calif.). The medium was supplemented with 2 μM glutamine (Irvine Scientific), 0.01 mM 2-mercaptoethanol (Sigma, St. Louis, Mo.), 100 U of penicillin (Irvine Scientific) per ml, 100 μg of streptomycin (Irvine Scientific) per ml, and 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, Ga.). The erythrocytes in the spleen cell homogenates were lysed with ACK lysing buffer (0.14 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA [pH 7.2 to 7.4]). The cells were washed three times in RPMI medium, inoculated into 50-ml polypropylene tubes at a concentration of 2 × 106 cells/ml in a total volume of 5 to 10 ml, and incubated at 37°C in the presence of 5% CO2.
Stimulation of cells.
Whole spleen cells (2 × 107 cells) were cultured with or without concanavalin A (ConA) (10 μg/ml; 24 h; Sigma), lipopolysaccharide (LPS) (10 μg/ml; 1.5 h; Sigma), different concentrations of PPD (5 to 25 μg/ml; 24 h; Statens Seruminstitut), phorbol myristate acetate (PMA) (10 ng/ml; 24 h; Sigma) plus ionomycin (500 ng/ml; 24 h; Calbiochem, San Diego, Calif.), M. tuberculosis H37Ra (= ATCC 25177 [American Type Culture Collection, Rockville, Md.]) (multiplicity of infection [MOI] [ratio of number of bacteria to number of cells], 1:30, 1:10, or 3:10; 24 h), or M. tuberculosis H37Rv (= ATCC 27294) (MOI, 1:30, 1:10, or 3:10; 24 h). Each mycobacterial suspension was briefly sonicated to disrupt bacterial clumps with an Ultrasonics sonicator (Heat Systems-Ultrasonics, Inc., Plainview, N.Y.) for 45 to 60 s at an output control setting of 8.0, and the appropriate volume was added from a stock preparation containing 108 CFU per ml. At the end of the incubation period, the medium was removed by centrifugation, and total RNA was extracted from the cells with the Trizol reagent (Life Technologies, Grand Island, N.Y.). The untreated control cells were maintained in culture during the whole experiment and were processed in the same manner as the treated cells.
Harvesting of PEC.
The macrophages used in this study were derived from PEC harvested from the peritoneal cavity by using a modification of methods described previously (17). Briefly, BCG-vaccinated and nonvaccinated guinea pigs were injected intraperitoneally with 5 ml of 2% sodium thioglycolate (Becton Dickinson, Cockeysville, Md.). Four days later, the guinea pigs were euthanized as described above, and the peritoneal cavity of each animal was flushed three times with 20 ml of RPMI medium containing 20 U of heparin. The cells were washed in RPMI medium two or three times, counted, and suspended at a concentration of 5 × 106 cells/ml in RPMI medium containing 2-mercaptoethanol, glutamine, antibiotics, and 10% fetal bovine serum. A total of 2 × 107 cells were allowed to adhere for 24 h to 100-mm-diameter plastic petri dishes (Becton Dickinson Labware, Franklin Lakes, N.J.) which had been precoated with inactivated guinea pig serum (Harlan Bioproducts for Science, Indianapolis, Ind.) for 30 min, and the petri dishes were incubated for 24 h at 37°C in the presence of 5% CO2. The nonadherent cells were removed, and RPMI medium without antibiotics was added to each culture. The monolayers obtained in this way had predominantly macrophages (more than 95%) in their populations, as determined by nonspecific esterase staining (44).
Infection of macrophages.
The macrophages were infected at different MOIs (1:30, 1:10, and 3:10) with M. bovis BCG (Danish 1331 strain; Statens Seruminstitut), Mycobacterium avium ATCC 25291, or a strain of M. tuberculosis (H37Ra, H37Rv, or Erdman [= ATCC 35801]) for 4 to 48 h. The extracellular bacteria were removed at the end of the phagocytic period, Trizol reagent was added, and the adherent macrophages were scraped off each plate with cell scrapers (Costar Corporation, Cambridge, Mass.) and used for RNA extraction. The viabilities of cells after of 24 to 48 h of infection with the attenuated and virulent strains of M. tuberculosis were determined by a trypan blue exclusion assay or by the MTT assay (16). Using these two methods, we found that more than 95% of the adherent cells were viable after 24 and 48 h of infection with the bacteria.
RNA isolation and Northern analysis.
Total RNA was extracted by using the Trizol reagent as recommended by the manufacturer. An RNA pellet was suspended in sterile distilled water containing 0.1% diethyl pyrocarbonate (Sigma) and was stored at −80°C. For Northern analysis, 8 to 12 μg of denatured RNA from approximately 15 × 106 to 20 × 106 cells was separated electrophoretically on 1.2 to 1.5% agarose-formaldehyde gels. The separated RNA was then transferred onto nylon membranes by using a Turboblotter (Schleicher & Schuell, Keene, N.H.) and was UV cross-linked with a Stratalinker (120 mJ; Stratagene, La Jolla, Calif.). Each membrane was prehybridized as described previously (45). Briefly, the blot was washed in a solution containing 2× SSC and 0.5% sodium dodecyl sulfate (SDS) for 1 h (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Prehybridization was carried out in a solution containing 30% formamide, 5× SSC, 50 μg of sheared denatured salmon sperm DNA (Research Genetics, Inc., Huntsville, Ala.) per ml, 5× Denhardt's reagent (Sigma), and 0.5% SDS for 2 to 3 h at 37°C. IL-1β and RANTES cDNA clones were obtained by screening a guinea pig-specific cDNA library as previously described (3, 46). The cDNA were 32P labeled by random priming (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) using 25 ng of DNA according to the manufacturer's instructions. The unincorporated nucleotides after labeling were removed by using Sephadex G-50 columns (5 prime-3 prime, Inc., Boulder, Colo.). The membrane was hybridized overnight in a prehybridizing solution that contained guinea pig IL-1β (1.4-kb) or RANTES (0.6-kb) cDNA probes. The filters were washed twice (15 min each) in 2× SSC containing 0.5% SDS at room temperature and finally in 0.3× SSC-0.5% SDS for 30 min at 50°C. The blots were analyzed with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Following analysis, the blots were stripped by using boiling distilled water and reprobed with a second radiolabeled cDNA and finally with antisense 18S rRNA (Ambion, Austin, Tex.) as an internal standard to ensure equal RNA loading. The sums of counts above the background value were analyzed by using the Imagequant software (Molecular Dynamics). The percentage of basal (unstimulated naïve) RNA was calculated by using the following formula: [(unstimulated culture cytokine mRNA/18S rRNA)/(stimulated culture cytokine mRNA/18S rRNA)] × 100. Each experiment was repeated at least three times. The results shown below include a representative RNA blot and the results of a combined densitometric analysis of three or more independent experiments. The densitometric results for unstimulated naïve or unstimulated BCG-vaccinated cultures were set to 100% basal.
Statistics.
The densitometric data are expressed below as percentages (means ± standard errors). The significance of the differences between naïve and BCG-vaccinated groups was determined by analysis of variance (ANOVA). The differences within the naïve and BCG-vaccinated groups were examined by using Student's two-tailed t test and Dunnett's test, respectively.
RESULTS
Effects of BCG vaccination and stimuli on mRNA expression in splenocytes.
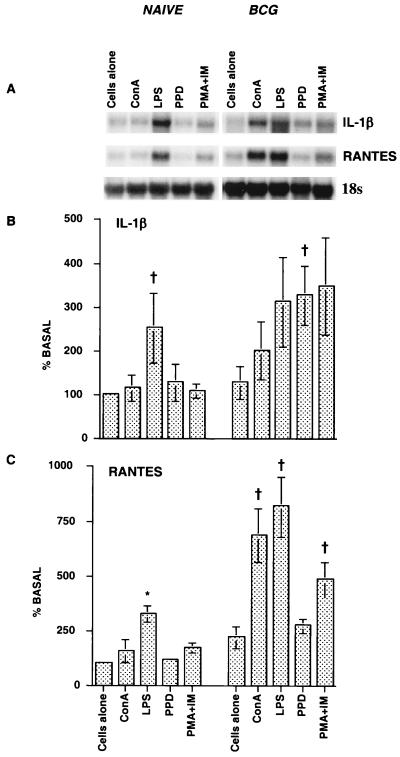
We first evaluated IL-1β and RANTES mRNA expression in spleen cells following stimulation with a variety of agonists. Whole spleen cells from naïve or BCG-vaccinated guinea pigs were stimulated with ConA, LPS, PMA plus ionomycin, or PPD for specific periods of time, and IL-1β and RANTES mRNA expression was examined by Northern blotting (Fig. 1). Low levels of IL-1β and RANTES mRNA were expressed in the spleen cells of naïve guinea pigs. Stimulation of cells with LPS resulted in significant increases in both IL-1β (P < 0.02) and RANTES (P < 0.001) mRNA expression, while stimulation with PPD affected only IL-1β expression. ConA and PMA plus ionomycin had only marginal effects on IL-1β and RANTES mRNA expression. Following BCG vaccination, stimulation with ConA, LPS, and PMA plus ionomycin resulted in significantly increased IL-1β and RANTES mRNA expression in the spleen cells. Although the IL-1β mRNA expression induced by PPD was similar to the IL-1β mRNA expression induced by the other agonists, it was significantly different from the IL-1β mRNA expression in the unstimulated group (P < 0.05), while the PPD-induced RANTES mRNA levels were lower than the RANTES mRNA levels induced by the other stimulants (P < 0.05). Overall, BCG vaccination resulted in significant increases in both IL-1β (P < 0.009) and RANTES (P < 0.0001) mRNA expression in spleen cells. There was a one- to threefold increase in IL-1β mRNA expression and a two- to fourfold increase in RANTES mRNA expression in spleen cells after stimulation with the nonspecific agonists or antigen-specific PPD compared to the expression in cells from naïve animals.
FIG. 1.
Effects of BCG vaccination and stimuli on IL-1β and RANTES mRNA expression in splenocytes. Whole spleen cells (2 × 107 cells) from naïve and BCG-vaccinated guinea pigs (4 to 6 weeks after vaccination) were stimulated in vitro with ConA or PMA plus ionomycin (IM) for 24 h or with LPS for 1.5 h. The total RNA extracted was subjected to Northern blot analysis, and the levels of expression were analyzed with a phosphorimager. (A) Representative RNA blot; (B) densitometric analysis of IL-1β; (C) densitometric analysis of RANTES. The densitometric results for unstimulated naïve cultures are set to 100% basal. Each bar represents the mean based on four experiments, and the error bars indicate standard errors. The differences between naïve and BCG-vaccinated groups were examined by ANOVA (P < 0.009). The differences within the naïve and BCG-vaccinated groups were analyzed by Student's t test or Dunnett's test. A dagger indicates that the P value was <0.02 to 0.05 for a comparison with the data obtained for unstimulated cells, and an asterisk indicates that the P value was <0.001 in comparison with the data obtained for unstimulated cells.
A PPD dose-response study indicated that 5 μg of PPD induced the maximum RANTES mRNA response; lower levels of RANTES mRNA were observed with the higher doses of PPD (10 to 25 μg/ml). However, at the higher doses the levels were still significantly higher (P < 0.05, as determined by Dunnett's test) than the levels in unstimulated cultures (data not shown).
Effect of BCG vaccination on mRNA expression after in vitro infection of macrophages with live mycobacteria.
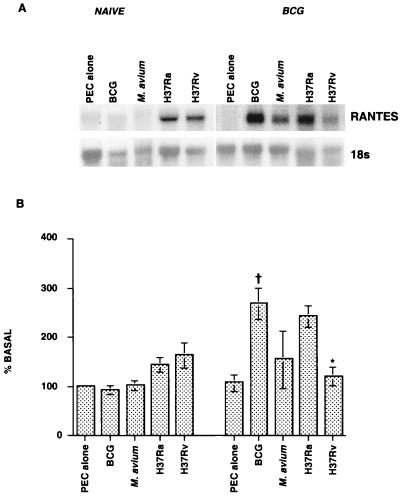
We examined whether the virulence of the organism affects expression of IL-1β and RANTES mRNA in macrophages derived from adherence-purified PEC. Cells from naïve and BCG-vaccinated animals were infected in vitro for 24 h with different strains of mycobacteria at MOIs ranging from 1:30 to 1:10. As shown in Fig. 2, uninfected macrophages from naïve and BCG-vaccinated animals expressed low levels of RANTES mRNA. After infection with M. tuberculosis H37Ra or H37Rv, there was an increase in RANTES mRNA expression in the macrophages from naïve animals, while BCG or M. avium infection had no apparent effect. In contrast, the levels of expression increased significantly (P < 0.01) when macrophages from BCG-vaccinated guinea pigs were infected with the different strains of mycobacteria (Fig. 2). The mRNA levels were higher in macrophages infected with the attenuated strains, BCG (P < 0.05) and M. tuberculosis H37Ra, than in macrophages infected within the more virulent organisms M. avium and M. tuberculosis H37Rv. However, the level of RANTES mRNA expression was significantly lower in macrophages infected with M. tuberculosis H37Rv than in macrophages infected with the attenuated H37Ra strain (P < 0.01), and this difference was not seen in the cells from naïve animals infected with the two strains. A similar trend was observed for IL-1β mRNA expression in macrophages from BCG-vaccinated guinea pigs infected with different strains of mycobacteria (data not shown).
FIG. 2.
Effect of BCG vaccination on RANTES mRNA expression in macrophages after in vitro infection with live mycobacteria. Macrophages (2 × 107 cells) from naïve and BCG-vaccinated guinea pigs were infected in vitro at MOIs ranging from 1:30 to 1:10 for 24 h with BCG, M. avium, or M. tuberculosis H37Ra or H37Rv. The RNA levels were analyzed as described in the text. (A) Representative RNA blot; (B) densitometric analysis of the results of three experiments. The differences between naïve and BCG-vaccinated groups were examined by ANOVA (P < 0.01), and the differences within groups were analyzed by Student's t test or by Dunnett's test. A dagger indicates that the P value was <0.05 for a comparison with the data obtained for unstimulated cultures, and an asterisk indicates that the P value was <0.01 in comparison with the data obtained with M. tuberculosis H37Ra.
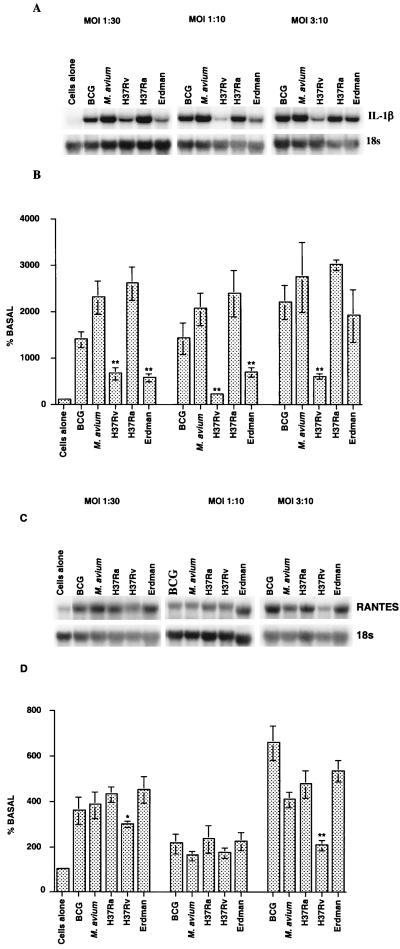
Effect of different MOI on IL-1β and RANTES mRNA expression.
In our preliminary studies, infection with virulent M. tuberculosis induced lower IL-1β and RANTES mRNA levels in macrophages than infection with the attenuated strain induced. However, the MOIs used in the experiment whose results are shown in Fig. 2 for different strains were not the same and ranged from 1:30 to 1:10. Therefore, macrophages from BCG-vaccinated guinea pigs were infected at three MOIs with various strains of mycobacteria. PEC cultures with bacterium/macrophage ratios of 1:30, 1:10, or 3:10 were established, and total RNA was harvested from these cultures 24 h after infection and used for Northern analysis. As shown in Fig. 3, the levels of IL-1β mRNA were significantly higher (P < 0.0001) in the cultures infected with BCG, M. avium, or M. tuberculosis H37Ra, H37Rv, or Erdman at all three MOIs (Fig. 3A and B) than in the uninfected macrophages. Infection with BCG, M. avium, or the attenuated H37Ra strain of M. tuberculosis induced the maximum response. However, IL-1β mRNA expression was significantly lower (P < 0.0001) in macrophages after infection with M. tuberculosis H37Rv or the Erdman strain at all three MOIs (except for infection with the Erdman strain at an MOI of 3:10) than after infection with the attenuated strain. Compared to the data obtained for the attenuated H37Ra strain, the percentages of reduction for the responses after infection with M. tuberculosis H37Rv were about 90, 75, and 80% at MOIs of 1:30, 1:10, and 3:10, respectively, and the percentages of reduction for the responses after infection with the Erdman strain were about 70, 80, and 35%, respectively. A dose-response relationship between the number of bacteria and IL-1β mRNA expression was not evident at the MOIs tested in these experiments.
FIG. 3.
Effects of different MOIs with various mycobacterial strains on IL-1β and RANTES mRNA expression in macrophages. Macrophages from BCG-vaccinated guinea pigs were cultured and infected in vitro with the different mycobacterial strains at an MOI of 1:30, 1:10, or 3:10 for 24 h. Representative RNA blots and densitometric analysis of IL-1β (A and B) and RANTES (C and D) mRNA expression (means based on three experiments) are shown. The differences between uninfected cultures and infected cultures were examined by ANOVA (P <0.001 to 0.0001). Within groups, the differences between M. tuberculosis H37Ra and H37Rv or Erdman were significant at P values of <0.001 (one asterisk) and <0.0001 to 0.0002 (two asterisks), as determined by Student's two-tailed t test.
Macrophages also exhibited RANTES mRNA expression after infection with various mycobacterial strains. The levels of RANTES mRNA were higher after infection with BCG (P < 0.0001 at an MOI of 3:10), M. avium (P < 0.003 at an MOI of 3:10), M. tuberculosis H37Ra (P < 0.002 at an MOI of 1:30 and P < 0.0001 at an MOI of 3:10), M. tuberculosis H37Rv (not significant), and M. tuberculosis Erdman (P < 0.001 at an MOI of 1:30 and P < 0.0006 at an MOI of 3:10) than they were in the uninfected cultures (Fig. 3C and D). However, BCG, M. avium, and M. tuberculosis H37Ra and Erdman induced higher levels of mRNA at an MOI of 1:30 or 3:10 than at an MOI of 1:10. Moreover, similar levels of mRNA were induced at MOIs of 1:30 and 3:10 by all of the strains except BCG, which induced a stronger response at an MOI of 3:10. Similar to the effect on IL-1β mRNA expression, infection of macrophages with M. tuberculosis H37Rv induced lower levels of RANTES mRNA than infection of macrophages with the attenuated H37Ra strain induced at MOIs of 1:30 (P < 0.001) and 3:10 (P < 0.0002). Infection with M. tuberculosis Erdman did not result in any reduction in the mRNA level at any of the infective doses. The percentages of reduction in the response obtained with the H37Rv strain were about 25, 30, and 60% at MOIs of 1:30, 1:10, and 3:10, respectively. The viabilities of macrophages after infection with M. tuberculosis H37Rv at all these MOIs were assessed by either trypan blue staining or the MTT assay after 48 h of infection. The results indicated that more than 95% of the cells were viable as determined by these two methods (data not shown). Therefore, the decreases in the cytokine or chemokine responses seen after virulent M. tuberculosis infection occurred in the absence of cytotoxicity.
Kinetics of IL-1β and RANTES mRNA expression in cultured macrophages.
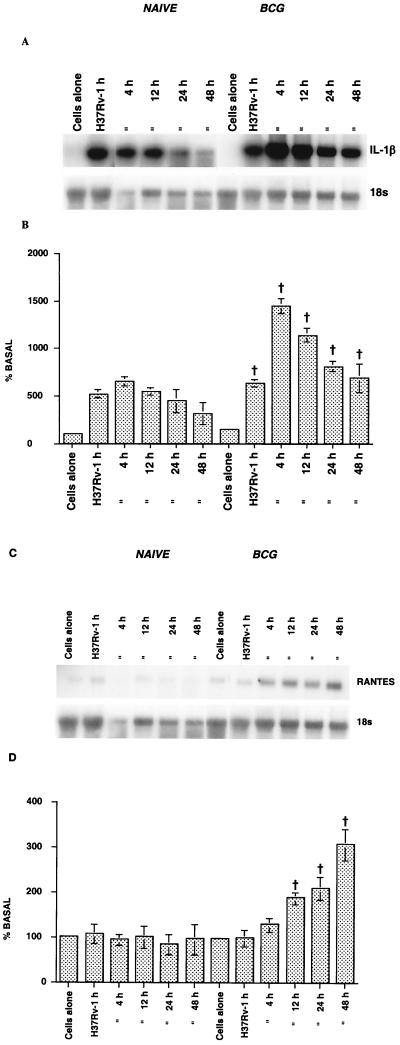
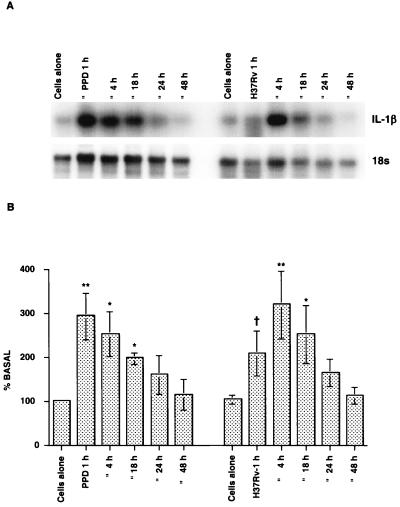
The kinetics of IL-1β and RANTES mRNA induction were assessed with macrophages from both naïve and BCG-vaccinated guinea pigs. The macrophages were infected with the virulent H37Rv strain of M. tuberculosis at an MOI of 1:30 for various periods of time. Figure 4A and B show that upon infection with the M. tuberculosis H37Rv strain, macrophages from both naïve and BCG-vaccinated guinea pigs exhibited a significant increase in IL-1β mRNA expression compared to uninfected cultures (P < 0.05). However, the macrophages from BCG-vaccinated guinea pigs exhibited an enhanced response compared to the cells from naïve animals (P < 0.0001). In both groups, the mRNA was induced as soon as 1 h after infection, the level peaked at 4 h, and then the level decreased with time over 48 h. The level of mRNA expression in macrophages fell to the basal level by 48 h in the naïve animals, but it was still elevated after 48 h in cells from the BCG-vaccinated animals.
FIG. 4.
Kinetics of IL-1β (A and B) and RANTES (C and D) mRNA expression in macrophages after M. tuberculosis H37Rv infection. Macrophages from naïve and BCG-vaccinated guinea pigs were infected in vitro with M. tuberculosis H37Rv at an MOI of 1:30 for specific periods of time. At regular intervals, adherent cells from the cultures were used for RNA analysis. The results are representative of the results of RNA blot analyses (A and C) and densitometric analyses (B and D) in three separate experiments. When the naïve and BCG-vaccinated groups were compared, the P value was <0.0001, as determined by ANOVA. A dagger indicates that the P value for a comparison of uninfected macrophages and an infected culture was <0.05, as determined by Dunnett's test.
The kinetics of RANTES mRNA induction in macrophages are shown in Fig. 4C and D. Uninfected macrophages from both naïve and BCG-vaccinated guinea pigs contained low levels of RANTES mRNA. Infection with the M. tuberculosis H37Rv strain did not alter the chemokine mRNA profile for the naïve group, whereas the levels were significantly enhanced (P < 0.0001) in the BCG-vaccinated group. In the latter group, upregulation of mRNA was evident 4 h after infection, and the levels at 18, 24, and 48 h were significantly different (P < 0.05, as determined by Dunnett's test) from the control levels; the maximum response occurred at 48 h. It was clear from other experiments that RANTES mRNA expression occurred even 72 h after infection, and the level of expression fell to the basal level by 120 h (data not shown).
Kinetics of IL-1β mRNA expression in splenocytes.
In order to determine whether IL-1β mRNA induction occurs in splenocytes as well as in macrophages, the kinetics of IL-1β mRNA expression in spleen cells from BCG-vaccinated animals after stimulation with PPD (15 μg/ml) or infection with M. tuberculosis H37Rv (MOI, 1:30) were determined (Fig. 5). Compared to unstimulated cultures, IL-1β mRNA was significantly induced as soon as 1 h after PPD stimulation (P < 0.0005), and the level of IL-1β mRNA remained well above the basal level after 4 h (P < 0.001) and 18 h (P < 0.009) in culture and began to decline by 24 to 48 h. Following infection with virulent M. tuberculosis, low levels of IL-1β mRNA were seen at 1 h (P < 0.01), a maximum response was seen at 4 h (P < 0.0005), and the levels were still elevated at 18 h after infection (P < 0.003) and declined by 48 h. Thus, the kinetics of IL-1β mRNA induction in the spleen cells somewhat paralleled the kinetics of IL-1β mRNA induction in PEC after infection with M. tuberculosis.
FIG. 5.
Kinetics of IL-1β mRNA expression in splenocytes after PPD or M. tuberculosis H37Rv stimulation. Whole spleen cells from BCG-vaccinated guinea pigs were stimulated with either PPD (15 μg/ml) or M. tuberculosis H37Rv (MOI, 1:30) for specific periods of time. At the end of each incubation period, cells were harvested and used for RNA analysis, and the levels of expression were analyzed as described in the text. (A) Representative RNA blot; (B) densitometric analysis of the means and standard errors from three experiments. A dagger indicates that the P value was <0.01 in comparison with the data obtained for the unstimulated cultures, as determined by Student's t test; one asterisk indicates that the P value was <0.001 to 0.009 in comparison with the data obtained for the unstimulated cultures, as determined by Student's t test; and two asterisks indicate that the P value was <0.0005 in comparison with the data obtained for the unstimulated cultures, as determined by Student's t test.
DISCUSSION
Previous work in our laboratory demonstrated that BCG vaccination induced a strong delayed-type hypersensitivity response to PPD in guinea pigs, which was associated with the ability of lymphocytes to proliferate and produce IL-2 in response to PPD (28, 30). Furthermore, prior vaccination with BCG protected guinea pigs against aerosol infection with virulent M. tuberculosis (29). Because IL-1β and RANTES are known to be associated with protective immune responses against mycobacteria, studies were undertaken to elucidate the effect of BCG vaccination on expression of these molecules in cultured guinea pig cells stimulated in vitro with various agonists, including mycobacterial antigens (PPD), or living mycobacteria.
The effects of the agonists used in this study (Fig. 1) on the ability of guinea pig spleen cells to induce IL-1β and RANTES mRNA expression are quite comparable to what has been reported for other systems. Peripheral blood leukocytes from normal human subjects stimulated with LPS or ConA produced IL-1β (35), and in mouse macrophages production of RANTES mRNA was induced following stimulation with LPS (22). PMA alone or in combination with ionomycin elicited secretion of RANTES and upregulated RANTES promoter activity in human lymphocytes or macrophages (34, 41). Similarly, stimulation of human peripheral blood leukocytes with PPD is known to result in production of RANTES protein (8). Our data indicate that guinea pig spleen cells, which consist of both lymphocytes and macrophages, respond in a similar manner when they are compared to other systems for induction of IL-1β and RANTES mRNA and when they are stimulated with PPD or agonists in vitro.
In the present study, it was observed that peritoneal macrophages from BCG-vaccinated animals infected with different mycobacterial strains in vitro exhibited significantly greater responses for both IL-1β and RANTES mRNA than cells from naïve animals exhibited (Fig. 2). Drapier and Hibbs (14) reported that in vivo infection of mice with BCG induced activated macrophages in the peritoneal cavity as these macrophages killed tumor targets more effectively than cells from uninfected mice killed tumor targets. Alternatively, it is possible that our macrophage preparations contained contaminating lymphocytes (less than 5%) that contributed to this effect.
There is ample evidence which indicates that IL-1β plays an important role in host resistance to mycobacteria, and expression of IL-1β has been associated with protective immunity (49). In humans, increased gene expression and release of IL-1β and TNF-α were seen with the BAL cells of tuberculosis patients when they were compared with cells from normal subjects (25). In other studies, live BCG induced production of proinflammatory cytokines, such as IL-1β or TNF-α, in tuberculosis patients and healthy individuals irrespective of their tuberculin skin status (43). Both M. tuberculosis and its cell wall components, as well as heat shock proteins from M. tuberculosis, Mycobacterium leprae, and M. bovis, induced mRNA for IL-1β, TNF-α, and granulocyte-macrophage colony-stimulating factor in human monocytes (19, 36). In our study of guinea pig macrophages, IL-1β mRNA expression also increased in response to mycobacterial infection in vitro, and this response was enhanced following BCG vaccination (Fig. 4A and B). These findings are consistent with a protective proinflammatory cytokine response following infection, and the response appears to mimic the response in humans infected with M. tuberculosis.
It is known that T cells and monocyte/macrophages, the integral components of a granuloma reaction, migrate in response to RANTES (3, 39). During the acute phase of tuberculosis, the levels of RANTES, IL-8, and monocyte chemoattractant protein 1 (MCP-1) were markedly increased in BAL fluid, and the concentration of RANTES correlated significantly with the absolute number of CD4+ cells (23). RANTES expression has been detected in the granulomatous lesions of both sarcoidosis and tuberculosis patients (13), and expression of RANTES was predominant in the macrophages and endothelial cells in the lesions. The role of RANTES in antimycobacterial immunity has also been studied in mouse systems. In IFN-γ knockout mice, decreased levels of TNF-α and RANTES mRNA were found after granulomas were elicited by beads coated with PPD (6). Similarly, in mouse models of type 1 and type 2 cell-mediated pulmonary granulomas elicited with M. bovis or Schistosoma mansoni egg antigen-coated beads, type 1 lesions had higher levels of RANTES protein and mRNA production than type 2 lesions. Injection of anti-RANTES antibody decreased the type 1 lesions and increased the type 2 lesions (7). These findings suggest that expression of RANTES is associated with the presence of protective cytokines and that RANTES contributes to mediation of an effective immune response.
Despite the rather extensive studies regarding the immune responses in mice and humans, very few studies have addressed this question with guinea pigs. Vaccination of inbred guinea pigs with M. bovis BCG resulted in an increase in the proportion of CD8+ T cells in the spleens at 8 days postvaccination (21). Expression of RANTES and IFN-γ mRNA was detected in the spleens of both naïve and BCG-vaccinated animals by reverse transcription-PCR. Furthermore, after vaccination, CD8+ T cells but not CD4+ cells from the lymph nodes expressed RANTES and IFN-γ mRNA (21). It is difficult to compare our results with the results of this previous study because the two studies differed in the strain of animals used, the dose of BCG used for vaccination, the time point at which the assays were done, and the assay with which mRNA expression was determined. Additionally, the cells used in the previous study were not cultured or further stimulated with any antigens, as the cells used in our studies were.
The time course of IL-1β and RANTES mRNA expression seems to differ after virulent M. tuberculosis infection, as IL-1β mRNA expression was observed as soon as 1 to 4 h after infection (Fig. 4A and B and 5). The time courses of IL-1β mRNA expression are similar in spleen cells and macrophages after virulent mycobacterial stimulation. Furthermore, PPD and viable mycobacteria induce similar kinetics for IL-1β mRNA expression in splenocytes. Unlike IL-1β mRNA induction, induction of RANTES mRNA was delayed, and the response peaked only at 48 h (Fig. 4C and D). Our results are consistent with other reports as IL-1β is a proinflammatory cytokine and is known to be induced in human monocytes as soon as 3 h after infection with M. avium (18). In contrast, the release of RANTES protein was delayed until 24 h when human epithelial cells were infected with M. avium (38), and the maximum response occurred 72 h after infection. Thus, human and guinea pig cells appear to respond similarly to mycobacterial stimulation of induction of IL-1β and RANTES mRNA.
Although no clear dose-response effect was seen in the MOI experiments, it is interesting that virulent M. tuberculosis induced lower levels of mRNA expression irrespective of the MOI used. It is not clear why virulent M. tuberculosis H37Rv induced less IL-1β and RANTES mRNA than the attenuated H37Ra strain induced in the BCG-vaccinated hosts but not in the naïve guinea pigs (Fig. 2). Macrophages infected with the Erdman strain expressed high levels of RANTES mRNA comparable to the level expressed by cells infected with an avirulent strain. We found that M. tuberculosis Erdman was slightly more virulent than the H37Rv strain in guinea pigs (unpublished data); however, the differences in the peak bacterial levels in the lungs and spleens 3 to 5 weeks after low-dose pulmonary infection were not statistically significant. Several reports have shown that the levels of certain cytokines, such as TNF-α or IL-1β, produced after mycobacterial stimulation seem to depend on the virulence of the organism and that this might be related to pathogenesis. It is known that the attenuated H37Ra strain of M. tuberculosis is capable of inducing a larger amount of NO in cultured human peripheral blood mononuclear cells than is the virulent H37Rv strain (24). In the case of AIDS patients, less IL-1α and less IL-1β were produced by monocytes in response to virulent M. avium than were produced by cells from healthy subjects. In both control and AIDS patients, infection of monocytes with the less virulent organism M. avium induced the release of more IL-1β (18). Other reports indicated that infection of human monocytes or macrophages with virulent M. avium downregulated production of proinflammatory cytokines (IL-1β, TNF-α, IL-6, and granulocyte-macrophage colony-stimulating factor) compared to infection with less virulent bacteria (33). Similar observations have been made with mice as lipoarabinomannan (LAM) from an attenuated strain of M. tuberculosis induced macrophage activation and TNF-α production, whereas LAM from the virulent Erdman strain was less active (2). Several factors, including differences in the LAM structure at the nonreducing termini in virulent and nonvirulent mycobacteria (5), differential induction of early genes (37), or different receptors that mediate phagocytosis (40), have all been used to explain this effect. It seems that virulent mycobacterial strains disarm host defenses by failing to induce proinflammatory cytokines that are necessary for resistance (33).
The mechanism of IL-1β or RANTES mRNA inhibition by virulent mycobacteria is not well understood. Several immunosuppressive cytokines, such as IL-10 or TGF-β, have been implicated in the pathogenesis of tuberculosis in mice, humans, and guinea pigs (11, 42). Previous results in our laboratory indicated that peritoneal macrophages from protein-malnourished guinea pigs produced high TGF-β levels after in vitro infection with virulent M. tuberculosis (11) compared to the levels of TGF-β produced by macrophages from normally fed animals. TGF-β is known to inhibit LPS-induced IL-1β in human peripheral blood mononuclear cells in a dose-dependent manner (4). Production of other cytokines, such as TNF-α induced by M. bovis BCG, was also inhibited by TGF-β in human mononuclear cells (32). It is possible that excessive levels of TGF-β produced by monocytes infected with virulent mycobacteria downregulate IL-1β, TNF-α, and other mediators of protective immunity. Our laboratory is now examining the immunomodulatory role that TGF-β might play in M. tuberculosis infection in guinea pigs.
In conclusion, these initial studies strongly indicate that BCG vaccination enhances cytokine responses in guinea pig cells towards agonists and living mycobacteria. In addition, this system can be successfully used to demonstrate individual differences in cytokine profiles after stimulation of cells with virulent mycobacteria and with attenuated strains. Furthermore, we provide evidence that virulent mycobacteria may interfere with proinflammatory cytokine and chemokine responses in vitro. However, the cellular source(s) of the cytokine and chemokine was not clearly determined in this study. With the help of recombinant proteins, as well as antibodies to these molecules, future studies will focus on characterizing the cells that are specifically involved in upregulation of IL-1β and RANTES mRNA. Since the guinea pig model is gaining importance as a tuberculosis model, detailed studies of immunoregulation by cytokines and chemokines in this species should allow us to characterize the effects of candidate vaccines against M. tuberculosis.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 AI 15495 to D.N.M.
We are indebted to Kyeong Eun Lee and workers at the Biostatistics Core of the Center for Environmental and Rural Health for their expertise in statistical analysis and to Susan Phalen for her suggestions and ideas throughout this study.
REFERENCES
- 1.Barnes, P. F., S. J. Fong, P. J. Brennan, P. E. Twomey, A. Mazumder, and R. L. Modlin. 1990. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J. Immunol. 145:149-154. [PubMed] [Google Scholar]
- 2.Brown, M. C., and S. M. Taffet. 1995. Lipoarabinomannans derived from different strains of Mycobacterium tuberculosis differentially stimulate the activation of NF-κB and KBF1 in murine macrophages. Infect. Immun. 63:1960-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell, E. M., A. E. Proudfoot, T. Yoshimura, B. Allet, T. N. Wells, A. M. White, J. Westwick, and M. L. Watson. 1997. Recombinant guinea pig and human RANTES activate macrophages but not eosinophils in the guinea pig. J. Immunol. 159:1482-1489. [PubMed] [Google Scholar]
- 4.Chantry, D., M. Turner, E. Abney, and M. Feldmann. 1989. Modulation of cytokine production by transforming growth factor-beta. J. Immunol. 142:4295-4300. [PubMed] [Google Scholar]
- 5.Chatterjee, D., A. D. Roberts, K. Lowell, P. J. Brennan, and I. M. Orme. 1992. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect. Immun. 60:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chensue, S. W., K. Warmington, J. H. Ruth, N. Lukacs, and S. L. Kunkel. 1997. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J. Immunol. 159:3565-3573. [PubMed] [Google Scholar]
- 7.Chensue, S. W., K. S. Warmington, E. J. Allenspach, B. Lu, C. Gerard, S. L. Kunkel, and N. W. Lukacs. 1999. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J. Immunol. 163:165-173. [PubMed] [Google Scholar]
- 8.Cho, Y. Y., A. Astgen, H. Hendel, W. Issing, J. Y. Perrot, F. Schachter, J. Rappaport, and J. F. Zagury. 1997. Homeostasis of chemokines, interferon production and lymphocyte subsets: implications for AIDS pathogenesis. Biomed. Pharmacother. 51:221-229. [DOI] [PubMed] [Google Scholar]
- 9.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed]
- 10.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai, G., and D. N. McMurray. 1998. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect. Immun. 66:3562-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis, M., and E. Ghadirian. 1991. Transforming growth factor beta (TGF-β1) plays a detrimental role in the progression of experimental Mycobacterium avium infection; in vivo and in vitro evidence. Microb. Pathog. 11:367-372. [DOI] [PubMed] [Google Scholar]
- 13.Devergne, O., A. Marfaing-Koka, T. J. Schall, M. B. Leger-Ravet, M. Sadick, M. Peuchmaur, M. C. Crevon, K. J. Kim, T. T. Schall, and T. Kim. 1994. Production of the RANTES chemokine in delayed-type hypersensitivity reactions: involvement of macrophages and endothelial cells. J. Exp. Med. 179:1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drapier, J. C., and J. B. Hibbs, Jr. 1988. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. 140:2829-2838. [PubMed] [Google Scholar]
- 15.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, M. B., S. E. Nielsen, and K. Berg. 1989. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 119:203-210. [DOI] [PubMed] [Google Scholar]
- 17.Jeevan, A., C. D. Bucana, Z. Dong, V. V. Dizon, S. L. Thomas, T. E. Lloyd, and M. L. Kripke. 1995. Ultraviolet radiation reduces phagocytosis and intracellular killing of mycobacteria and inhibits nitric oxide production by macrophages in mice. J. Leukoc. Biol. 57:883-890. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. L., H. Shiratsuchi, Z. Toossi, and J. J. Ellner. 1997. Altered IL-1 expression and compartmentalization in monocytes from patients with AIDS stimulated with Mycobacterium avium complex. J. Clin. Immunol. 17:387-395. [DOI] [PubMed] [Google Scholar]
- 19.Juffermans, N. P., A. Verbon, J. T. Belisle, P. J. Hill, P. Speelman, S. J. van Deventer, and T. van der Poll. 2000. Mycobacterial lipoarabinomannan induces an inflammatory response in the mouse lung. A role for interleukin-1. Am. J. Respir. Crit. Care Med. 162:486-489. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, S. H. E. 1987. Towards new leprosy and tuberculosis vaccines. Microbiol. Sci. 4:324-328. [PubMed] [Google Scholar]
- 21.Klünner, T., T. Bartels, M. Vordermeier, R. Burger, and H. Schäfer. 2001. Immune reactions of CD4- and CD8-positive T cell subpopulations in spleen and lymph nodes of guinea pigs after vaccination with bacillus Calmette Guerin. Vaccine 19:1968-1977. [DOI] [PubMed] [Google Scholar]
- 22.Kopydlowski, K. M., C. A. Salkowski, M. J. Cody, N. van Rooijen, J. Major, T. A. Hamilton, and S. N. Vogel. 1999. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 163:1537-1544. [PubMed] [Google Scholar]
- 23.Kurashima, K., N. Mukaida, M. Fujimura, M. Yasui, Y. Nakazumi, T. Matsuda, and K. Matsushima. 1997. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am. J. Respir. Crit. Care Med. 155:1474-1477. [DOI] [PubMed] [Google Scholar]
- 24.Kwon, O. J. 1997. The role of nitric oxide in the immune response of tuberculosis. J. Korean Med. Sci. 12:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law, K., M. Weiden, T. Harkin, K. Tchou-Wong, C. Chi, and W. N. Rom. 1996. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 153:799-804. [DOI] [PubMed] [Google Scholar]
- 26.Mackaness, G. B. 1968. The immunology of antituberculous immunity. Am. Rev. Respir. Dis. 97:337-344. [DOI] [PubMed] [Google Scholar]
- 27.Marfaing-Koka, A., O. Devergne, G. Gorgone, A. Portier, T. J. Schall, P. Galanaud, and D. Emilie. 1995. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J. Immunol. 154:1870-1878. [PubMed] [Google Scholar]
- 28.McMurray, D. N., M. A. Carlomagno, and P. A. Cumberland. 1983. Respiratory infection with attenuated Mycobacterium tuberculosis H37Ra in malnourished guinea pigs. Infect. Immun. 39:793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray, D. N., M. A. Carlomagno, C. L. Mintzer, and C. L. Tetzlaff. 1985. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect. Immun. 50:555-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMurray, D. N., C. L. Mintzer, R. A. Bartow, and R. L. Parr. 1989. Dietary protein deficiency and Mycobacterium bovis BCG affect interleukin-2 activity in experimental pulmonary tuberculosis. Infect. Immun. 57:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurray, D. N., and E. A. Yetley. 1983. Response to Mycobacterium bovis BCG vaccination in protein- and zinc-deficient guinea pigs. Infect. Immun. 39:755-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Méndez-Samperio, P., M. Hernandez-Garay, and A. Nuñez Vazquez. 1998. Inhibition of Mycobacterium bovis BCG-induced tumor necrosis factor alpha secretion in human cells by transforming growth factor β. Clin. Diagn. Lab. Immunol. 5:588-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelini-Norris, M. B., D. K. Blanchard, C. A. Pearson, and J. Y. Djeu. 1992. Differential release of interleukin (IL)-1 alpha, IL-1 beta, and IL-6 from normal human monocytes stimulated with a virulent and an avirulent isogenic variant of Mycobacterium avium-intracellulare complex. J. Infect. Dis. 165:702-709. [DOI] [PubMed] [Google Scholar]
- 34.Moriuchi, H., M. Moriuchi, and A. S. Fauci. 1997. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 158:3483-3491. [PubMed] [Google Scholar]
- 35.Pyne, D. V., W. A. McDonald, D. S. Morton, J. P. Swigget, M. Foster, G. Sonnenfeld, and J. A. Smith. 2000. Inhibition of interferon, cytokine, and lymphocyte proliferative responses in elite swimmers with altitude exposure. J. Interferon Cytokine Res. 20:411-418. [DOI] [PubMed] [Google Scholar]
- 36.Retzlaff, C., Y. Yamamoto, P. S. Hoffman, H. Friedman, and T. W. Klein. 1994. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect. Immun. 62:5689-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roach, T. I., C. H. Barton, D. Chatterjee, and J. M. Blackwell. 1993. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-alpha. J. Immunol. 150:1886-1896. [PubMed] [Google Scholar]
- 38.Sangari, F. J., M. Petrofsky, and L. E. Bermudez. 1999. Mycobacterium avium infection of epithelial cells results in inhibition or delay in the release of interleukin-8 and RANTES. Infect. Immun. 67:5069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669-671. [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 41.Sotsios, Y., P. J. Blair, J. Westwick, and S. G. Ward. 2000. Disparate effects of phorbol esters, CD3 and the costimulatory receptors CD2 and CD28 on RANTES secretion by human T lymphocytes. Immunology 101:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toossi, Z., T. G. Young, L. E. Averill, B. D. Hamilton, H. Shiratsuchi, and J. J. Ellner. 1995. Induction of transforming growth factor beta 1 by purified protein derivative of Mycobacterium tuberculosis. Infect. Immun. 63:224-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Crevel, R., J. van der Ven-Jongekrijg, M. G. Netea, W. de Lange, B. J. Kullberg, and J. W. van der Meer. 1999. Disease-specific ex vivo stimulation of whole blood for cytokine production: applications in the study of tuberculosis. J. Immunol. Methods 222:145-153. [DOI] [PubMed] [Google Scholar]
- 44.Yam, L. T., C. Y. Li, and W. H. Crosby. 1971. Cytochemical identification of monocytes and granulocytes. Am. J. Clin. Pathol. 55:283-290. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura, T., and D. G. Johnson. 1993. cDNA cloning and expression of guinea pig neutrophil attractant protein-1 (NAP-1). NAP-1 is highly conserved in guinea pig. J. Immunol. 151:6225-6236. [PubMed] [Google Scholar]
- 46.Yoshimura, T., M. Takeya, H. Ogata, S. Yamashiro, W. S. Modi, and R. Gillitzer. 1999. Molecular cloning of the guinea pig GRO gene and its rapid expression in the tissues of lipopolysaccharide-injected guinea pigs. Int. Arch. Allergy Immunol. 119:101-111. [DOI] [PubMed] [Google Scholar]
- 47.Young, L. S., C. B. Inderlied, O. G. Berlin, and M. S. Gottlieb. 1986. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev. Infect. Dis. 8:1024-1033. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Y., M. Doerfler, T. C. Lee, B. Guillemin, and W. N. Rom. 1993. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J. Clin. Investig. 91:2076-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, Y., and W. N. Rom. 1993. Regulation of the interleukin-1β (IL-1β) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol. Cell. Biol. 13:3831-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]